 Found 224 hits with Last Name = 'arunachalam' and Initial = 'pn'
Found 224 hits with Last Name = 'arunachalam' and Initial = 'pn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM412734

(N-(3-fluoropyridin-4-yl)-2-(6-methylpyridin-2-yl)-...)Show InChI InChI=1S/C17H13FN6/c1-10-3-2-4-14(21-10)17-23-15-11(5-8-20-15)16(24-17)22-13-6-7-19-9-12(13)18/h2-9H,1H3,(H2,19,20,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50454871
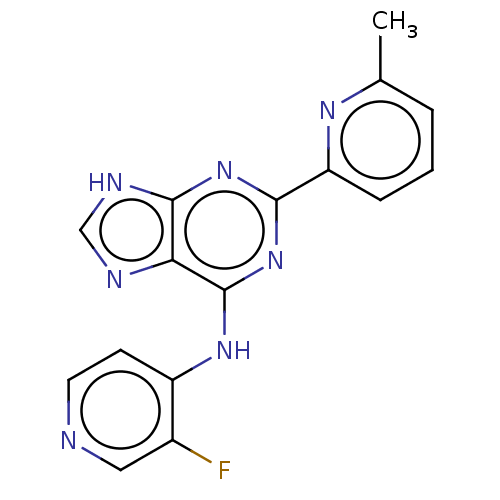
(CHEMBL4209835)Show InChI InChI=1S/C16H12FN7/c1-9-3-2-4-12(21-9)14-23-15-13(19-8-20-15)16(24-14)22-11-5-6-18-7-10(11)17/h2-8H,1H3,(H2,18,19,20,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM412755
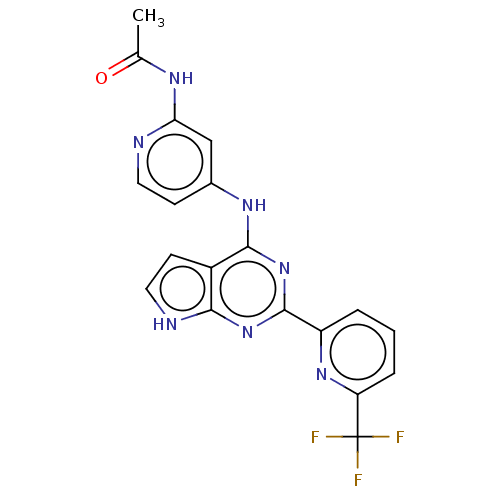
(N-(4-((2-(6-(trifluoromethyl)pyridin-2-yl)-7H-pyrr...)Show SMILES CC(=O)Nc1cc(Nc2nc(nc3[nH]ccc23)-c2cccc(n2)C(F)(F)F)ccn1 Show InChI InChI=1S/C19H14F3N7O/c1-10(30)25-15-9-11(5-7-23-15)26-17-12-6-8-24-16(12)28-18(29-17)13-3-2-4-14(27-13)19(20,21)22/h2-9H,1H3,(H3,23,24,25,26,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM412745
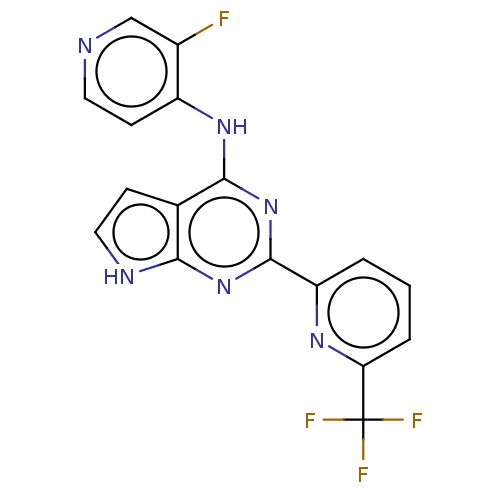
(N-(3-fluoropyridin-4-yl)-2-(6-(trifluoromethyl)pyr...)Show SMILES Fc1cnccc1Nc1nc(nc2[nH]ccc12)-c1cccc(n1)C(F)(F)F Show InChI InChI=1S/C17H10F4N6/c18-10-8-22-6-5-11(10)25-15-9-4-7-23-14(9)26-16(27-15)12-2-1-3-13(24-12)17(19,20)21/h1-8H,(H2,22,23,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| PDB
Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239718
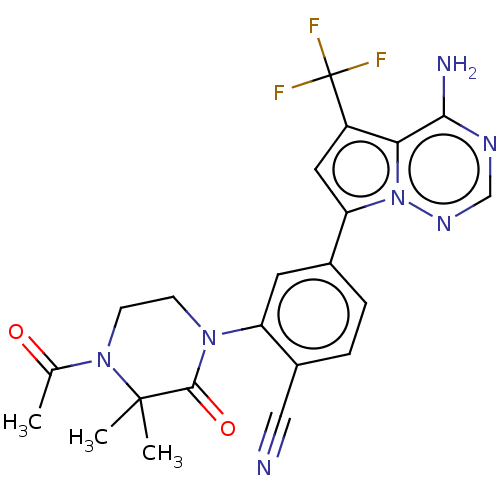
(CHEMBL4064666 | US10214537, Example 639)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H20F3N7O2/c1-12(33)31-7-6-30(20(34)21(31,2)3)16-8-13(4-5-14(16)10-26)17-9-15(22(23,24)25)18-19(27)28-11-29-32(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50232433
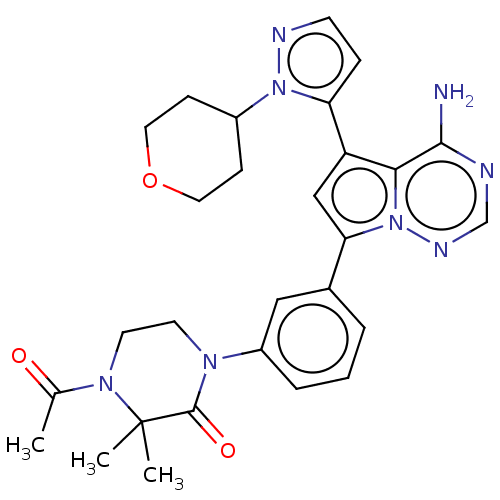
(CHEMBL4068514 | US10214537, Example 619)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2ccnn2C2CCOCC2)c2c(N)ncnn12 Show InChI InChI=1S/C28H32N8O3/c1-18(37)34-12-11-33(27(38)28(34,2)3)21-6-4-5-19(15-21)24-16-22(25-26(29)30-17-32-36(24)25)23-7-10-31-35(23)20-8-13-39-14-9-20/h4-7,10,15-17,20H,8-9,11-14H2,1-3H3,(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM412745

(N-(3-fluoropyridin-4-yl)-2-(6-(trifluoromethyl)pyr...)Show SMILES Fc1cnccc1Nc1nc(nc2[nH]ccc12)-c1cccc(n1)C(F)(F)F Show InChI InChI=1S/C17H10F4N6/c18-10-8-22-6-5-11(10)25-15-9-4-7-23-14(9)26-16(27-15)12-2-1-3-13(24-12)17(19,20)21/h1-8H,(H2,22,23,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122323
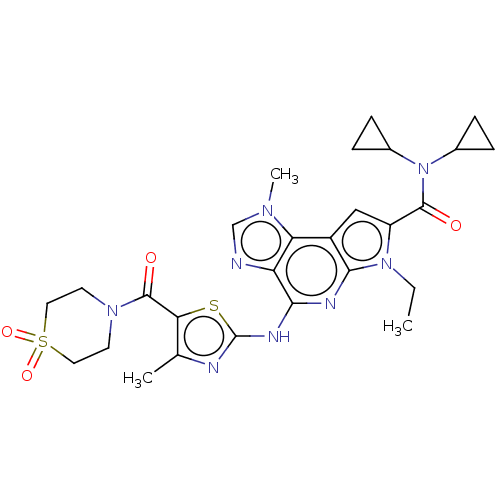
(CHEMBL3622146)Show SMILES CCn1c(cc2c1nc(Nc1nc(C)c(s1)C(=O)N1CCS(=O)(=O)CC1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C27H32N8O4S2/c1-4-34-19(25(36)35(16-5-6-16)17-7-8-17)13-18-21-20(28-14-32(21)3)23(30-24(18)34)31-27-29-15(2)22(40-27)26(37)33-9-11-41(38,39)12-10-33/h13-14,16-17H,4-12H2,1-3H3,(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472
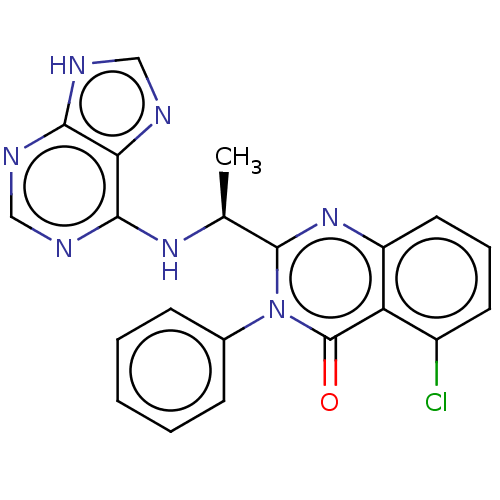
(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122326
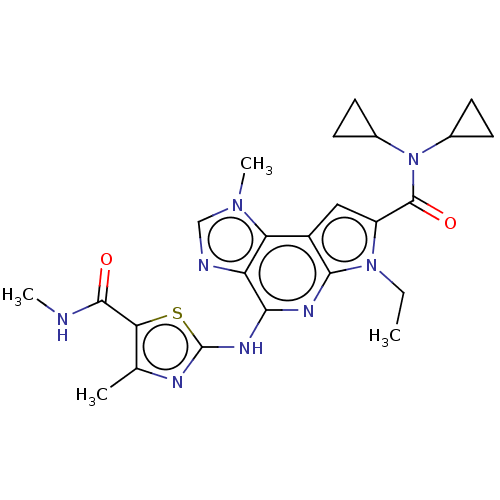
(CHEMBL3622143)Show SMILES CCn1c(cc2c1nc(Nc1nc(C)c(s1)C(=O)NC)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C24H28N8O2S/c1-5-31-16(23(34)32(13-6-7-13)14-8-9-14)10-15-18-17(26-11-30(18)4)20(28-21(15)31)29-24-27-12(2)19(35-24)22(33)25-3/h10-11,13-14H,5-9H2,1-4H3,(H,25,33)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM406774
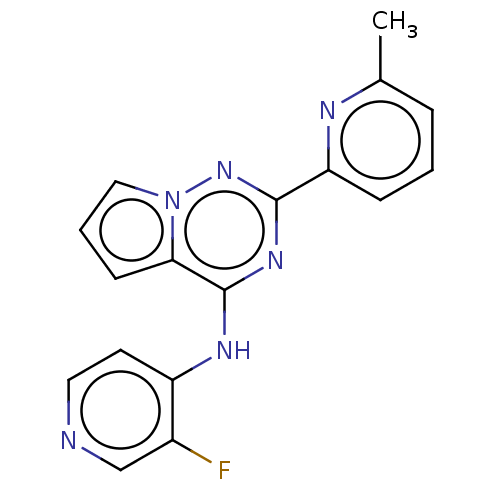
(3-fluoro-N-[2-(6-methylpyridin-2-yl)pyrrolo[2,1-f]...)Show InChI InChI=1S/C17H13FN6/c1-11-4-2-5-14(20-11)16-22-17(15-6-3-9-24(15)23-16)21-13-7-8-19-10-12(13)18/h2-10H,1H3,(H,19,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239736
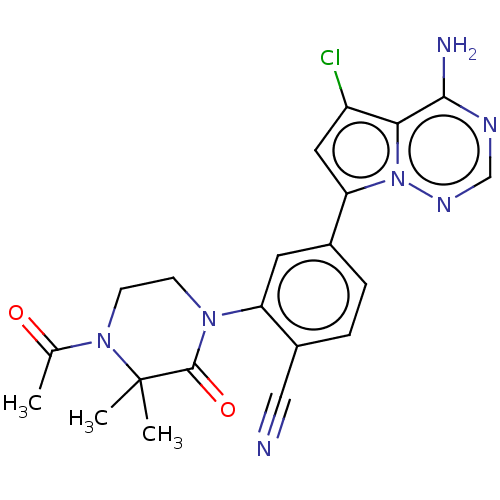
(CHEMBL4074315 | US10214537, Example 637)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H20ClN7O2/c1-12(30)28-7-6-27(20(31)21(28,2)3)16-8-13(4-5-14(16)10-23)17-9-15(22)18-19(24)25-11-26-29(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM412755

(N-(4-((2-(6-(trifluoromethyl)pyridin-2-yl)-7H-pyrr...)Show SMILES CC(=O)Nc1cc(Nc2nc(nc3[nH]ccc23)-c2cccc(n2)C(F)(F)F)ccn1 Show InChI InChI=1S/C19H14F3N7O/c1-10(30)25-15-9-11(5-7-23-15)26-17-12-6-8-24-16(12)28-18(29-17)13-3-2-4-14(27-13)19(20,21)22/h2-9H,1H3,(H3,23,24,25,26,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239752
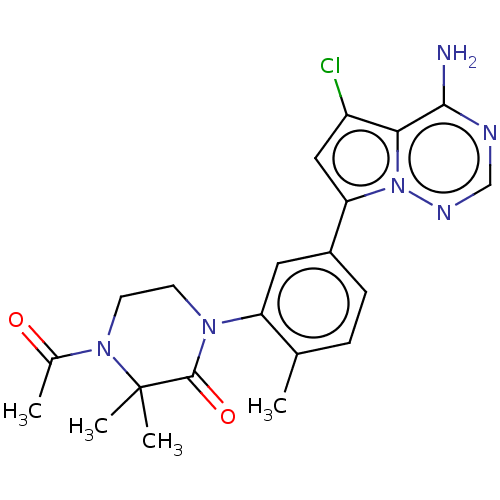
(CHEMBL4067315 | US10214537, Example 585)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H23ClN6O2/c1-12-5-6-14(17-10-15(22)18-19(23)24-11-25-28(17)18)9-16(12)26-7-8-27(13(2)29)21(3,4)20(26)30/h5-6,9-11H,7-8H2,1-4H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239744
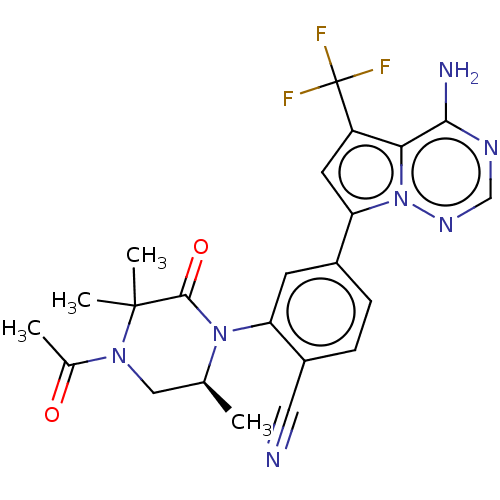
(CHEMBL4071965 | US10214537, Example 643)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C23H22F3N7O2/c1-12-10-31(13(2)34)22(3,4)21(35)32(12)17-7-14(5-6-15(17)9-27)18-8-16(23(24,25)26)19-20(28)29-11-30-33(18)19/h5-8,11-12H,10H2,1-4H3,(H2,28,29,30)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50454872
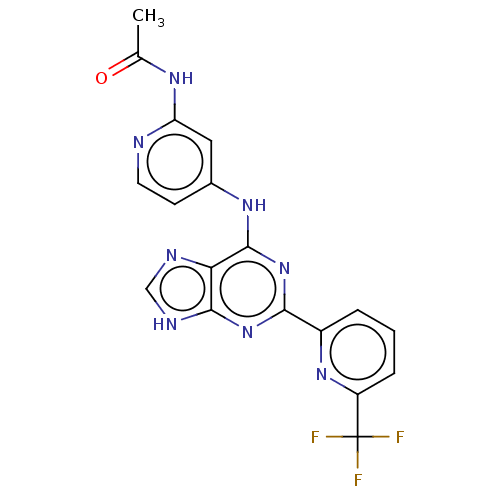
(CHEMBL4205456)Show SMILES CC(=O)Nc1cc(Nc2nc(nc3[nH]cnc23)-c2cccc(n2)C(F)(F)F)ccn1 Show InChI InChI=1S/C18H13F3N8O/c1-9(30)25-13-7-10(5-6-22-13)26-17-14-16(24-8-23-14)28-15(29-17)11-3-2-4-12(27-11)18(19,20)21/h2-8H,1H3,(H3,22,23,24,25,26,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122318
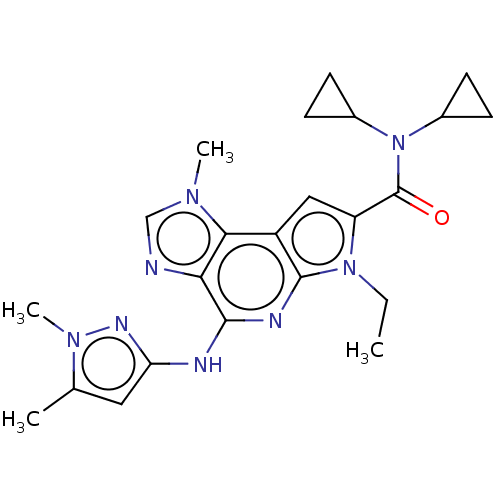
(BMS-911543)Show SMILES CCn1c(cc2c1nc(Nc1cc(C)n(C)n1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C23H28N8O/c1-5-30-17(23(32)31(14-6-7-14)15-8-9-15)11-16-20-19(24-12-28(20)3)21(26-22(16)30)25-18-10-13(2)29(4)27-18/h10-12,14-15H,5-9H2,1-4H3,(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122319
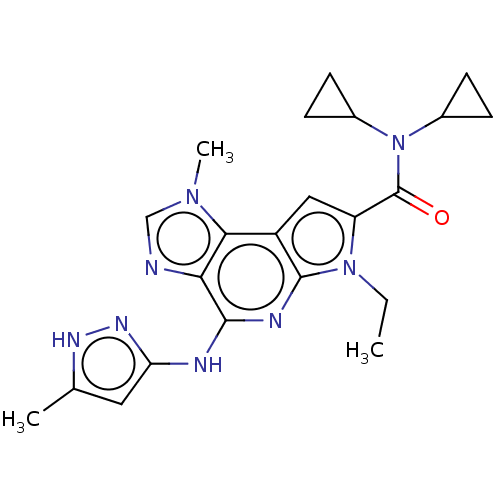
(CHEMBL3622150)Show SMILES CCn1c(cc2c1nc(Nc1cc(C)[nH]n1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C22H26N8O/c1-4-29-16(22(31)30(13-5-6-13)14-7-8-14)10-15-19-18(23-11-28(19)3)20(25-21(15)29)24-17-9-12(2)26-27-17/h9-11,13-14H,4-8H2,1-3H3,(H2,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM406766
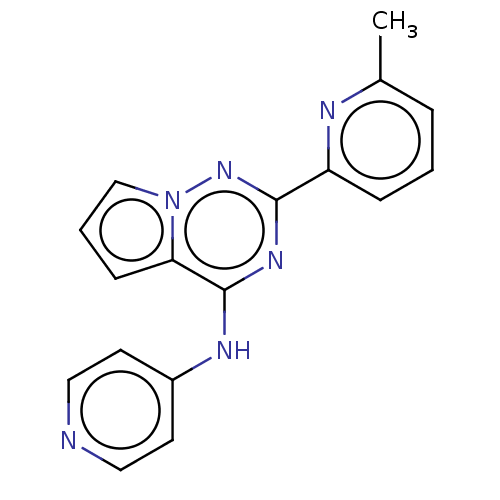
(N-[2-(6-methylpyridin-2-yl)pyrrolo[2,1-f][1,2,4]tr...)Show InChI InChI=1S/C17H14N6/c1-12-4-2-5-14(19-12)16-21-17(15-6-3-11-23(15)22-16)20-13-7-9-18-10-8-13/h2-11H,1H3,(H,18,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239741
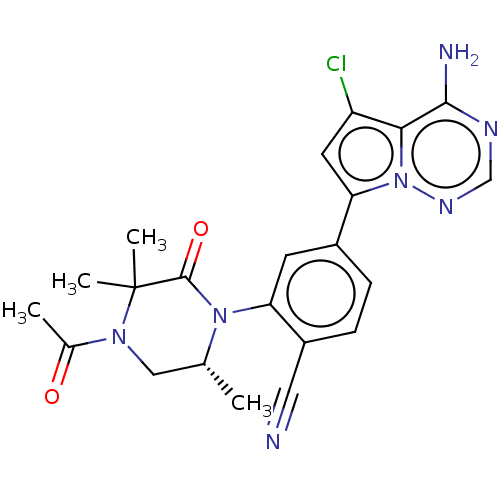
(CHEMBL4095752)Show SMILES C[C@@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C22H22ClN7O2/c1-12-10-28(13(2)31)22(3,4)21(32)29(12)17-7-14(5-6-15(17)9-24)18-8-16(23)19-20(25)26-11-27-30(18)19/h5-8,11-12H,10H2,1-4H3,(H2,25,26,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122327
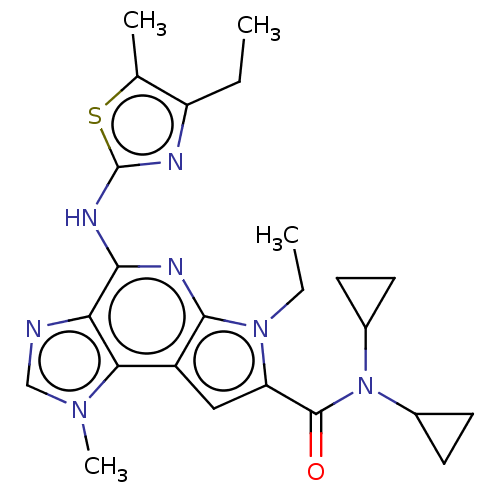
(CHEMBL3622142)Show SMILES CCc1nc(Nc2nc3n(CC)c(cc3c3n(C)cnc23)C(=O)N(C2CC2)C2CC2)sc1C Show InChI InChI=1S/C24H29N7OS/c1-5-17-13(3)33-24(26-17)28-21-19-20(29(4)12-25-19)16-11-18(30(6-2)22(16)27-21)23(32)31(14-7-8-14)15-9-10-15/h11-12,14-15H,5-10H2,1-4H3,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122324

(CHEMBL3622145)Show SMILES CCn1c(cc2c1nc(Nc1nc(C)c(s1)S(C)(=O)=O)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-5-29-16(21(31)30(13-6-7-13)14-8-9-14)10-15-18-17(24-11-28(18)3)19(26-20(15)29)27-23-25-12(2)22(34-23)35(4,32)33/h10-11,13-14H,5-9H2,1-4H3,(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239746
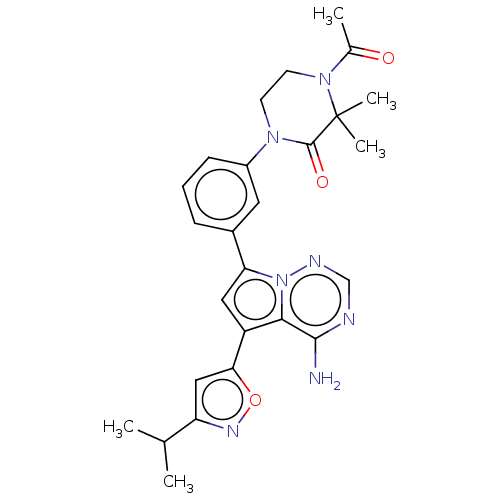
(CHEMBL4094693)Show SMILES CC(C)c1cc(on1)-c1cc(-c2cccc(c2)N2CCN(C(C)=O)C(C)(C)C2=O)n2ncnc(N)c12 Show InChI InChI=1S/C26H29N7O3/c1-15(2)20-13-22(36-30-20)19-12-21(33-23(19)24(27)28-14-29-33)17-7-6-8-18(11-17)31-9-10-32(16(3)34)26(4,5)25(31)35/h6-8,11-15H,9-10H2,1-5H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM406735
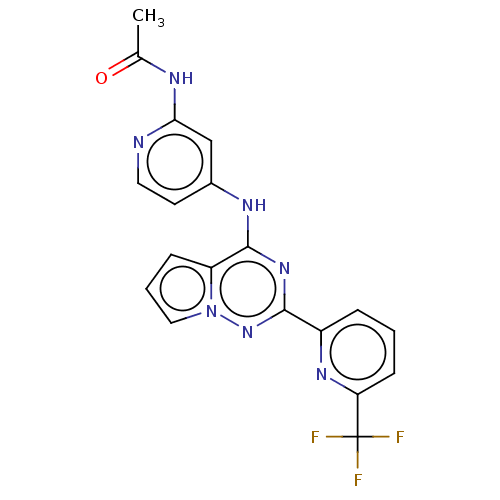
(N-(4-((2-(6-(trifluoromethyl)pyridin-2-yl)pyrrolo[...)Show SMILES CC(=O)Nc1cc(Nc2nc(nn3cccc23)-c2cccc(n2)C(F)(F)F)ccn1 Show InChI InChI=1S/C19H14F3N7O/c1-11(30)24-16-10-12(7-8-23-16)25-18-14-5-3-9-29(14)28-17(27-18)13-4-2-6-15(26-13)19(20,21)22/h2-10H,1H3,(H2,23,24,25,27,28,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239723
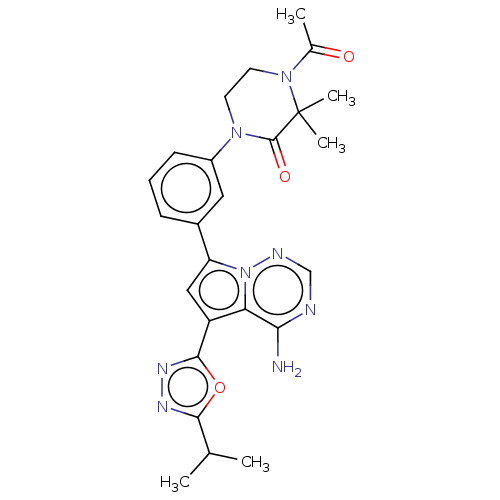
(CHEMBL4074193 | US10214537, Example 478)Show SMILES CC(C)c1nnc(o1)-c1cc(-c2cccc(c2)N2CCN(C(C)=O)C(C)(C)C2=O)n2ncnc(N)c12 Show InChI InChI=1S/C25H28N8O3/c1-14(2)22-29-30-23(36-22)18-12-19(33-20(18)21(26)27-13-28-33)16-7-6-8-17(11-16)31-9-10-32(15(3)34)25(4,5)24(31)35/h6-8,11-14H,9-10H2,1-5H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239734
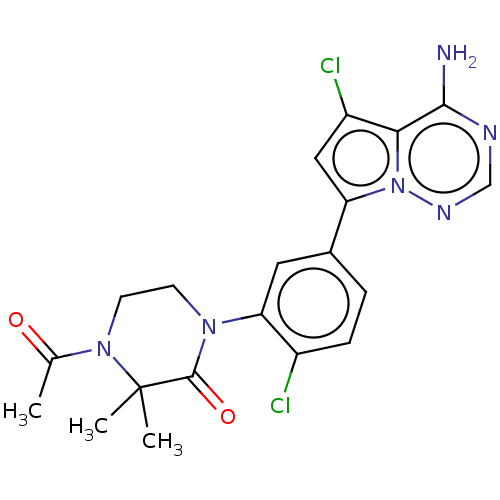
(CHEMBL4097222 | US10214537, Example 628)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1Cl)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C20H20Cl2N6O2/c1-11(29)27-7-6-26(19(30)20(27,2)3)16-8-12(4-5-13(16)21)15-9-14(22)17-18(23)24-10-25-28(15)17/h4-5,8-10H,6-7H2,1-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122321
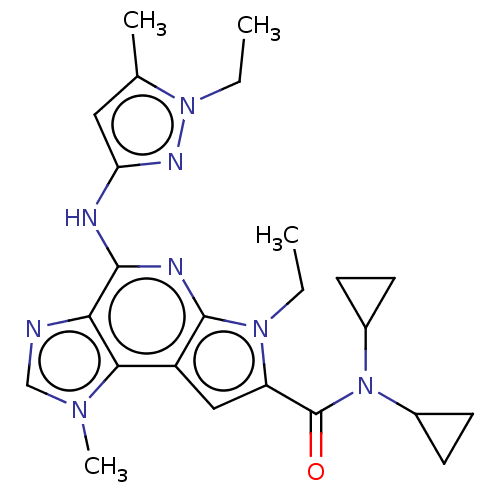
(CHEMBL3622148)Show SMILES CCn1nc(Nc2nc3n(CC)c(cc3c3n(C)cnc23)C(=O)N(C2CC2)C2CC2)cc1C Show InChI InChI=1S/C24H30N8O/c1-5-30-18(24(33)32(15-7-8-15)16-9-10-16)12-17-21-20(25-13-29(21)4)22(27-23(17)30)26-19-11-14(3)31(6-2)28-19/h11-13,15-16H,5-10H2,1-4H3,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239716
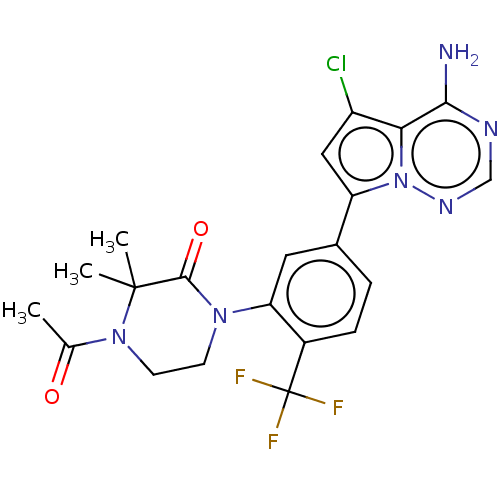
(CHEMBL4078237 | US10214537, Example 587)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C(F)(F)F)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H20ClF3N6O2/c1-11(32)30-7-6-29(19(33)20(30,2)3)16-8-12(4-5-13(16)21(23,24)25)15-9-14(22)17-18(26)27-10-28-31(15)17/h4-5,8-10H,6-7H2,1-3H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2:PS as substrate after 3 hrs by ADP-Glo assay |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM387317
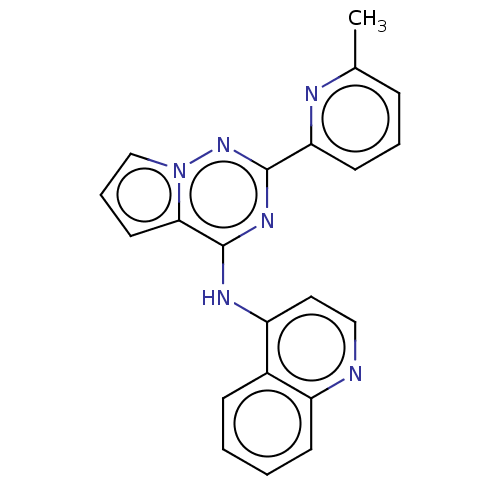
(N-[2-(6-methylpyridin-2-yl)pyrrolo[2,1-f][1,2,4]tr...)Show InChI InChI=1S/C21H16N6/c1-14-6-4-9-18(23-14)20-25-21(19-10-5-13-27(19)26-20)24-17-11-12-22-16-8-3-2-7-15(16)17/h2-13H,1H3,(H,22,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239718

(CHEMBL4064666 | US10214537, Example 639)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H20F3N7O2/c1-12(33)31-7-6-30(20(34)21(31,2)3)16-8-13(4-5-14(16)10-26)17-9-15(22(23,24)25)18-19(27)28-11-29-32(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM406773
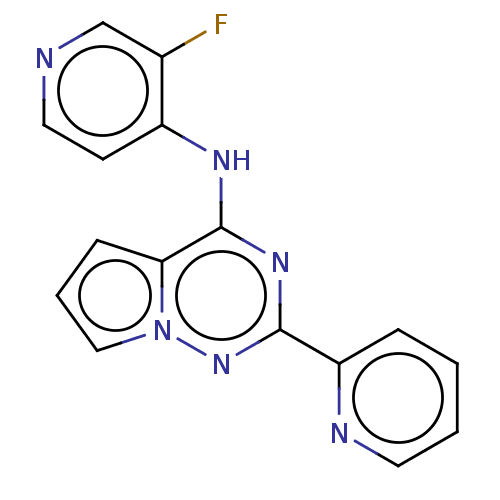
(3-fluoro-N-[2-(pyridin-2-yl)pyrrolo[2,1-f][1,2,4]t...)Show InChI InChI=1S/C16H11FN6/c17-11-10-18-8-6-12(11)20-16-14-5-3-9-23(14)22-15(21-16)13-4-1-2-7-19-13/h1-10H,(H,18,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239743
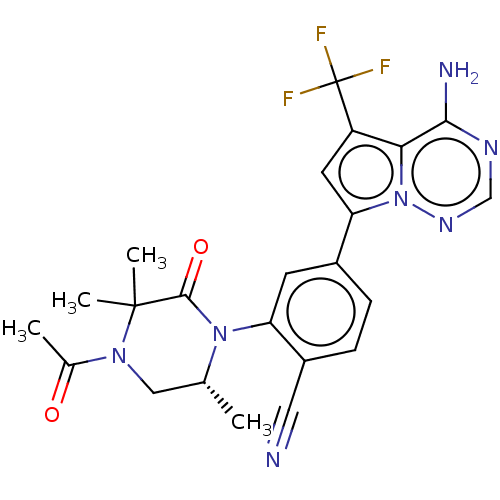
(CHEMBL4083358 | US10214537, Example 644)Show SMILES C[C@@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C23H22F3N7O2/c1-12-10-31(13(2)34)22(3,4)21(35)32(12)17-7-14(5-6-15(17)9-27)18-8-16(23(24,25)26)19-20(28)29-11-30-33(18)19/h5-8,11-12H,10H2,1-4H3,(H2,28,29,30)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50122319

(CHEMBL3622150)Show SMILES CCn1c(cc2c1nc(Nc1cc(C)[nH]n1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C22H26N8O/c1-4-29-16(22(31)30(13-5-6-13)14-7-8-14)10-15-19-18(23-11-28(19)3)20(25-21(15)29)24-17-9-12(2)26-27-17/h9-11,13-14H,4-8H2,1-3H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TYK2 (unknown origin) by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239715
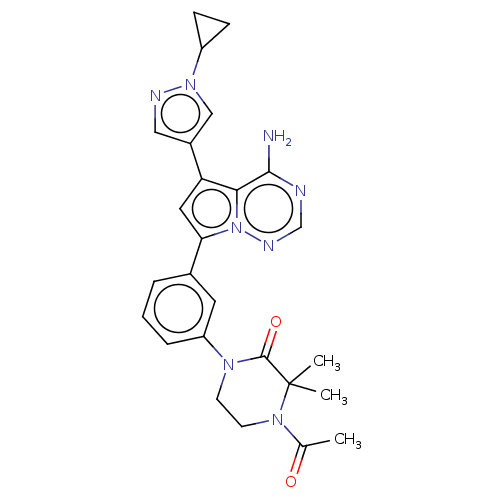
(CHEMBL4087215 | US10214537, Example 349)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2cnn(c2)C2CC2)c2c(N)ncnn12 Show InChI InChI=1S/C26H28N8O2/c1-16(35)32-10-9-31(25(36)26(32,2)3)20-6-4-5-17(11-20)22-12-21(23-24(27)28-15-30-34(22)23)18-13-29-33(14-18)19-7-8-19/h4-6,11-15,19H,7-10H2,1-3H3,(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2:PS as substrate after 3 hrs by ADP-Glo assay |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239737
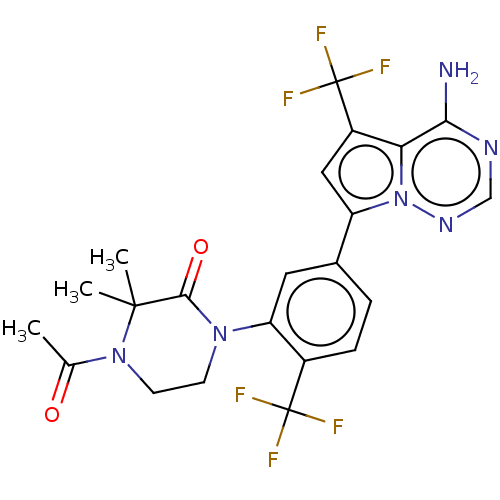
(CHEMBL4094814 | US10214537, Example 573)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C(F)(F)F)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H20F6N6O2/c1-11(35)33-7-6-32(19(36)20(33,2)3)16-8-12(4-5-13(16)21(23,24)25)15-9-14(22(26,27)28)17-18(29)30-10-31-34(15)17/h4-5,8-10H,6-7H2,1-3H3,(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239739
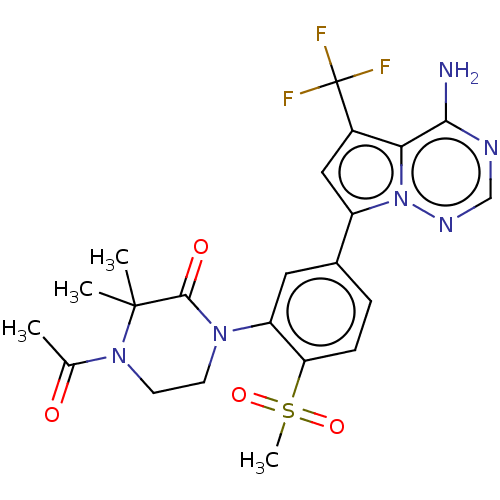
(CHEMBL4073255 | US10214537, Example 629)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1S(C)(=O)=O)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H23F3N6O4S/c1-12(32)30-8-7-29(20(33)21(30,2)3)16-9-13(5-6-17(16)36(4,34)35)15-10-14(22(23,24)25)18-19(26)27-11-28-31(15)18/h5-6,9-11H,7-8H2,1-4H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239728
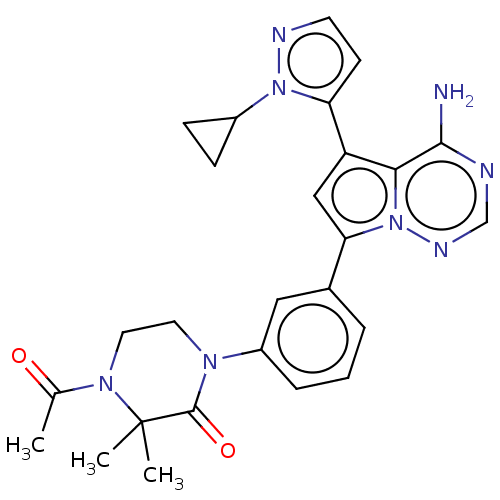
(CHEMBL4081766 | US10214537, Example 328)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2ccnn2C2CC2)c2c(N)ncnn12 Show InChI InChI=1S/C26H28N8O2/c1-16(35)32-12-11-31(25(36)26(32,2)3)19-6-4-5-17(13-19)22-14-20(23-24(27)28-15-30-34(22)23)21-9-10-29-33(21)18-7-8-18/h4-6,9-10,13-15,18H,7-8,11-12H2,1-3H3,(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
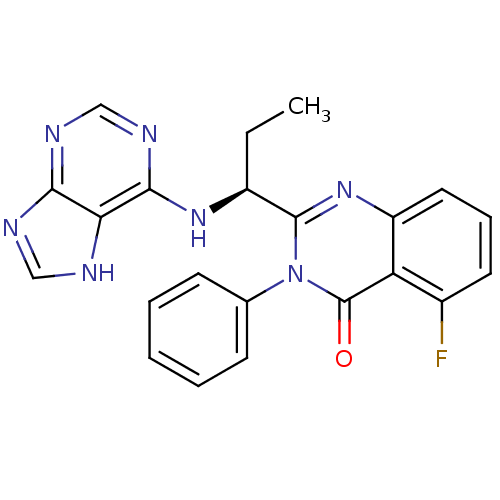
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239730
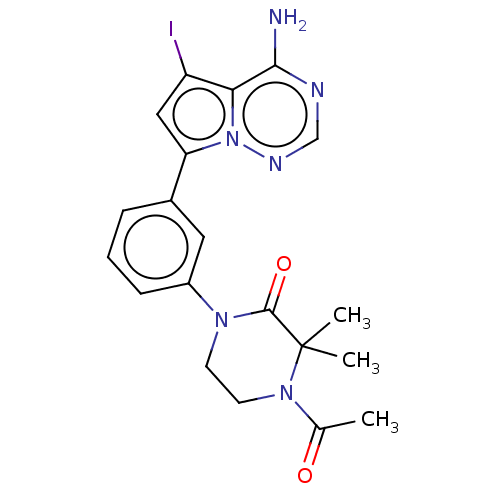
(CHEMBL4093883 | US10214537, Example 550)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(I)c2c(N)ncnn12 Show InChI InChI=1S/C20H21IN6O2/c1-12(28)26-8-7-25(19(29)20(26,2)3)14-6-4-5-13(9-14)16-10-15(21)17-18(22)23-11-24-27(16)17/h4-6,9-11H,7-8H2,1-3H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239748
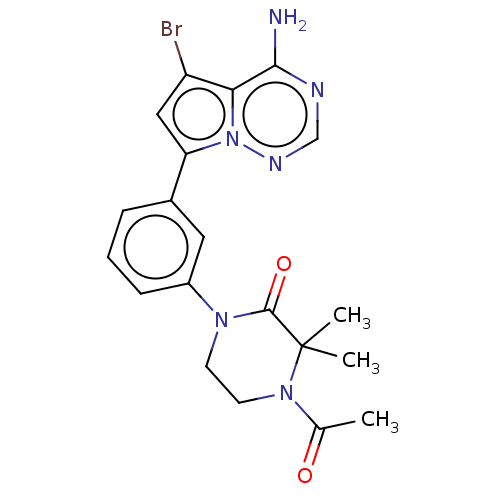
(CHEMBL4066316 | US10214537, Example 548)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(Br)c2c(N)ncnn12 Show InChI InChI=1S/C20H21BrN6O2/c1-12(28)26-8-7-25(19(29)20(26,2)3)14-6-4-5-13(9-14)16-10-15(21)17-18(22)23-11-24-27(16)17/h4-6,9-11H,7-8H2,1-3H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM407035
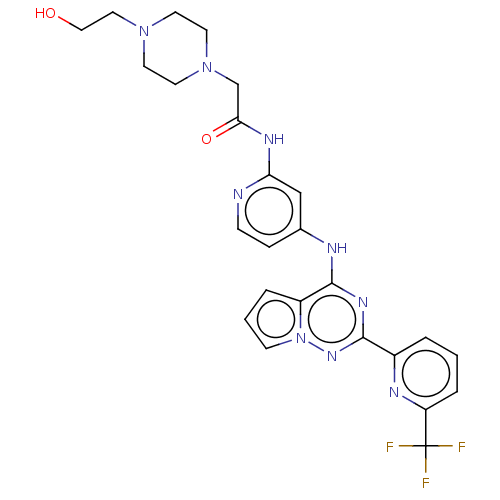
(2-[4-(2-hydroxyethyl)piperazin-1-yl]-N-[4-({2-[6-(...)Show SMILES OCCN1CCN(CC(=O)Nc2cc(Nc3nc(nn4cccc34)-c3cccc(n3)C(F)(F)F)ccn2)CC1 Show InChI InChI=1S/C25H26F3N9O2/c26-25(27,28)20-5-1-3-18(31-20)23-33-24(19-4-2-8-37(19)34-23)30-17-6-7-29-21(15-17)32-22(39)16-36-11-9-35(10-12-36)13-14-38/h1-8,15,38H,9-14,16H2,(H2,29,30,32,33,34,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239733
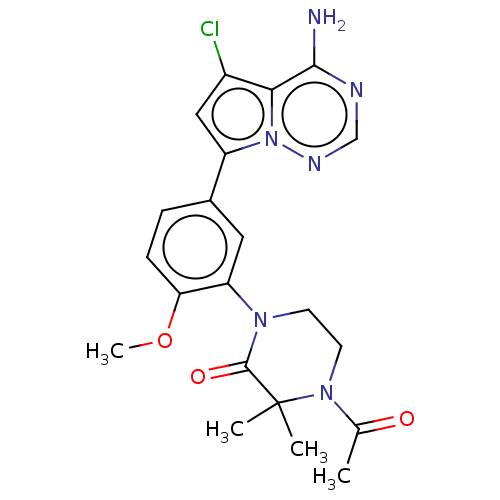
(CHEMBL4070029 | US10214537, Example 594)Show SMILES COc1ccc(cc1N1CCN(C(C)=O)C(C)(C)C1=O)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H23ClN6O3/c1-12(29)27-8-7-26(20(30)21(27,2)3)16-9-13(5-6-17(16)31-4)15-10-14(22)18-19(23)24-11-25-28(15)18/h5-6,9-11H,7-8H2,1-4H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239750
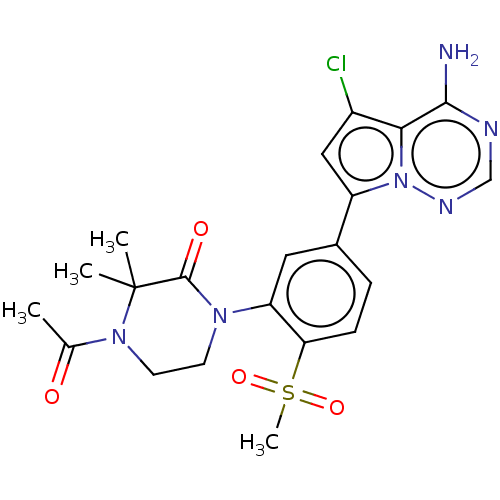
(CHEMBL4084604)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1S(C)(=O)=O)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H23ClN6O4S/c1-12(29)27-8-7-26(20(30)21(27,2)3)16-9-13(5-6-17(16)33(4,31)32)15-10-14(22)18-19(23)24-11-25-28(15)18/h5-6,9-11H,7-8H2,1-4H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239742
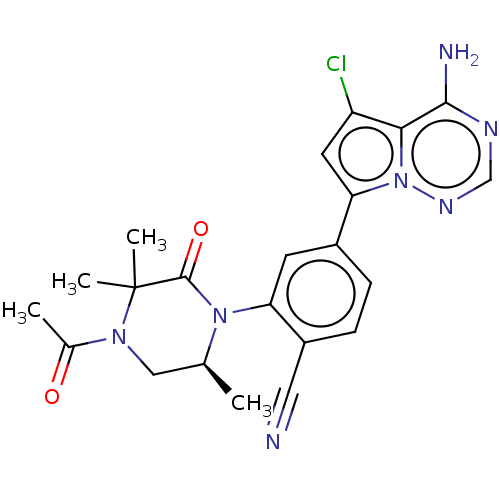
(CHEMBL4068408 | US10214537, Example 642)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C22H22ClN7O2/c1-12-10-28(13(2)31)22(3,4)21(32)29(12)17-7-14(5-6-15(17)9-24)18-8-16(23)19-20(25)26-11-27-30(18)19/h5-8,11-12H,10H2,1-4H3,(H2,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
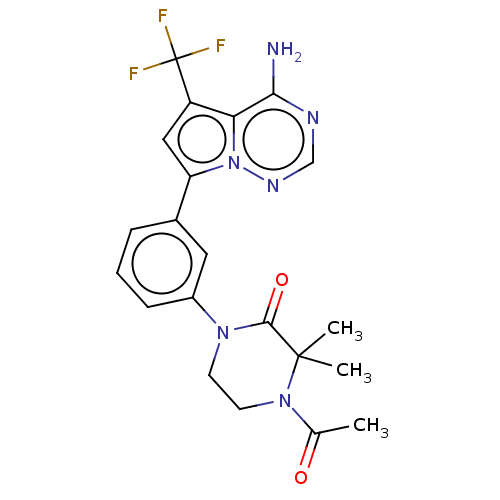
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239747
(CHEMBL4064543 | US10214537, Example 600)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C21H21F3N6O2/c1-12(31)29-8-7-28(19(32)20(29,2)3)14-6-4-5-13(9-14)16-10-15(21(22,23)24)17-18(25)26-11-27-30(16)17/h4-6,9-11H,7-8H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM406696
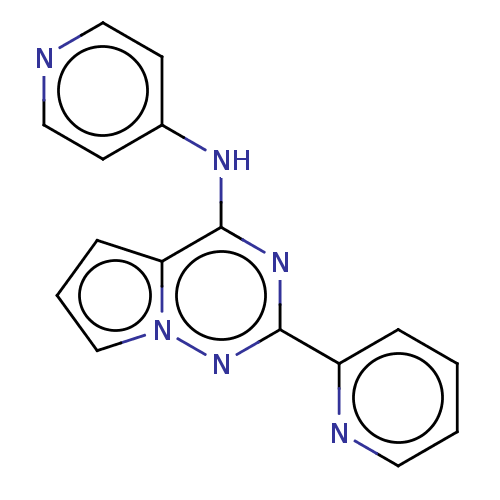
(N-[2-(pyridin-2-yl)pyrrolo[2,1-f][1,2,4]triazin-4-...)Show InChI InChI=1S/C16H12N6/c1-2-8-18-13(4-1)15-20-16(14-5-3-11-22(14)21-15)19-12-6-9-17-10-7-12/h1-11H,(H,17,19,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239738

(CHEMBL4100099 | US10214537, Example 638)Show SMILES COc1ccc(cc1N1CCN(C(C)=O)C(C)(C)C1=O)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H23F3N6O3/c1-12(32)30-8-7-29(20(33)21(30,2)3)16-9-13(5-6-17(16)34-4)15-10-14(22(23,24)25)18-19(26)27-11-28-31(15)18/h5-6,9-11H,7-8H2,1-4H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239717
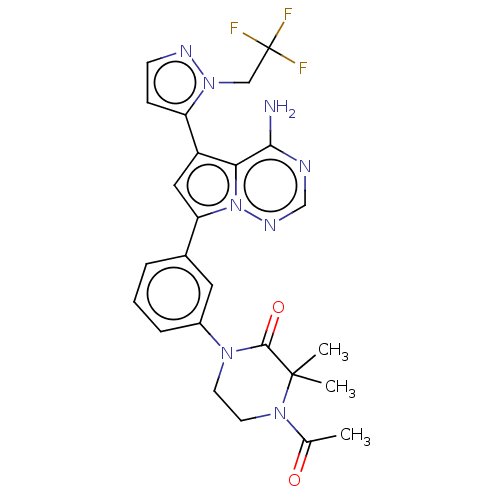
(CHEMBL4059848 | US10214537, Example 657)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2ccnn2CC(F)(F)F)c2c(N)ncnn12 Show InChI InChI=1S/C25H25F3N8O2/c1-15(37)34-10-9-33(23(38)24(34,2)3)17-6-4-5-16(11-17)20-12-18(21-22(29)30-14-32-36(20)21)19-7-8-31-35(19)13-25(26,27)28/h4-8,11-12,14H,9-10,13H2,1-3H3,(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239729

(CHEMBL4086128 | US10214537, Example 581)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C20H21ClN6O2/c1-12(28)26-8-7-25(19(29)20(26,2)3)14-6-4-5-13(9-14)16-10-15(21)17-18(22)23-11-24-27(16)17/h4-6,9-11H,7-8H2,1-3H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122325
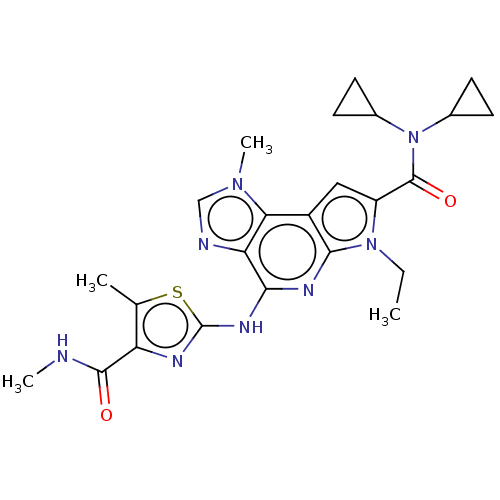
(CHEMBL3622144)Show SMILES CCn1c(cc2c1nc(Nc1nc(C(=O)NC)c(C)s1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C24H28N8O2S/c1-5-31-16(23(34)32(13-6-7-13)14-8-9-14)10-15-19-18(26-11-30(19)4)20(28-21(15)31)29-24-27-17(12(2)35-24)22(33)25-3/h10-11,13-14H,5-9H2,1-4H3,(H,25,33)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data