

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410424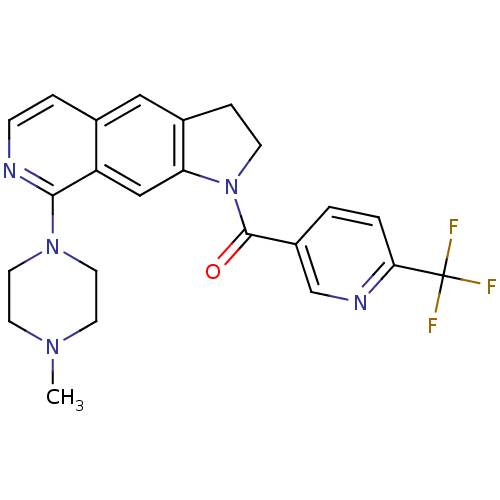 (CHEMBL199088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50410428 (CHEMBL380812) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410426 (CHEMBL370852) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50410424 (CHEMBL199088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410427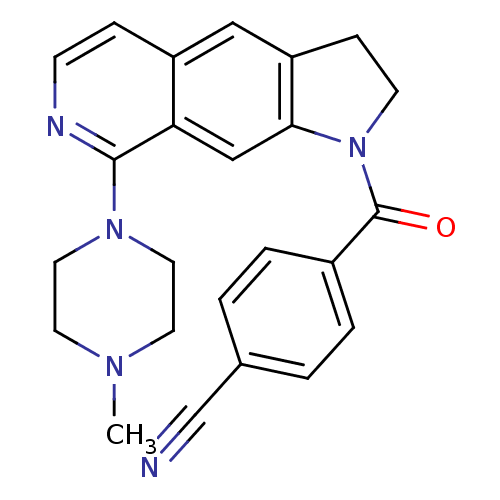 (CHEMBL194647) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50410426 (CHEMBL370852) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50410440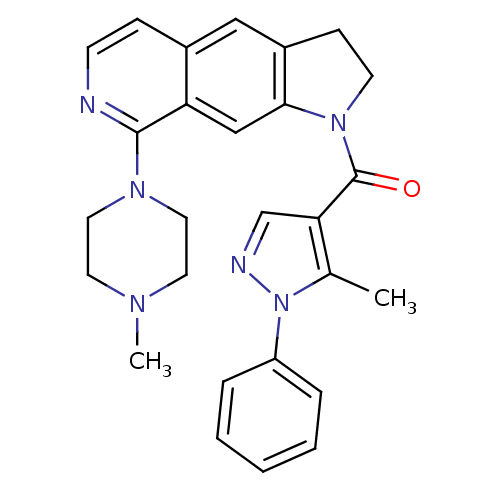 (CHEMBL198975) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410440 (CHEMBL198975) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50410440 (CHEMBL198975) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50410430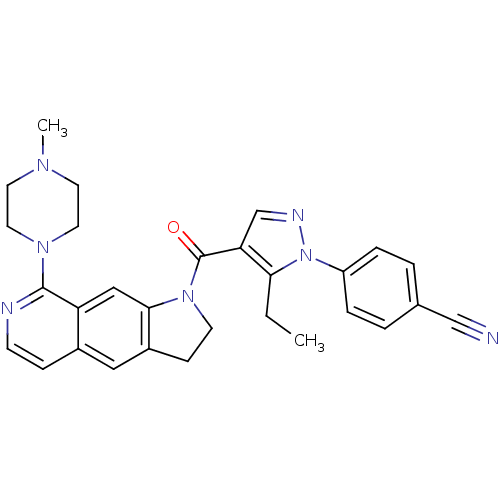 (CHEMBL372743) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50410426 (CHEMBL370852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50410427 (CHEMBL194647) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410437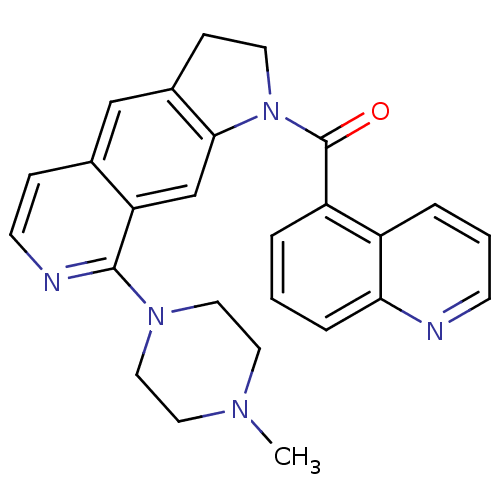 (CHEMBL370625) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474384 (CHEMBL2113364) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410428 (CHEMBL380812) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50410427 (CHEMBL194647) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474398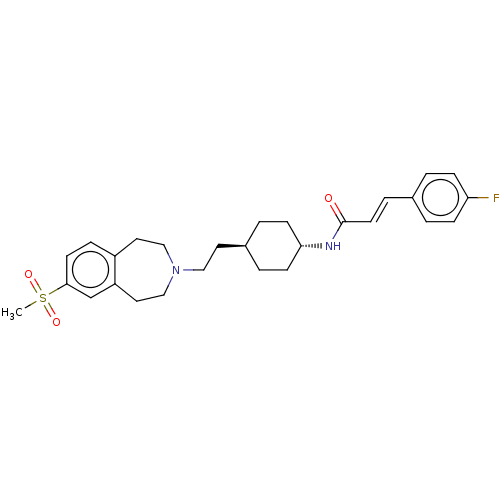 (CHEMBL2368629) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50410432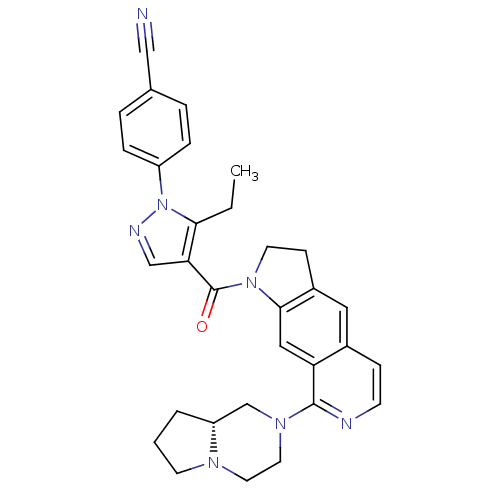 (CHEMBL197947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410430 (CHEMBL372743) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410432 (CHEMBL197947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474396 (CHEMBL2113356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410429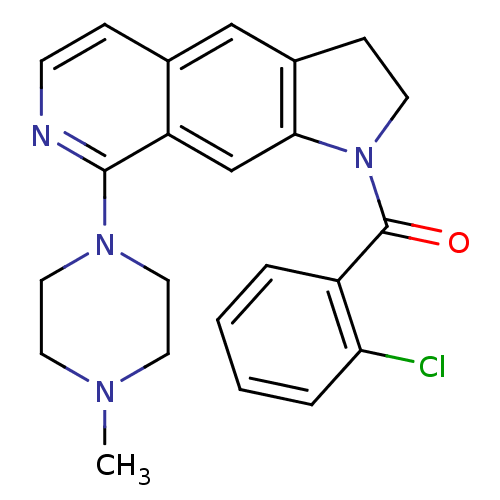 (CHEMBL194809) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50410436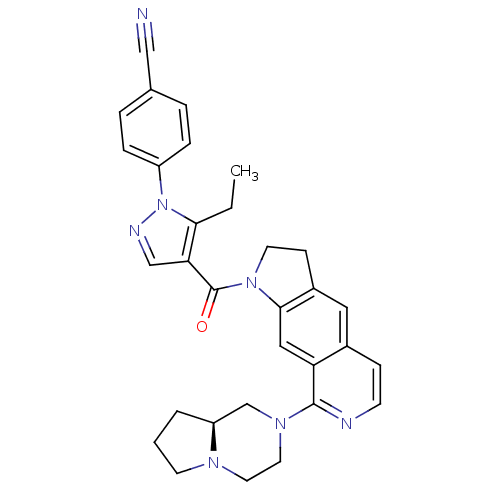 (CHEMBL382078) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50410434 (CHEMBL372651) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50410430 (CHEMBL372743) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50410428 (CHEMBL380812) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474403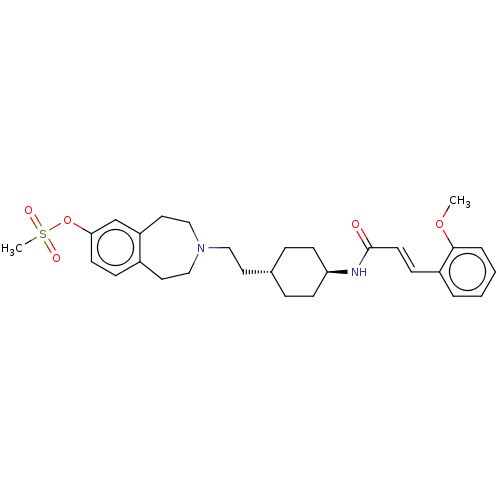 (CHEMBL2368633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474395 (CHEMBL2368626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474389 (CHEMBL3084597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474399 (CHEMBL2368625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474406 (CHEMBL3084598) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474412 (CHEMBL2368622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50410432 (CHEMBL197947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410433 (CHEMBL426285) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474410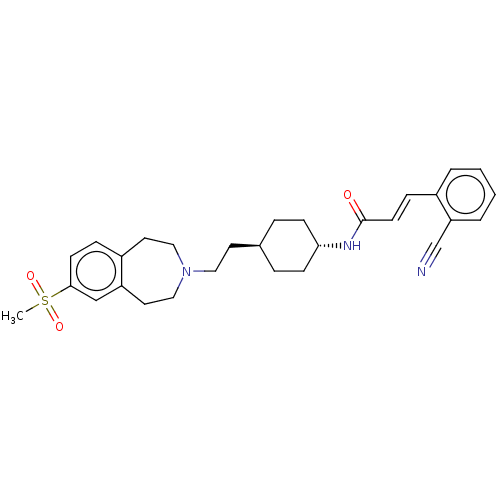 (CHEMBL2368620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50000022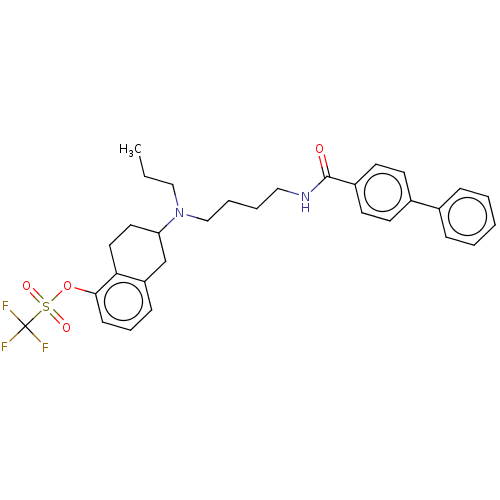 (CHEMBL57219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50000027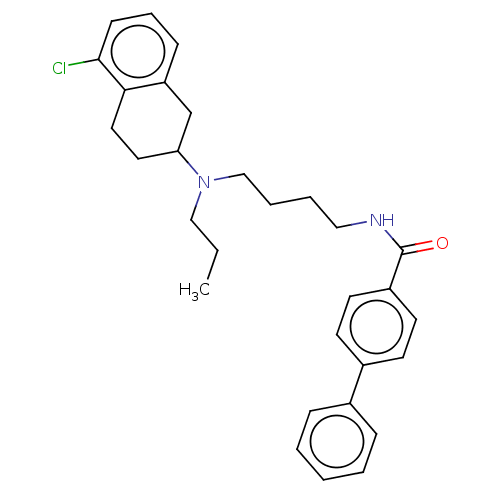 (CHEMBL51977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474393 (CHEMBL2368623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50217172 (CHEMBL52724) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474392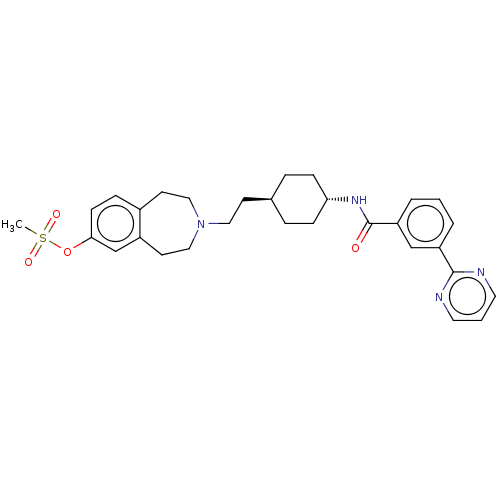 (CHEMBL3084601) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50410435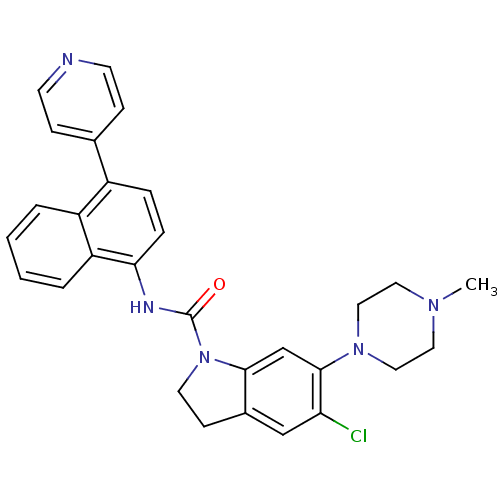 (CHEMBL191971 | SB-272183) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474407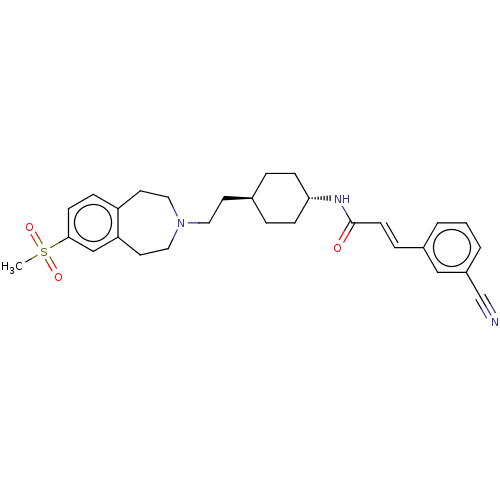 (CHEMBL2368624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474404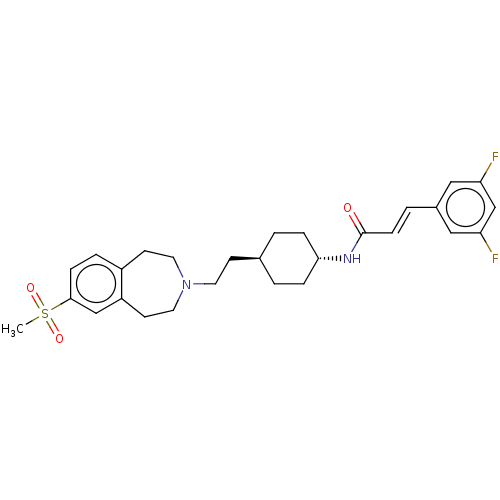 (CHEMBL2368627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410436 (CHEMBL382078) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474401 (CHEMBL2368618) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474413 (CHEMBL3084626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50217155 (CHEMBL291545) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474414 (CHEMBL2368617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50410439 (CHEMBL199175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand | Bioorg Med Chem Lett 15: 4370-4 (2005) Article DOI: 10.1016/j.bmcl.2005.06.042 BindingDB Entry DOI: 10.7270/Q2319X3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474388 (CHEMBL3084625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 309 total ) | Next | Last >> |