

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM97464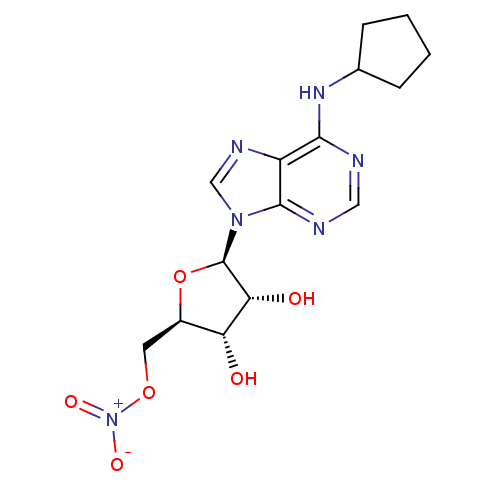 (US8470800, A | US8609833, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99192 (US8501708, 12) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99205 (US8501708, 29) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM97465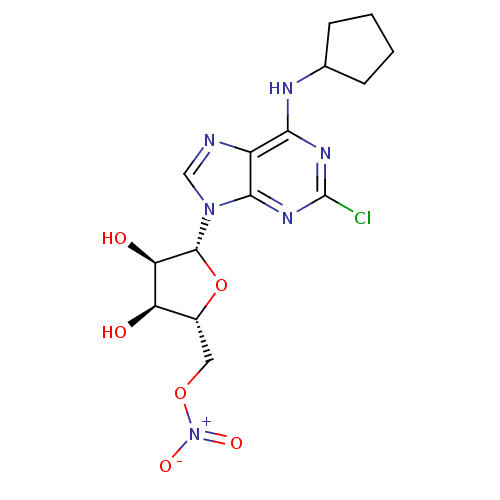 (US8470800, B | US8609833, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99204 (US8501708, 28) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99197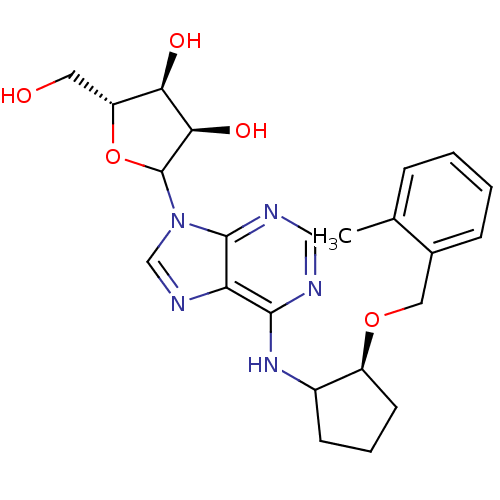 (US8501708, 17) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99193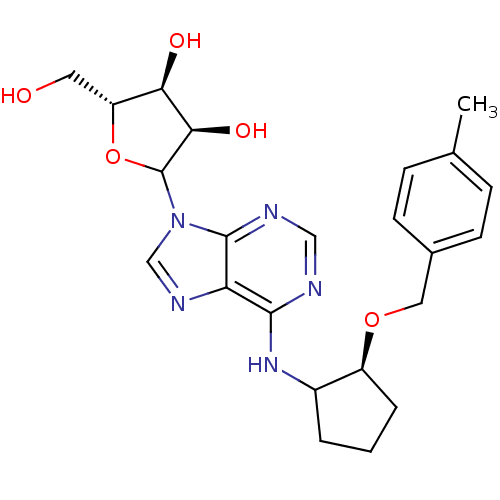 (US8501708, 13) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99206 (US8501708, 30) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99199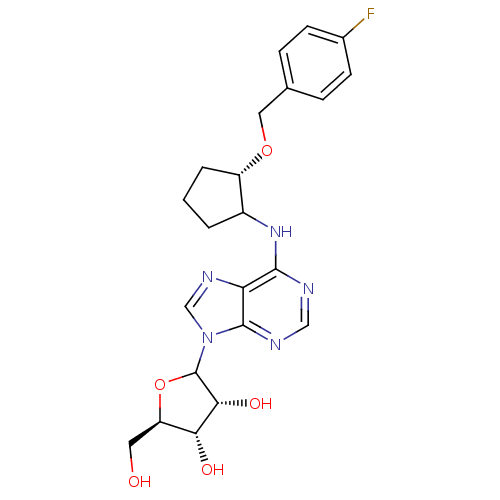 (US8501708, 19) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99202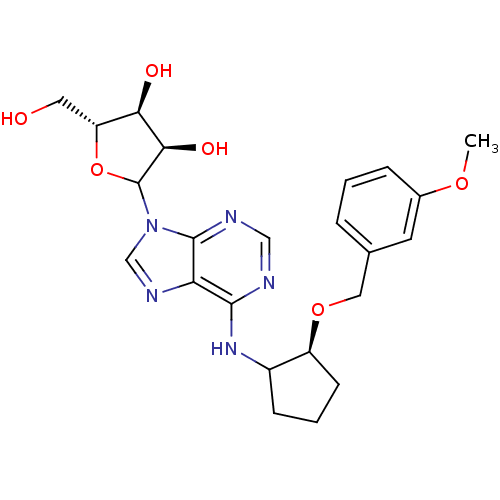 (US8501708, 22) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM108258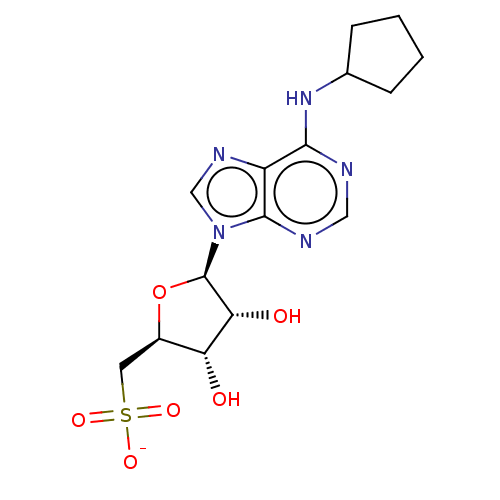 (US8609833, 91) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 4.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99210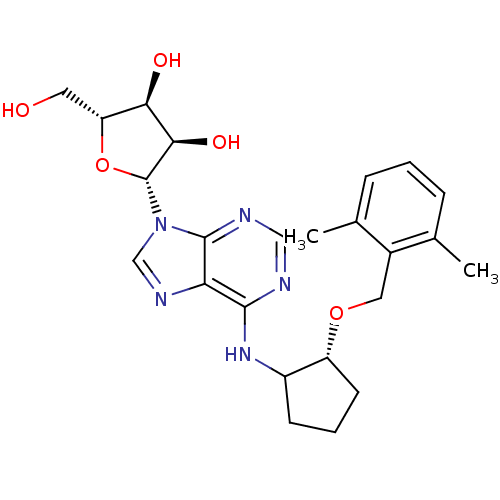 (US8501708, 34) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99209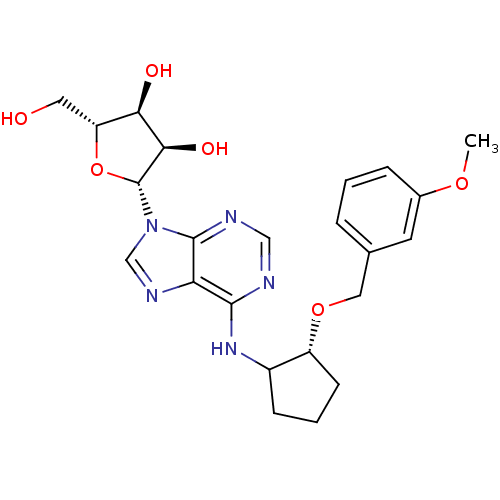 (US8501708, 33) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM108256 (CHEMBL2205239 | US8609833, 87) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 5.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99208 (US8501708, 32) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99207 (US8501708, 31) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM108257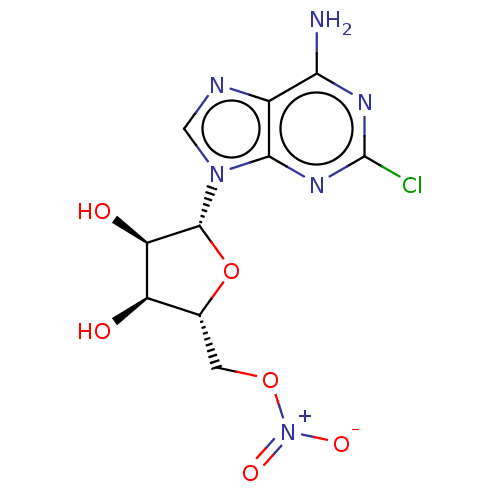 (US8609833, 88) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM97467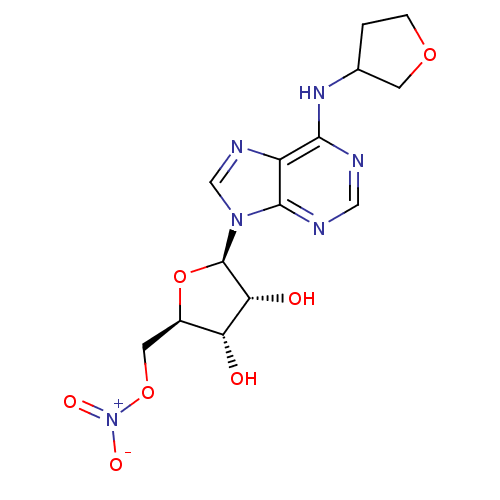 (US8470800, D | US8609833, 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99203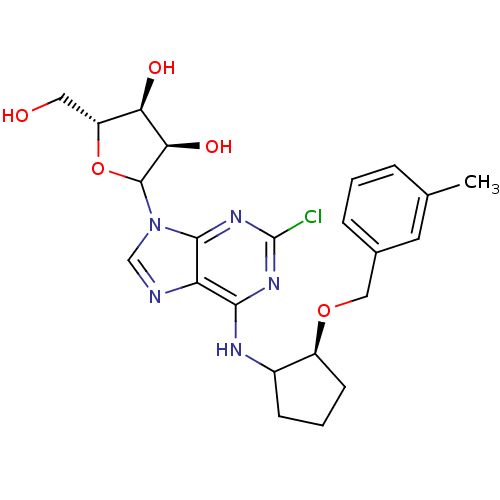 (US8501708, 23) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99198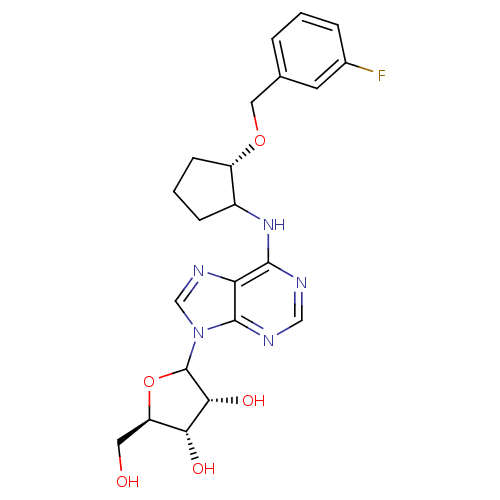 (US8501708, 18) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99200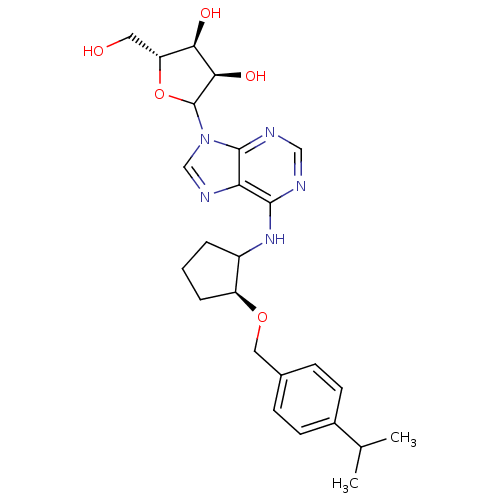 (US8501708, 20) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50141447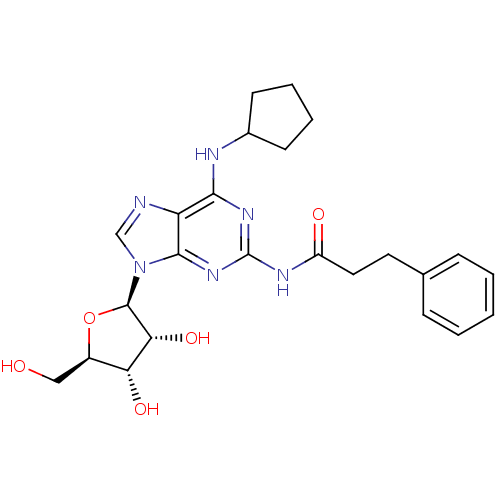 (CHEMBL279391 | N-[6-Cyclopentylamino-9-((2R,3R,4S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99201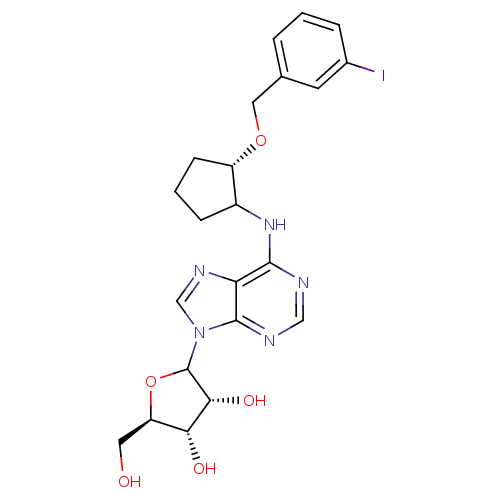 (US8501708, 21) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 28.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99194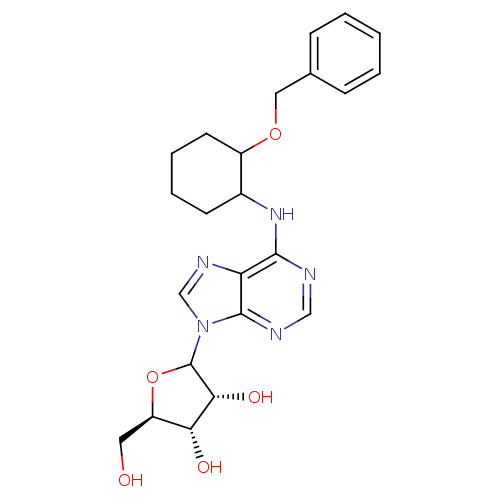 (US8501708, 14 | US8501708, 15) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50141452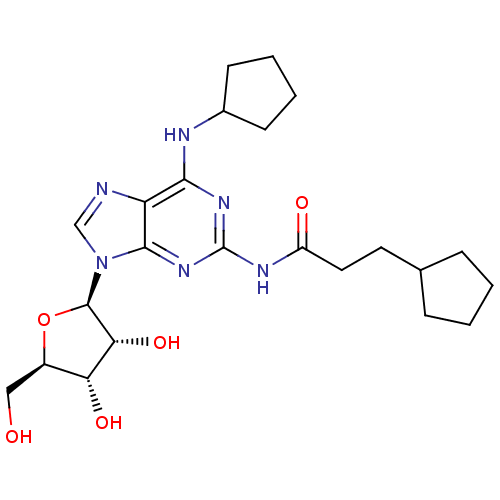 (3-Cyclopentyl-N-[6-cyclopentylamino-9-((2R,3R,4S,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human adenosine A3 receptor in stably transfected HEK cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Oryctolagus cuniculus (Rabbit)) | BDBM99194 (US8501708, 14 | US8501708, 15) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 53.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50141455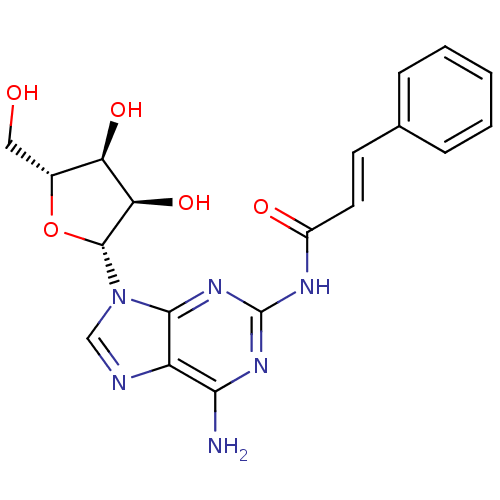 (CHEMBL285251 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50141453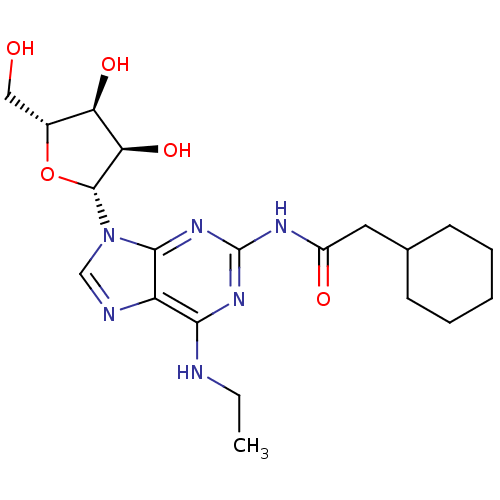 (2-Cyclohexyl-N-[9-((2R,3R,4S,5R)-3,4-dihydroxy-5-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human adenosine A3 receptor in stably transfected HEK cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50141453 (2-Cyclohexyl-N-[9-((2R,3R,4S,5R)-3,4-dihydroxy-5-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM108256 (CHEMBL2205239 | US8609833, 87) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50141451 (CHEMBL36786 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50141449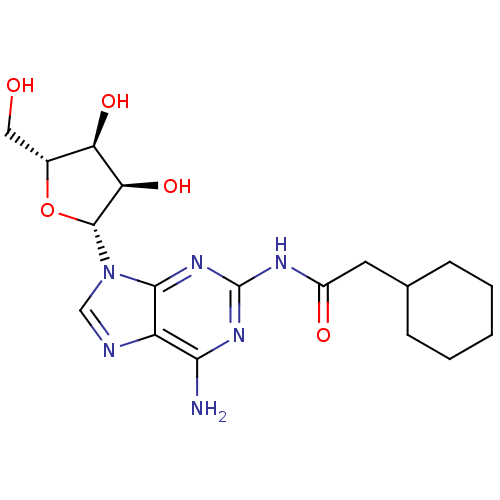 (CHEMBL36789 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2 (Oryctolagus cuniculus (rabbit)) | BDBM99205 (US8501708, 29) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50141456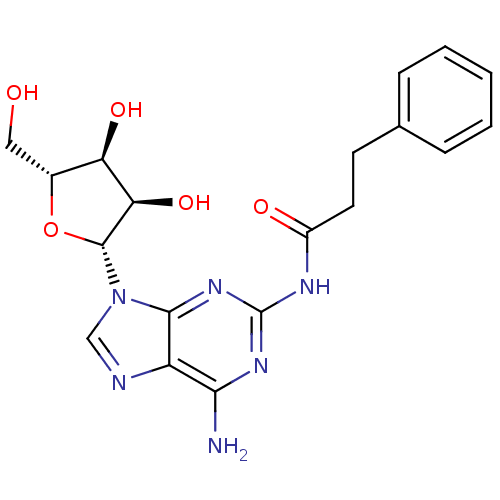 (CHEMBL289982 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 432 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2 (Oryctolagus cuniculus (rabbit)) | BDBM99192 (US8501708, 12) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM97465 (US8470800, B | US8609833, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Oryctolagus cuniculus (rabbit)) | BDBM99209 (US8501708, 33) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 551 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM97464 (US8470800, A | US8609833, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 704 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50141450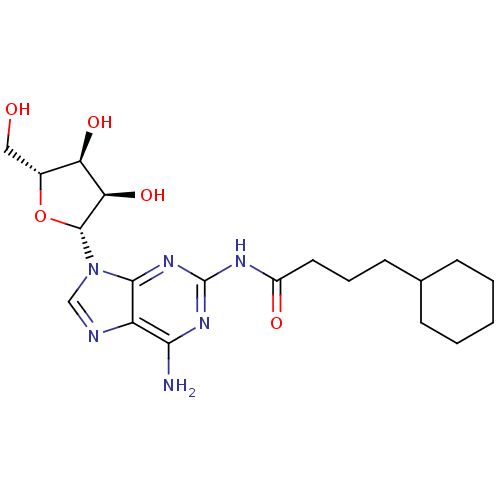 (CHEMBL36320 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Oryctolagus cuniculus (rabbit)) | BDBM99193 (US8501708, 13) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The compounds were studied in binding assays to determine selectivity and potency to A1, A2a and A3 adenosine receptors. | US Patent US8501708 (2013) BindingDB Entry DOI: 10.7270/Q2QN65CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50141455 (CHEMBL285251 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human adenosine A3 receptor in stably transfected HEK cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM108257 (US8609833, 88) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2 (Homo sapiens (Human)) | BDBM108256 (CHEMBL2205239 | US8609833, 87) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 951 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50141448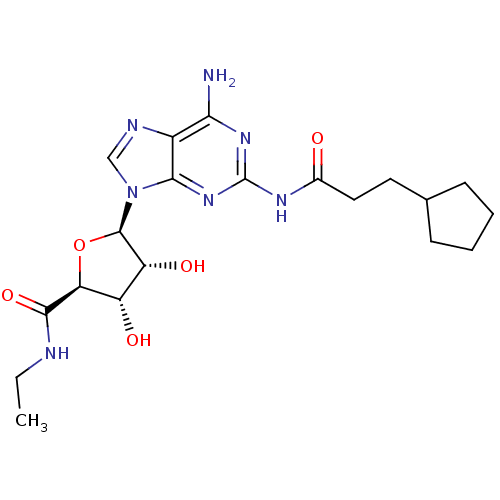 ((2S,3S,4R,5R)-5-[6-Amino-2-(3-cyclopentyl-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human adenosine A3 receptor in stably transfected HEK cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM108258 (US8609833, 91) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation US Patent | Assay Description The affinities of selected Purine Derivatives for the adenosine A1 receptor were determined by measuring the displacement of specific [3H] 2-chloro-N... | US Patent US8609833 (2013) BindingDB Entry DOI: 10.7270/Q2125R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50141451 (CHEMBL36786 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human adenosine A3 receptor in stably transfected HEK cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50141450 (CHEMBL36320 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human adenosine A3 receptor in stably transfected HEK cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50141449 (CHEMBL36789 | N-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inotek Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human adenosine A3 receptor in stably transfected HEK cells | Bioorg Med Chem Lett 14: 1495-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.011 BindingDB Entry DOI: 10.7270/Q27H1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 171 total ) | Next | Last >> |