 Found 198 hits with Last Name = 'schneider' and Initial = 'p'
Found 198 hits with Last Name = 'schneider' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254456
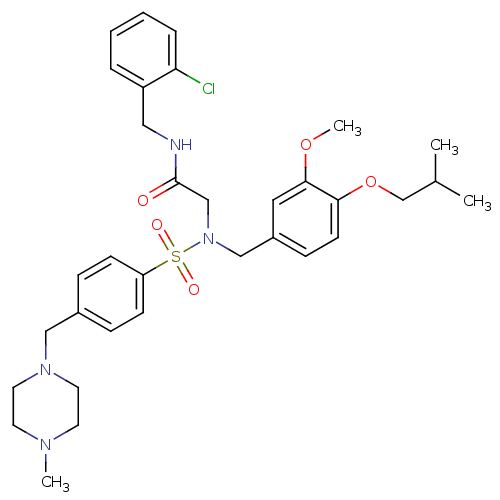
(CHEMBL447392 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES COc1cc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCN(C)CC3)cc2)ccc1OCC(C)C Show InChI InChI=1S/C33H43ClN4O5S/c1-25(2)24-43-31-14-11-27(19-32(31)42-4)22-38(23-33(39)35-20-28-7-5-6-8-30(28)34)44(40,41)29-12-9-26(10-13-29)21-37-17-15-36(3)16-18-37/h5-14,19,25H,15-18,20-24H2,1-4H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369348

(CHEMBL606286)Show SMILES NC(=O)c1c[se]c(n1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2Se/c20-15-9-17(23-4-22-15)26(5-24-9)19-13(30)11(28)8(39-19)2-37-42(34,35)40-41(32,33)36-1-7-10(27)12(29)14(38-7)18-25-6(3-43-18)16(21)31/h3-5,7-8,10-14,19,27-30H,1-2H2,(H2,21,31)(H,32,33)(H,34,35)(H2,20,22,23)/t7-,8-,10-,11-,12-,13-,14?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254457

(CHEMBL443207 | N-(2-chlorobenzyl)-2-(N-(3-chloro-4...)Show SMILES CC(C)COc1ccc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1Cl Show InChI InChI=1S/C32H40Cl2N4O4S/c1-24(2)23-42-31-13-10-26(18-30(31)34)21-38(22-32(39)35-19-27-6-4-5-7-29(27)33)43(40,41)28-11-8-25(9-12-28)20-37-16-14-36(3)15-17-37/h4-13,18,24H,14-17,19-23H2,1-3H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369348

(CHEMBL606286)Show SMILES NC(=O)c1c[se]c(n1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2Se/c20-15-9-17(23-4-22-15)26(5-24-9)19-13(30)11(28)8(39-19)2-37-42(34,35)40-41(32,33)36-1-7-10(27)12(29)14(38-7)18-25-6(3-43-18)16(21)31/h3-5,7-8,10-14,19,27-30H,1-2H2,(H2,21,31)(H,32,33)(H,34,35)(H2,20,22,23)/t7-,8-,10-,11-,12-,13-,14?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369348

(CHEMBL606286)Show SMILES NC(=O)c1c[se]c(n1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2Se/c20-15-9-17(23-4-22-15)26(5-24-9)19-13(30)11(28)8(39-19)2-37-42(34,35)40-41(32,33)36-1-7-10(27)12(29)14(38-7)18-25-6(3-43-18)16(21)31/h3-5,7-8,10-14,19,27-30H,1-2H2,(H2,21,31)(H,32,33)(H,34,35)(H2,20,22,23)/t7-,8-,10-,11-,12-,13-,14?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254458
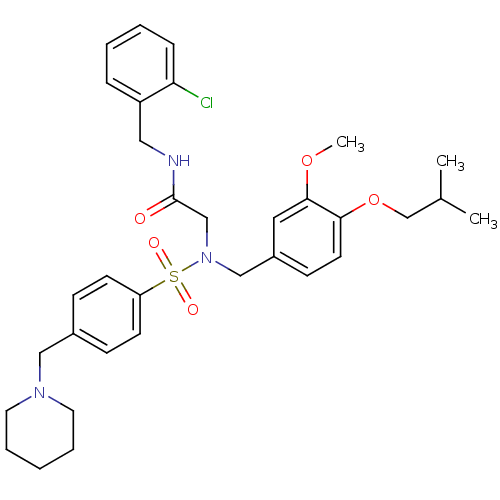
(CHEMBL452238 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES COc1cc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCCCC3)cc2)ccc1OCC(C)C Show InChI InChI=1S/C33H42ClN3O5S/c1-25(2)24-42-31-16-13-27(19-32(31)41-3)22-37(23-33(38)35-20-28-9-5-6-10-30(28)34)43(39,40)29-14-11-26(12-15-29)21-36-17-7-4-8-18-36/h5-6,9-16,19,25H,4,7-8,17-18,20-24H2,1-3H3,(H,35,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254456

(CHEMBL447392 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES COc1cc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCN(C)CC3)cc2)ccc1OCC(C)C Show InChI InChI=1S/C33H43ClN4O5S/c1-25(2)24-43-31-14-11-27(19-32(31)42-4)22-38(23-33(39)35-20-28-7-5-6-8-30(28)34)44(40,41)29-12-9-26(10-13-29)21-37-17-15-36(3)16-18-37/h5-14,19,25H,15-18,20-24H2,1-4H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369348

(CHEMBL606286)Show SMILES NC(=O)c1c[se]c(n1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2Se/c20-15-9-17(23-4-22-15)26(5-24-9)19-13(30)11(28)8(39-19)2-37-42(34,35)40-41(32,33)36-1-7-10(27)12(29)14(38-7)18-25-6(3-43-18)16(21)31/h3-5,7-8,10-14,19,27-30H,1-2H2,(H2,21,31)(H,32,33)(H,34,35)(H2,20,22,23)/t7-,8-,10-,11-,12-,13-,14?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50176996

(CHEMBL3813969)Show InChI InChI=1S/C22H20N6O/c29-22(27-19-7-5-18(6-8-19)14-28-16-24-15-26-28)20-3-1-2-4-21(20)25-13-17-9-11-23-12-10-17/h1-12,15-16,25H,13-14H2,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology (ETH)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
J Med Chem 59: 4077-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01849
BindingDB Entry DOI: 10.7270/Q2FN184P |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254459
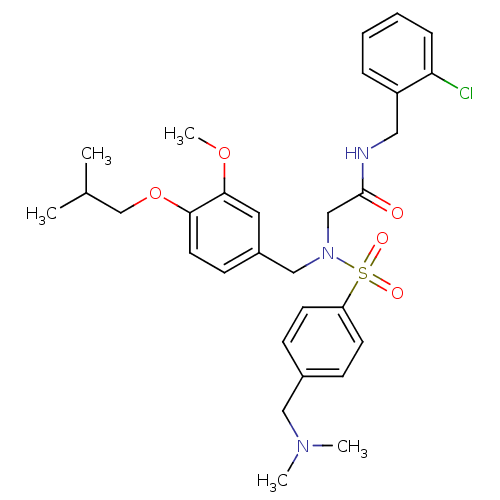
(CHEMBL512111 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES COc1cc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN(C)C)cc2)ccc1OCC(C)C Show InChI InChI=1S/C30H38ClN3O5S/c1-22(2)21-39-28-15-12-24(16-29(28)38-5)19-34(20-30(35)32-17-25-8-6-7-9-27(25)31)40(36,37)26-13-10-23(11-14-26)18-33(3)4/h6-16,22H,17-21H2,1-5H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254460
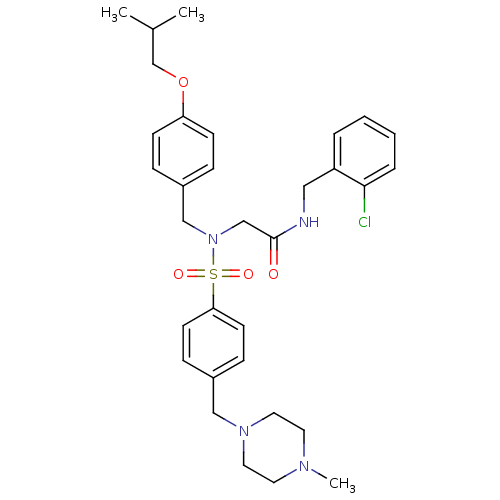
(CHEMBL499999 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES CC(C)COc1ccc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1 Show InChI InChI=1S/C32H41ClN4O4S/c1-25(2)24-41-29-12-8-27(9-13-29)22-37(23-32(38)34-20-28-6-4-5-7-31(28)33)42(39,40)30-14-10-26(11-15-30)21-36-18-16-35(3)17-19-36/h4-15,25H,16-24H2,1-3H3,(H,34,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254461
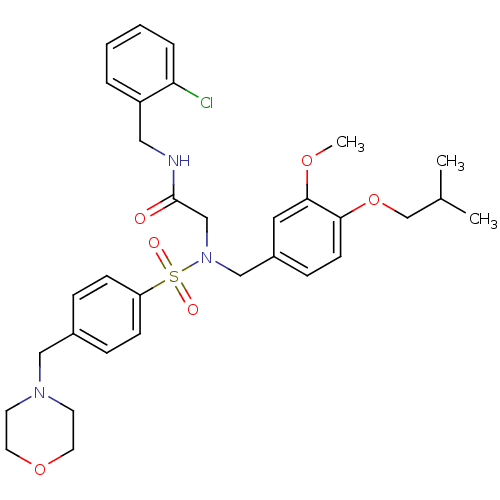
(CHEMBL507546 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES COc1cc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCOCC3)cc2)ccc1OCC(C)C Show InChI InChI=1S/C32H40ClN3O6S/c1-24(2)23-42-30-13-10-26(18-31(30)40-3)21-36(22-32(37)34-19-27-6-4-5-7-29(27)33)43(38,39)28-11-8-25(9-12-28)20-35-14-16-41-17-15-35/h4-13,18,24H,14-17,19-23H2,1-3H3,(H,34,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254457

(CHEMBL443207 | N-(2-chlorobenzyl)-2-(N-(3-chloro-4...)Show SMILES CC(C)COc1ccc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1Cl Show InChI InChI=1S/C32H40Cl2N4O4S/c1-24(2)23-42-31-13-10-26(18-30(31)34)21-38(22-32(39)35-19-27-6-4-5-7-29(27)33)43(40,41)28-11-8-25(9-12-28)20-37-16-14-36(3)15-17-37/h4-13,18,24H,14-17,19-23H2,1-3H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254462
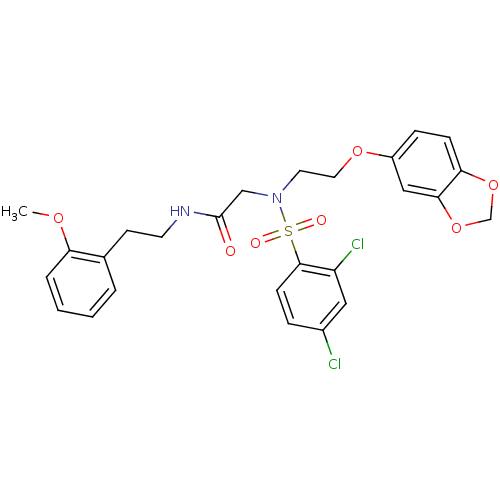
(CHEMBL468492 | N-(2-methoxyphenethyl)-2-(N-(2-(ben...)Show SMILES COc1ccccc1CCNC(=O)CN(CCOc1ccc2OCOc2c1)S(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H26Cl2N2O7S/c1-34-22-5-3-2-4-18(22)10-11-29-26(31)16-30(38(32,33)25-9-6-19(27)14-21(25)28)12-13-35-20-7-8-23-24(15-20)37-17-36-23/h2-9,14-15H,10-13,16-17H2,1H3,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369347

(CHEMBL605452)Show SMILES NC(=O)c1c[se]c(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O14P2Se/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)9(39-20)3-37-42(34,35)40-41(32,33)36-2-8-12(27)14(29)16(38-8)10-1-7(4-43-10)18(22)31/h1,4-6,8-9,12-16,20,27-30H,2-3H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t8-,9-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254458

(CHEMBL452238 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES COc1cc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCCCC3)cc2)ccc1OCC(C)C Show InChI InChI=1S/C33H42ClN3O5S/c1-25(2)24-42-31-16-13-27(19-32(31)41-3)22-37(23-33(38)35-20-28-9-5-6-10-30(28)34)43(39,40)29-14-11-26(12-15-29)21-36-17-7-4-8-18-36/h5-6,9-16,19,25H,4,7-8,17-18,20-24H2,1-3H3,(H,35,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369347

(CHEMBL605452)Show SMILES NC(=O)c1c[se]c(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O14P2Se/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)9(39-20)3-37-42(34,35)40-41(32,33)36-2-8-12(27)14(29)16(38-8)10-1-7(4-43-10)18(22)31/h1,4-6,8-9,12-16,20,27-30H,2-3H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t8-,9-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369349

(CHEMBL603997)Show SMILES NC(=O)c1csc(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O14P2S/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)9(39-20)3-37-42(34,35)40-41(32,33)36-2-8-12(27)14(29)16(38-8)10-1-7(4-43-10)18(22)31/h1,4-6,8-9,12-16,20,27-30H,2-3H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t8-,9-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369349

(CHEMBL603997)Show SMILES NC(=O)c1csc(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O14P2S/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)9(39-20)3-37-42(34,35)40-41(32,33)36-2-8-12(27)14(29)16(38-8)10-1-7(4-43-10)18(22)31/h1,4-6,8-9,12-16,20,27-30H,2-3H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t8-,9-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369349

(CHEMBL603997)Show SMILES NC(=O)c1csc(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O14P2S/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)9(39-20)3-37-42(34,35)40-41(32,33)36-2-8-12(27)14(29)16(38-8)10-1-7(4-43-10)18(22)31/h1,4-6,8-9,12-16,20,27-30H,2-3H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t8-,9-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369349

(CHEMBL603997)Show SMILES NC(=O)c1csc(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O14P2S/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)9(39-20)3-37-42(34,35)40-41(32,33)36-2-8-12(27)14(29)16(38-8)10-1-7(4-43-10)18(22)31/h1,4-6,8-9,12-16,20,27-30H,2-3H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t8-,9-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254463
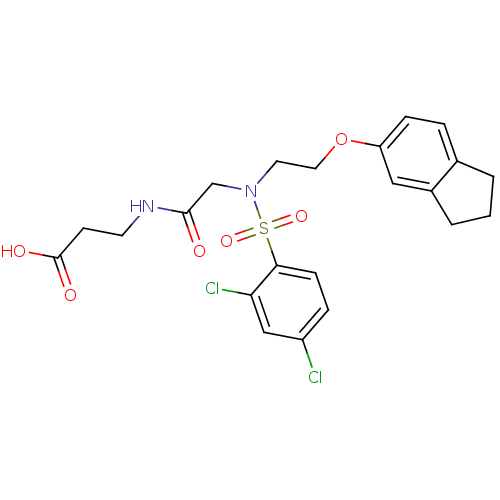
(3-(2-(2,4-dichloro-N-(2-(2,3-dihydro-1H-inden-5-yl...)Show SMILES OC(=O)CCNC(=O)CN(CCOc1ccc2CCCc2c1)S(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H24Cl2N2O6S/c23-17-5-7-20(19(24)13-17)33(30,31)26(14-21(27)25-9-8-22(28)29)10-11-32-18-6-4-15-2-1-3-16(15)12-18/h4-7,12-13H,1-3,8-11,14H2,(H,25,27)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50421763
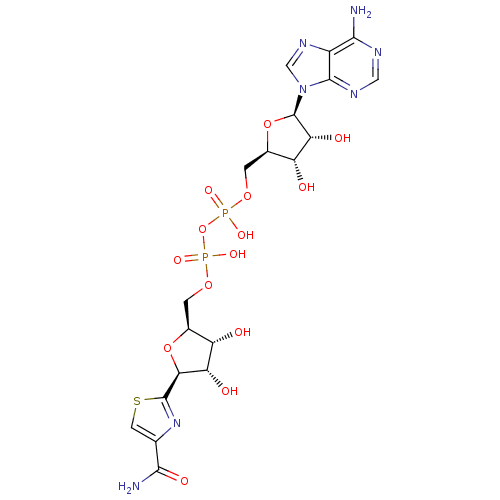
(CHEMBL2364562)Show SMILES NC(=O)c1csc(n1)[C@H]1O[C@@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2S/c20-15-9-17(23-4-22-15)26(5-24-9)19-13(30)11(28)8(39-19)2-37-42(34,35)40-41(32,33)36-1-7-10(27)12(29)14(38-7)18-25-6(3-43-18)16(21)31/h3-5,7-8,10-14,19,27-30H,1-2H2,(H2,21,31)(H,32,33)(H,34,35)(H2,20,22,23)/t7-,8+,10-,11+,12-,13+,14-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50421763

(CHEMBL2364562)Show SMILES NC(=O)c1csc(n1)[C@H]1O[C@@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2S/c20-15-9-17(23-4-22-15)26(5-24-9)19-13(30)11(28)8(39-19)2-37-42(34,35)40-41(32,33)36-1-7-10(27)12(29)14(38-7)18-25-6(3-43-18)16(21)31/h3-5,7-8,10-14,19,27-30H,1-2H2,(H2,21,31)(H,32,33)(H,34,35)(H2,20,22,23)/t7-,8+,10-,11+,12-,13+,14-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254461

(CHEMBL507546 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES COc1cc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN3CCOCC3)cc2)ccc1OCC(C)C Show InChI InChI=1S/C32H40ClN3O6S/c1-24(2)23-42-30-13-10-26(18-31(30)40-3)21-36(22-32(37)34-19-27-6-4-5-7-29(27)33)43(38,39)28-11-8-25(9-12-28)20-35-14-16-41-17-15-35/h4-13,18,24H,14-17,19-23H2,1-3H3,(H,34,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 463 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50421763

(CHEMBL2364562)Show SMILES NC(=O)c1csc(n1)[C@H]1O[C@@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2S/c20-15-9-17(23-4-22-15)26(5-24-9)19-13(30)11(28)8(39-19)2-37-42(34,35)40-41(32,33)36-1-7-10(27)12(29)14(38-7)18-25-6(3-43-18)16(21)31/h3-5,7-8,10-14,19,27-30H,1-2H2,(H2,21,31)(H,32,33)(H,34,35)(H2,20,22,23)/t7-,8+,10-,11+,12-,13+,14-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254459

(CHEMBL512111 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...)Show SMILES COc1cc(CN(CC(=O)NCc2ccccc2Cl)S(=O)(=O)c2ccc(CN(C)C)cc2)ccc1OCC(C)C Show InChI InChI=1S/C30H38ClN3O5S/c1-22(2)21-39-28-15-12-24(16-29(28)38-5)19-34(20-30(35)32-17-25-8-6-7-9-27(25)31)40(36,37)26-13-10-23(11-14-26)18-33(3)4/h6-16,22H,17-21H2,1-5H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369347

(CHEMBL605452)Show SMILES NC(=O)c1c[se]c(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O14P2Se/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)9(39-20)3-37-42(34,35)40-41(32,33)36-2-8-12(27)14(29)16(38-8)10-1-7(4-43-10)18(22)31/h1,4-6,8-9,12-16,20,27-30H,2-3H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t8-,9-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50421763

(CHEMBL2364562)Show SMILES NC(=O)c1csc(n1)[C@H]1O[C@@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C19H25N7O14P2S/c20-15-9-17(23-4-22-15)26(5-24-9)19-13(30)11(28)8(39-19)2-37-42(34,35)40-41(32,33)36-1-7-10(27)12(29)14(38-7)18-25-6(3-43-18)16(21)31/h3-5,7-8,10-14,19,27-30H,1-2H2,(H2,21,31)(H,32,33)(H,34,35)(H2,20,22,23)/t7-,8+,10-,11+,12-,13+,14-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254508
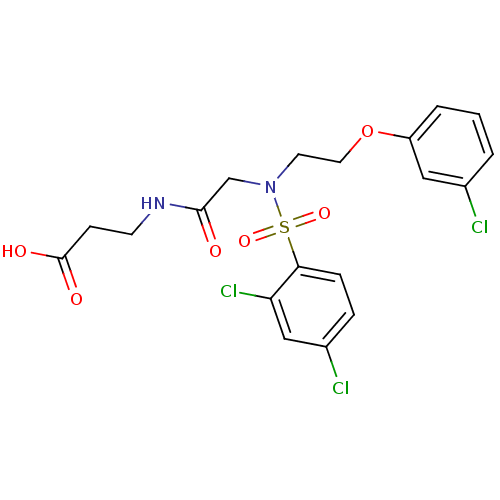
(3-(2-(2,4-dichloro-N-(2-(3-chlorophenoxy)ethyl)phe...)Show SMILES OC(=O)CCNC(=O)CN(CCOc1cccc(Cl)c1)S(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl3N2O6S/c20-13-2-1-3-15(10-13)30-9-8-24(12-18(25)23-7-6-19(26)27)31(28,29)17-5-4-14(21)11-16(17)22/h1-5,10-11H,6-9,12H2,(H,23,25)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369347

(CHEMBL605452)Show SMILES NC(=O)c1c[se]c(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O14P2Se/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)9(39-20)3-37-42(34,35)40-41(32,33)36-2-8-12(27)14(29)16(38-8)10-1-7(4-43-10)18(22)31/h1,4-6,8-9,12-16,20,27-30H,2-3H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t8-,9-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254632
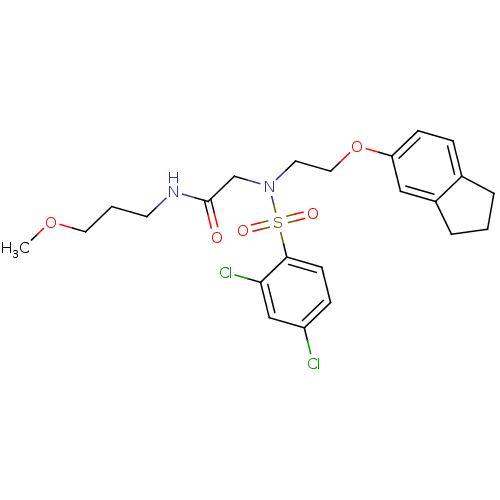
(2-(2,4-dichloro-N-(2-(2,3-dihydro-1H-inden-5-yloxy...)Show SMILES COCCCNC(=O)CN(CCOc1ccc2CCCc2c1)S(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H28Cl2N2O5S/c1-31-12-3-10-26-23(28)16-27(33(29,30)22-9-7-19(24)15-21(22)25)11-13-32-20-8-6-17-4-2-5-18(17)14-20/h6-9,14-15H,2-5,10-13,16H2,1H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254633
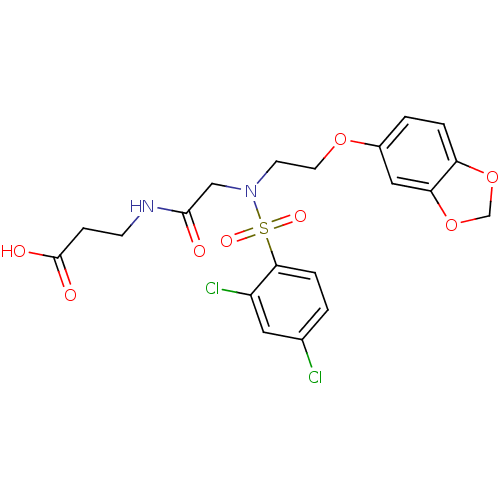
(3-(2-(N-(2-(benzo[d][1,3]dioxol-5-yloxy)ethyl)-2,4...)Show SMILES OC(=O)CCNC(=O)CN(CCOc1ccc2OCOc2c1)S(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C20H20Cl2N2O8S/c21-13-1-4-18(15(22)9-13)33(28,29)24(11-19(25)23-6-5-20(26)27)7-8-30-14-2-3-16-17(10-14)32-12-31-16/h1-4,9-10H,5-8,11-12H2,(H,23,25)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254634
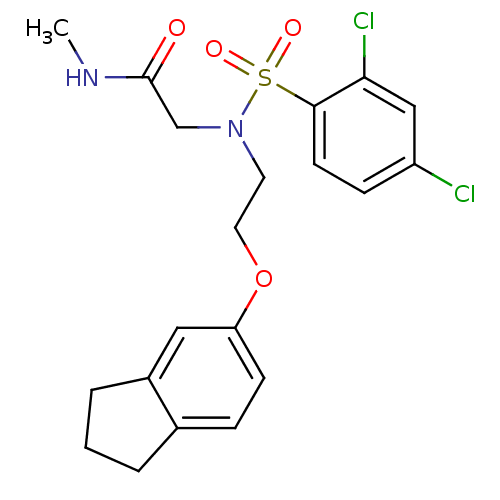
(2-(2,4-dichloro-N-(2-(2,3-dihydro-1H-inden-5-yloxy...)Show SMILES CNC(=O)CN(CCOc1ccc2CCCc2c1)S(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C20H22Cl2N2O4S/c1-23-20(25)13-24(29(26,27)19-8-6-16(21)12-18(19)22)9-10-28-17-7-5-14-3-2-4-15(14)11-17/h5-8,11-12H,2-4,9-10,13H2,1H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50176996

(CHEMBL3813969)Show InChI InChI=1S/C22H20N6O/c29-22(27-19-7-5-18(6-8-19)14-28-16-24-15-26-28)20-3-1-2-4-21(20)25-13-17-9-11-23-12-10-17/h1-12,15-16,25H,13-14H2,(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology (ETH)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 (unknown origin) |
J Med Chem 59: 4077-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01849
BindingDB Entry DOI: 10.7270/Q2FN184P |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50254635
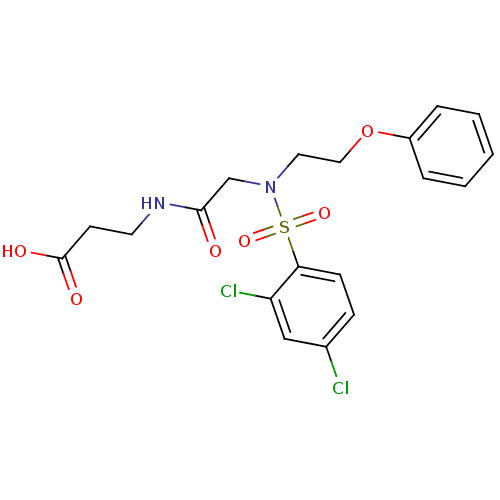
(3-(2-(2,4-dichloro-N-(2-phenoxyethyl)phenylsulfona...)Show SMILES OC(=O)CCNC(=O)CN(CCOc1ccccc1)S(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H20Cl2N2O6S/c20-14-6-7-17(16(21)12-14)30(27,28)23(13-18(24)22-9-8-19(25)26)10-11-29-15-4-2-1-3-5-15/h1-7,12H,8-11,13H2,(H,22,24)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]desArg from human B1 in human WI 38 cells |
Bioorg Med Chem Lett 19: 119-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.005
BindingDB Entry DOI: 10.7270/Q2KH0N6M |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369350
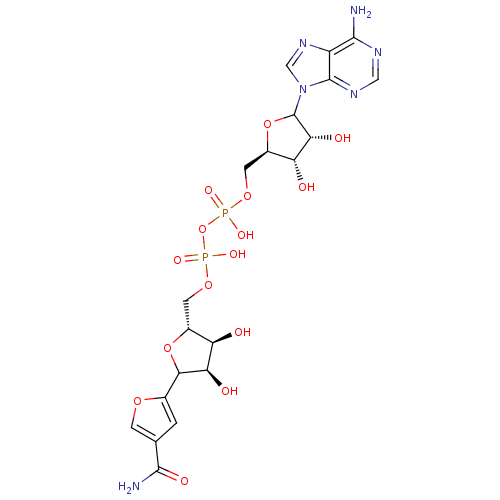
(CHEMBL606280)Show SMILES NC(=O)c1coc(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O15P2/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)10(40-20)4-38-43(34,35)41-42(32,33)37-3-9-12(27)14(29)16(39-9)8-1-7(2-36-8)18(22)31/h1-2,5-6,9-10,12-16,20,27-30H,3-4H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t9-,10-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369350

(CHEMBL606280)Show SMILES NC(=O)c1coc(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O15P2/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)10(40-20)4-38-43(34,35)41-42(32,33)37-3-9-12(27)14(29)16(39-9)8-1-7(2-36-8)18(22)31/h1-2,5-6,9-10,12-16,20,27-30H,3-4H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t9-,10-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards IMP(Inosine 5'-monophosphate) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 2
(Rattus norvegicus) | BDBM19473
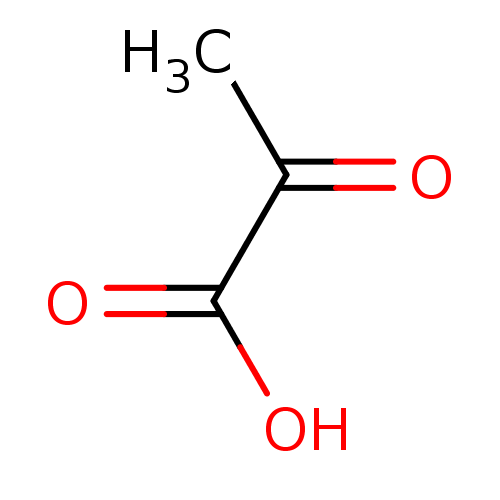
(2-Oxopropanoate | 2-oxopropanoic acid | Pyruvate)Show InChI InChI=1S/C3H4O3/c1-2(4)3(5)6/h1H3,(H,5,6) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes |
Biochem J 341: 529-35 (1999)
BindingDB Entry DOI: 10.7270/Q2125TQV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369350

(CHEMBL606280)Show SMILES NC(=O)c1coc(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O15P2/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)10(40-20)4-38-43(34,35)41-42(32,33)37-3-9-12(27)14(29)16(39-9)8-1-7(2-36-8)18(22)31/h1-2,5-6,9-10,12-16,20,27-30H,3-4H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t9-,10-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 2 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 2
(Rattus norvegicus) | BDBM50390988
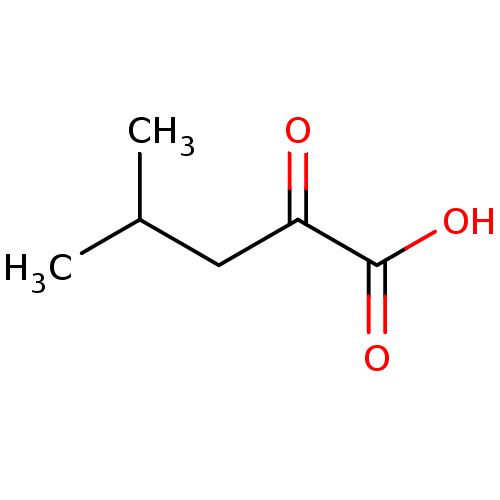
(CHEMBL445647)Show InChI InChI=1S/C6H10O3/c1-4(2)3-5(7)6(8)9/h4H,3H2,1-2H3,(H,8,9) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes |
Biochem J 341: 529-35 (1999)
BindingDB Entry DOI: 10.7270/Q2125TQV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369350

(CHEMBL606280)Show SMILES NC(=O)c1coc(c1)C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H26N6O15P2/c21-17-11-19(24-5-23-17)26(6-25-11)20-15(30)13(28)10(40-20)4-38-43(34,35)41-42(32,33)37-3-9-12(27)14(29)16(39-9)8-1-7(2-36-8)18(22)31/h1-2,5-6,9-10,12-16,20,27-30H,3-4H2,(H2,22,31)(H,32,33)(H,34,35)(H2,21,23,24)/t9-,10-,12-,13-,14-,15-,16?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition towards NMD (Nicotinamide adenine dinucleotide) substrate of Inosine-5'-monophosphate dehydrogenase 1 |
J Med Chem 41: 1702-7 (1998)
Article DOI: 10.1021/jm970772e
BindingDB Entry DOI: 10.7270/Q23J3DNP |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 2
(Rattus norvegicus) | BDBM50390989

(CHEMBL146554)Show InChI InChI=1S/C5H8O3/c1-3(2)4(6)5(7)8/h3H,1-2H3,(H,7,8) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes |
Biochem J 341: 529-35 (1999)
BindingDB Entry DOI: 10.7270/Q2125TQV |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 2
(Rattus norvegicus) | BDBM50390990

(CHEMBL2074691)Show InChI InChI=1S/C4H6O3/c1-3(5)2-4(6)7/h2H2,1H3,(H,6,7)/p-1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| PubMed
| 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes |
Biochem J 341: 529-35 (1999)
BindingDB Entry DOI: 10.7270/Q2125TQV |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 2
(Rattus norvegicus) | BDBM50270275

(3-OH butyrate | 3-OH-butyrate | 3-hydroxybutanoate...)Show InChI InChI=1S/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/p-1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes |
Biochem J 341: 529-35 (1999)
BindingDB Entry DOI: 10.7270/Q2125TQV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM24773
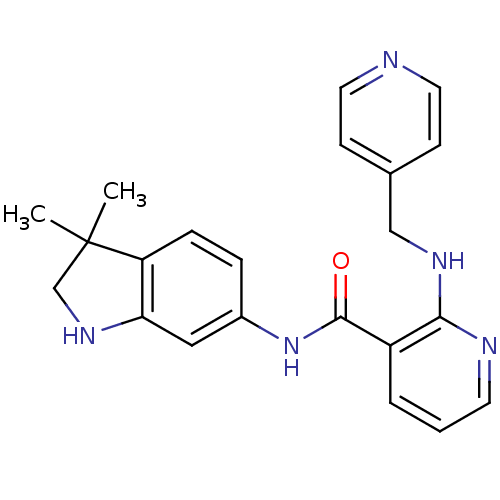
(AMG 706 | AMG-706 | Motesanib | N-(3,3-dimethyl-1,...)Show SMILES CC1(C)CNc2cc(NC(=O)c3cccnc3NCc3ccncc3)ccc12 Show InChI InChI=1S/C22H23N5O/c1-22(2)14-26-19-12-16(5-6-18(19)22)27-21(28)17-4-3-9-24-20(17)25-13-15-7-10-23-11-8-15/h3-12,26H,13-14H2,1-2H3,(H,24,25)(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology (ETH)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 (unknown origin) |
J Med Chem 59: 4077-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01849
BindingDB Entry DOI: 10.7270/Q2FN184P |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM24773

(AMG 706 | AMG-706 | Motesanib | N-(3,3-dimethyl-1,...)Show SMILES CC1(C)CNc2cc(NC(=O)c3cccnc3NCc3ccncc3)ccc12 Show InChI InChI=1S/C22H23N5O/c1-22(2)14-26-19-12-16(5-6-18(19)22)27-21(28)17-4-3-9-24-20(17)25-13-15-7-10-23-11-8-15/h3-12,26H,13-14H2,1-2H3,(H,24,25)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology (ETH)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
J Med Chem 59: 4077-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01849
BindingDB Entry DOI: 10.7270/Q2FN184P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13066
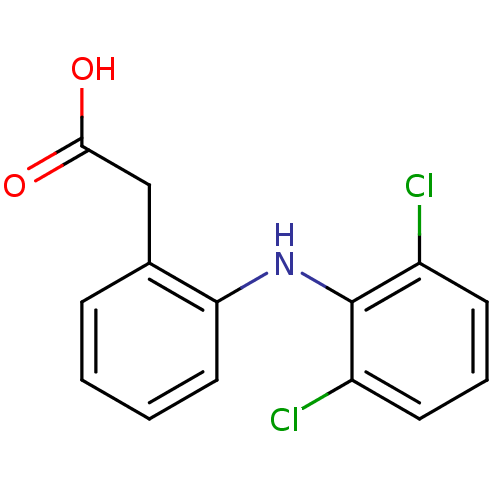
(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität
Curated by ChEMBL
| Assay Description
Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree C |
J Med Chem 48: 6997-7004 (2005)
Article DOI: 10.1021/jm050619h
BindingDB Entry DOI: 10.7270/Q2N29WH3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
S-adenosylmethionine decarboxylase proenzyme
(Rattus norvegicus) | BDBM50368644
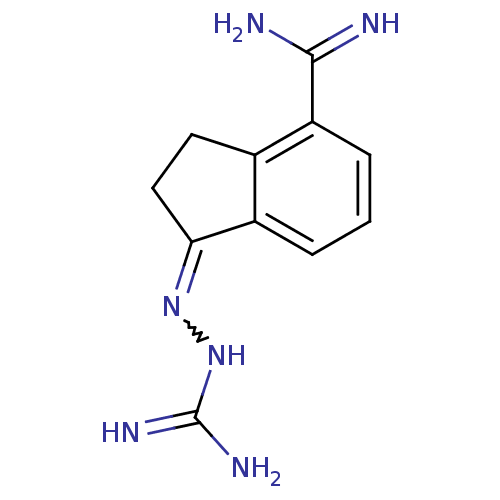
(CHEMBL1202793 | Sardomozide chloride)Show InChI InChI=1S/C11H14N6/c12-10(13)8-3-1-2-7-6(8)4-5-9(7)16-17-11(14)15/h1-3H,4-5H2,(H3,12,13)(H4,14,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against S- adenosylmethionine decarboxylase (SAMDC) from rat liver |
J Med Chem 36: 2168-71 (1993)
BindingDB Entry DOI: 10.7270/Q24Q7VM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
S-adenosylmethionine decarboxylase proenzyme
(Rattus norvegicus) | BDBM50046197
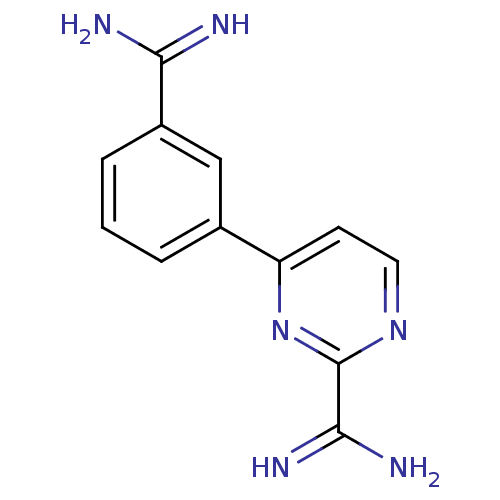
(4-{3-[(Z)-amino(imino)methyl]phenyl}pyrimidine-2-c...)Show InChI InChI=1S/C12H12N6/c13-10(14)8-3-1-2-7(6-8)9-4-5-17-12(18-9)11(15)16/h1-6H,(H3,13,14)(H3,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG
Curated by ChEMBL
| Assay Description
Inhibitory concentration of compound against S-Adenosylmethionine decarboxylase (SAMDC) from rat liver |
J Med Chem 36: 46-54 (1993)
BindingDB Entry DOI: 10.7270/Q2FT8K3B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data