

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422425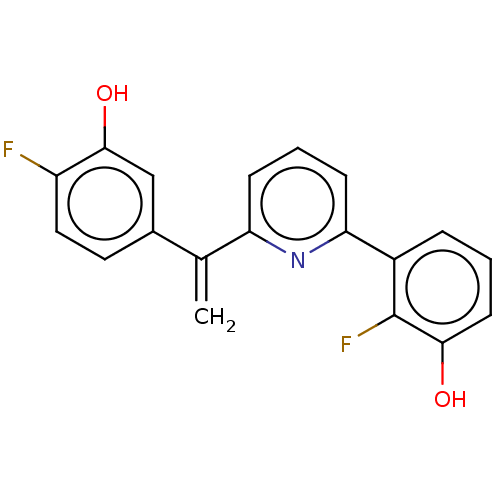 (CHEMBL4175585) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422397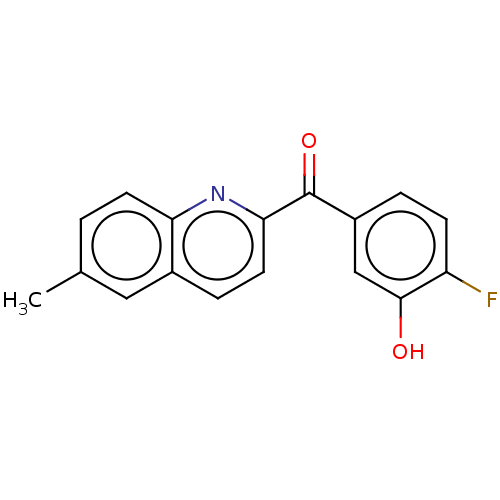 (CHEMBL4176263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422422 (CHEMBL4164239) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422417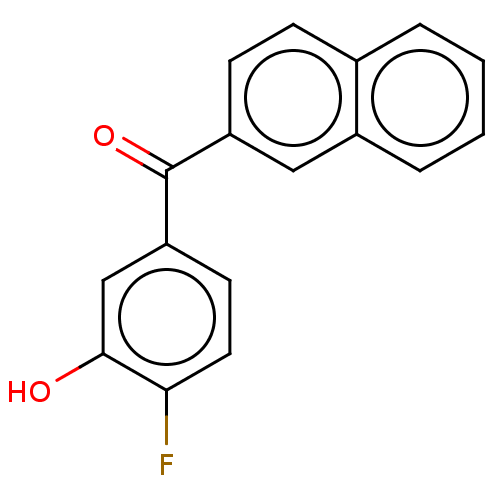 (CHEMBL4165659) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422419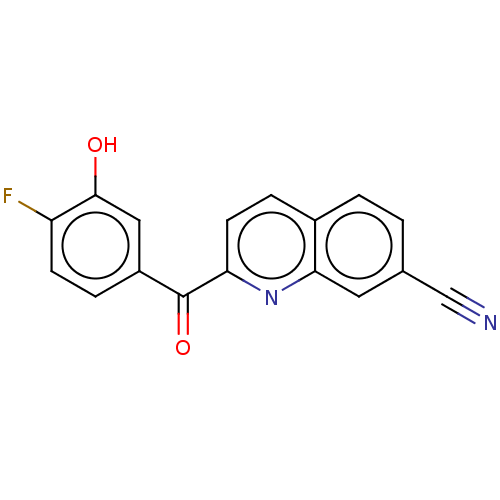 (CHEMBL4168004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422398 (CHEMBL4172463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422416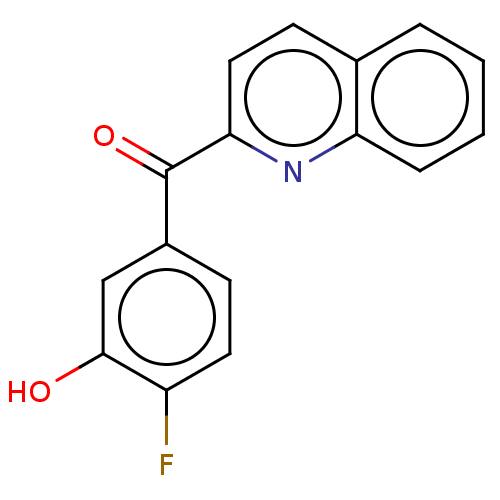 (CHEMBL4171902) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197831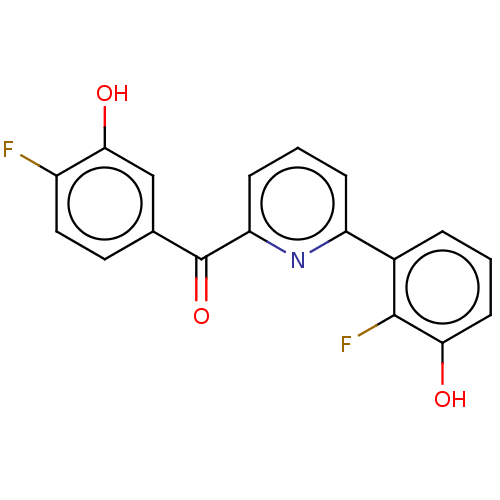 (CHEMBL3949996) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569927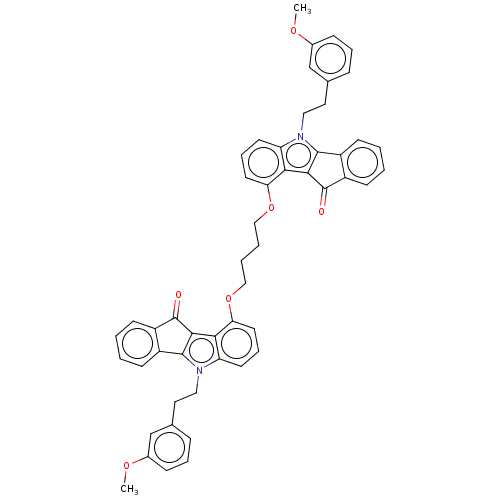 (CHEMBL4851961) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki2 for inhibition of mitoxantrone efflux measured after 30 min... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422421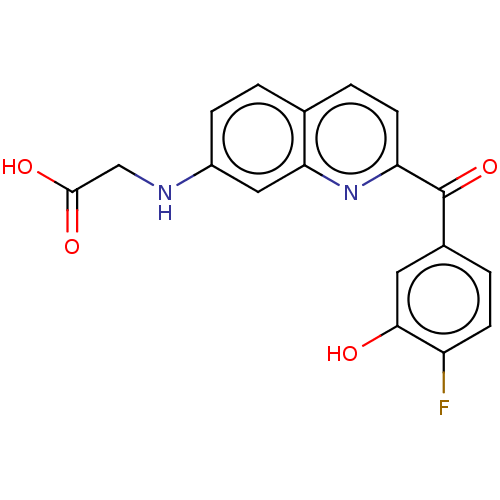 (CHEMBL4174579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569927 (CHEMBL4851961) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki2 for inhibition of Hoechst33342 efflux measured after 30 mins by FAC... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422420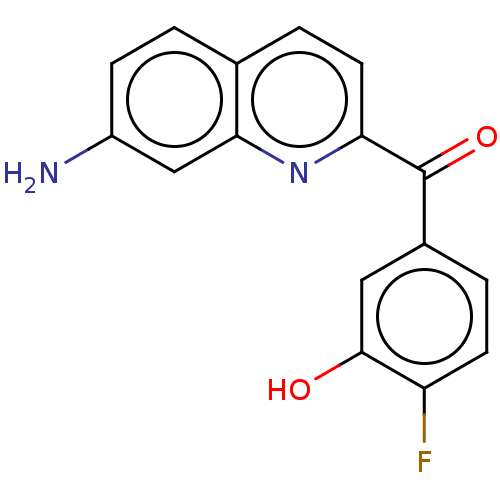 (CHEMBL4164576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569929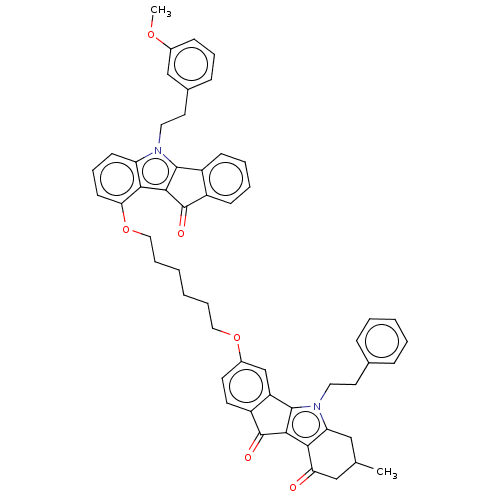 (CHEMBL4876781) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki2 for inhibition of mitoxantrone efflux measured after 30 min... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422426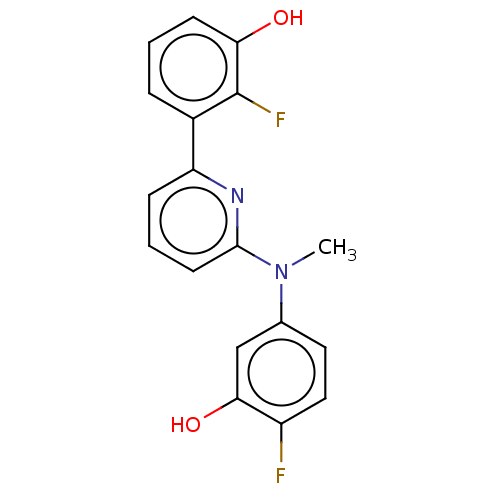 (CHEMBL4169325) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569929 (CHEMBL4876781) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki2 for inhibition of mitoxantrone efflux measured after 30 mins by FAC... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422427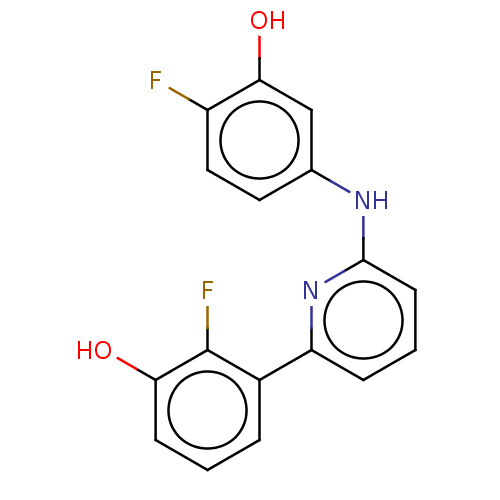 (CHEMBL4177499) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422402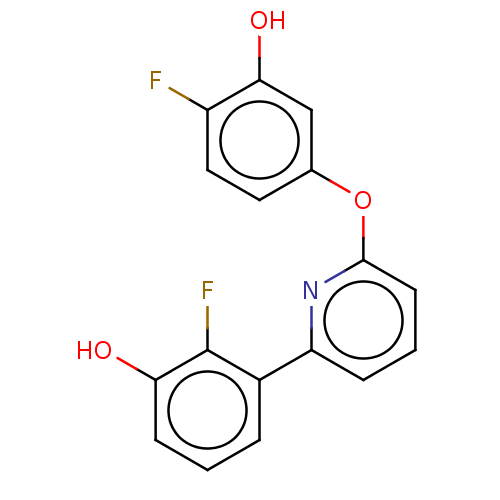 (CHEMBL4171183) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569927 (CHEMBL4851961) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki1 for inhibition of Hoechst33342 efflux measured after 30 mins by FAC... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422418 (CHEMBL4175870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569928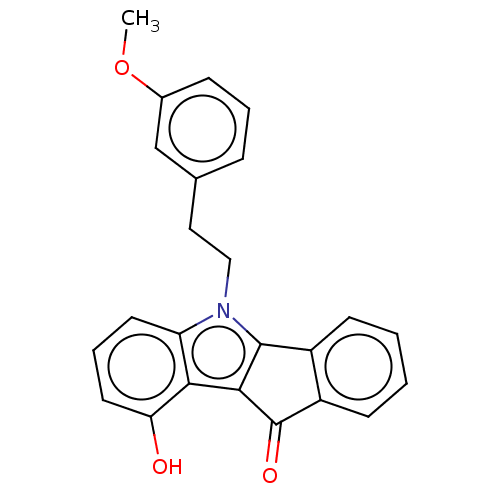 (CHEMBL4875810) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki1 for inhibition of mitoxantrone efflux measured after 30 mins by FAC... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569929 (CHEMBL4876781) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki1 for inhibition of mitoxantrone efflux measured after 30 mins by FAC... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569928 (CHEMBL4875810) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-competitive inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki2 for inhibition of mitoxantrone efflux measured after 30 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50569928 (CHEMBL4875810) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki2 for inhibition of mitoxantrone efflux measured after 30 mins by FAC... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50029739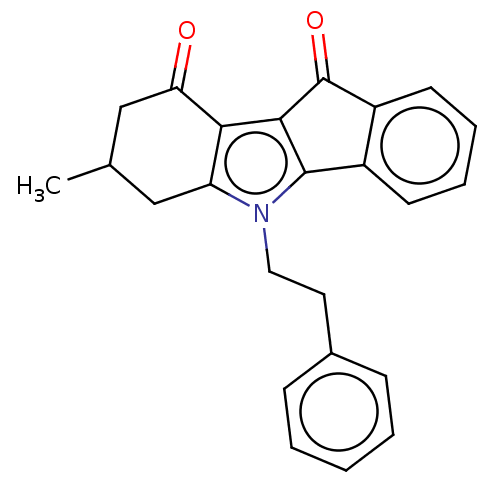 (CHEMBL3353414) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki1 for inhibition of mitoxantrone efflux measured after 30 mins by FAC... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422423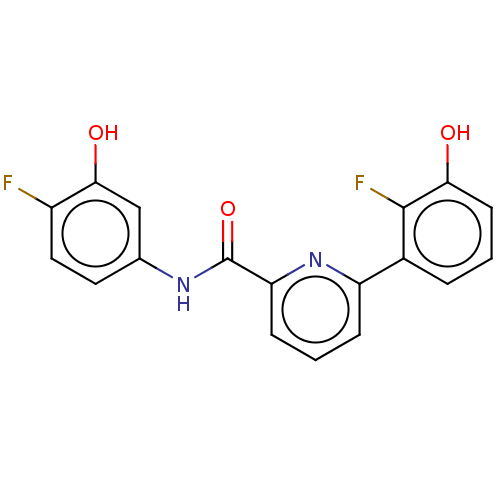 (CHEMBL4160081) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422399 (CHEMBL4167395) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 686 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50029739 (CHEMBL3353414) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 843 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of human ABCG2 transfected in human HEK293 cells assessed as Ki2 for inhibition of mitoxantrone efflux measured after 30 mins by FAC... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113017 BindingDB Entry DOI: 10.7270/Q2VX0M95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50422424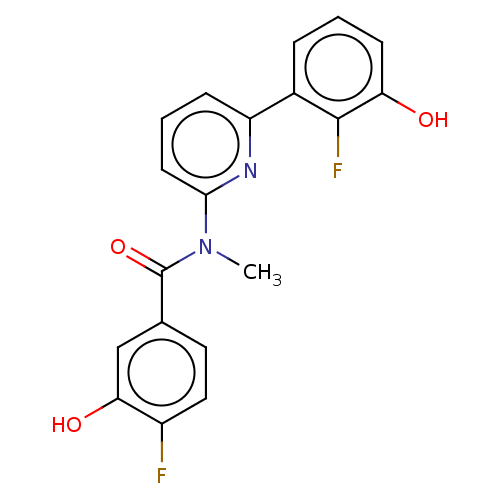 (CHEMBL4170719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... | Eur J Med Chem 155: 61-76 (2018) Article DOI: 10.1016/j.ejmech.2018.05.029 BindingDB Entry DOI: 10.7270/Q23F4S76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495680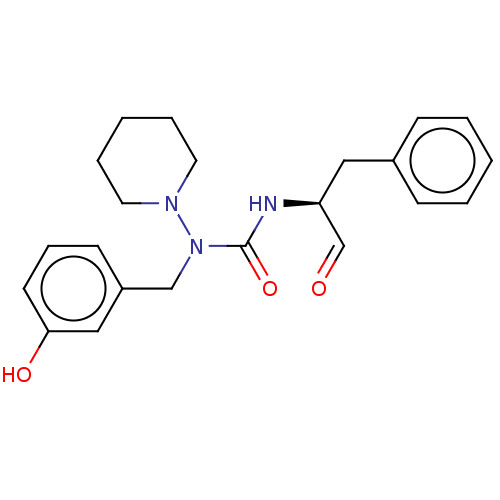 (CHEMBL3115093) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495686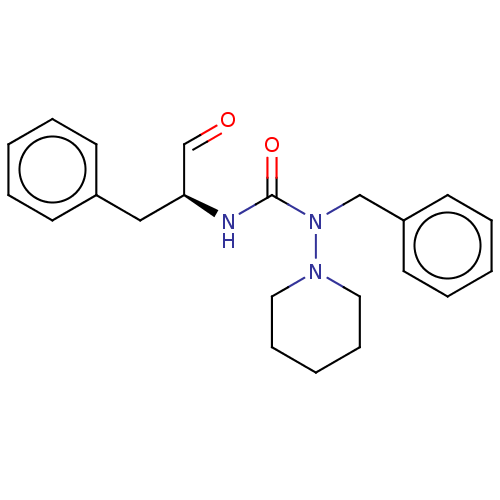 (CHEMBL3115090) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495680 (CHEMBL3115093) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495686 (CHEMBL3115090) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495680 (CHEMBL3115093) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495686 (CHEMBL3115090) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495681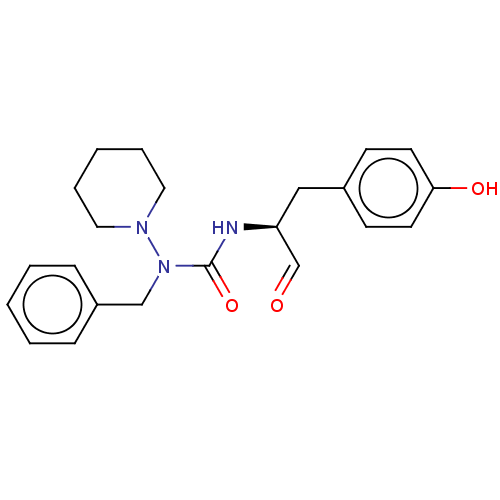 (CHEMBL3115092) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495684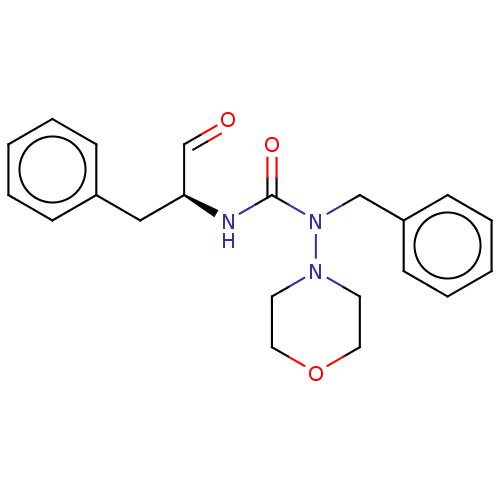 (CHEMBL3115091) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495683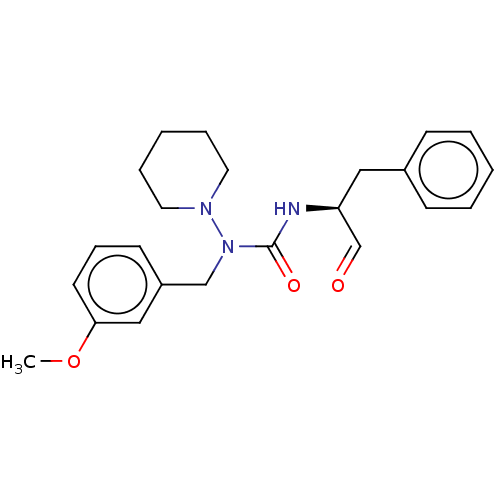 (CHEMBL3115094) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495683 (CHEMBL3115094) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495681 (CHEMBL3115092) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495684 (CHEMBL3115091) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495681 (CHEMBL3115092) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495685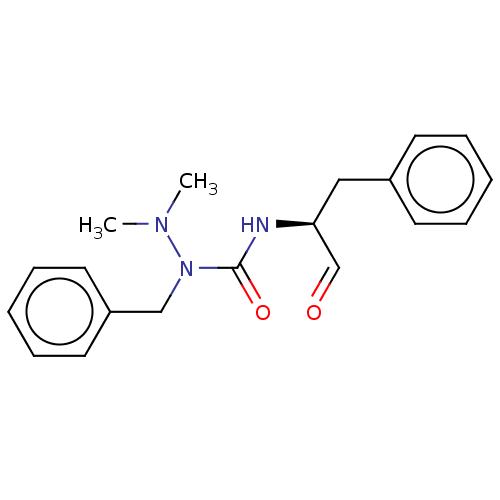 (CHEMBL3115089) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495683 (CHEMBL3115094) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495682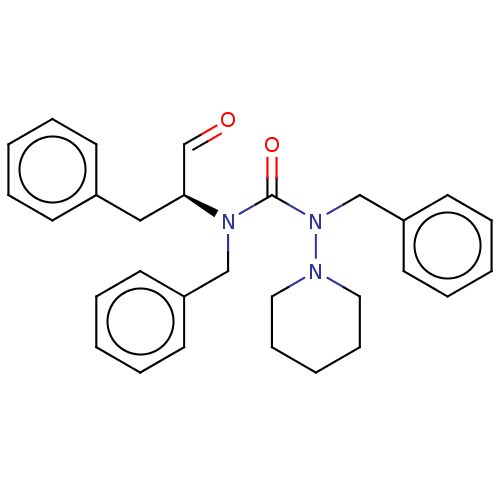 (CHEMBL3115095) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495685 (CHEMBL3115089) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495685 (CHEMBL3115089) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50495682 (CHEMBL3115095) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon - ENS Curated by ChEMBL | Assay Description Inhibition of HIV-1 recombinant protease using DABCYL-GABA-Ser-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as substrate incubated for 30 mins prior to substrate ad... | Bioorg Med Chem 21: 5407-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.018 BindingDB Entry DOI: 10.7270/Q2D50QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 137 total ) | Next | Last >> |