

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M4 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M2 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239278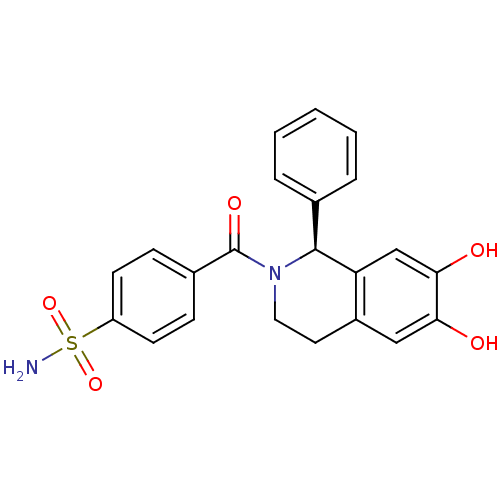 (CHEMBL4095957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239281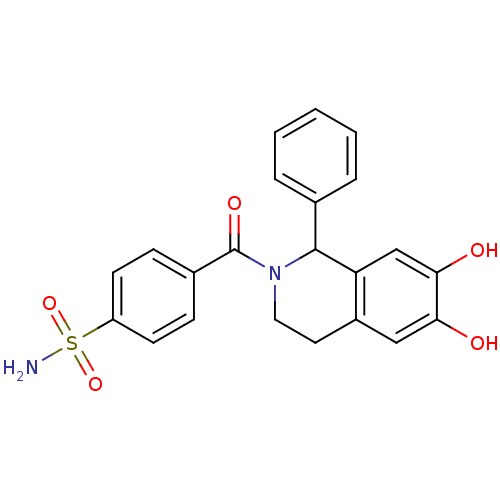 (CHEMBL4100218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M5 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239283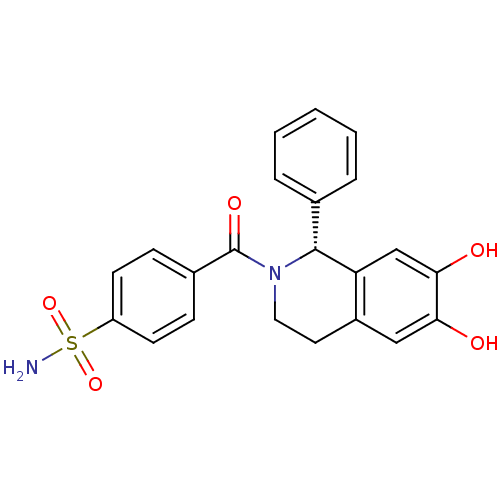 (CHEMBL4068642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50239275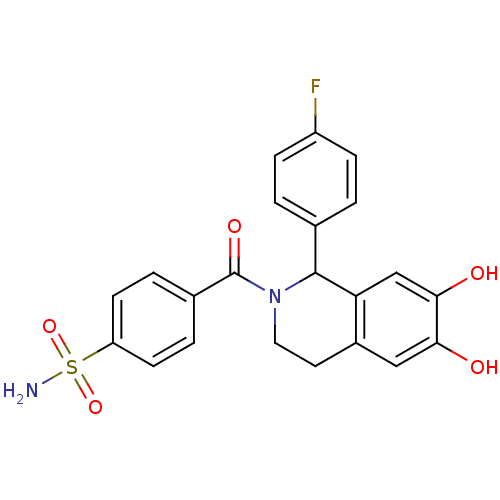 (CHEMBL4073364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50239282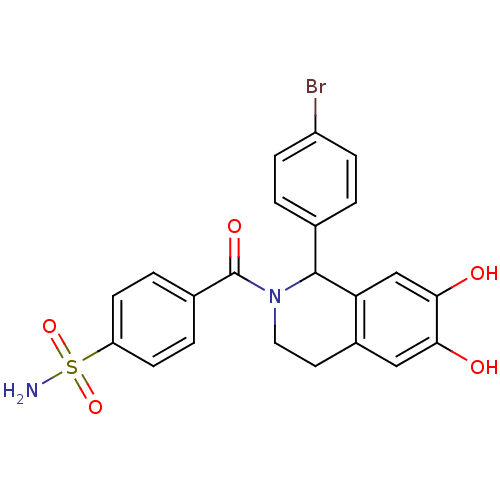 (CHEMBL4099267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50239277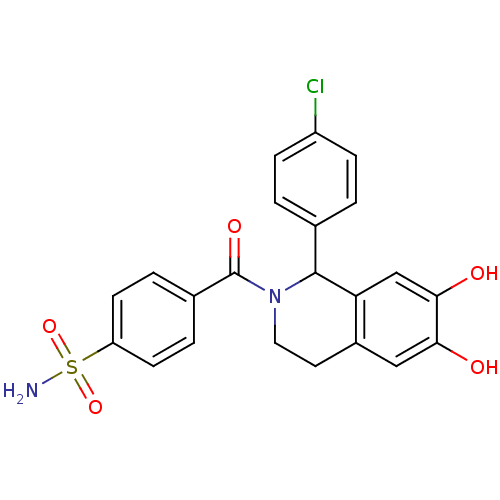 (CHEMBL4081248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239282 (CHEMBL4099267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239274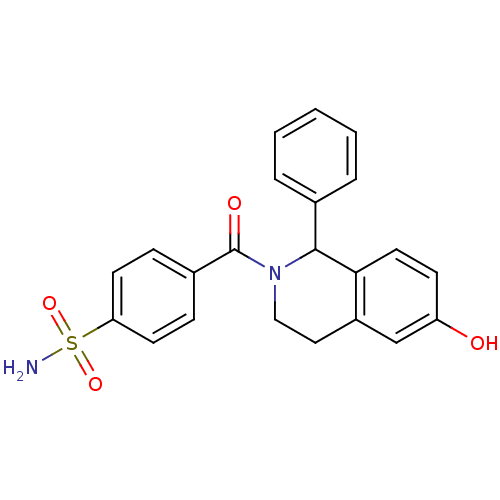 (CHEMBL4079453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239277 (CHEMBL4081248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239274 (CHEMBL4079453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239275 (CHEMBL4073364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239280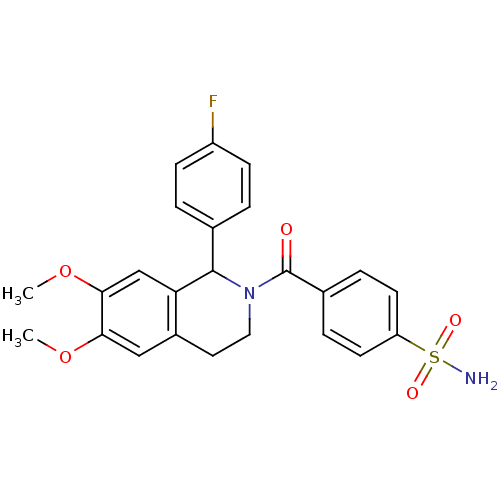 (CHEMBL4071251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50415153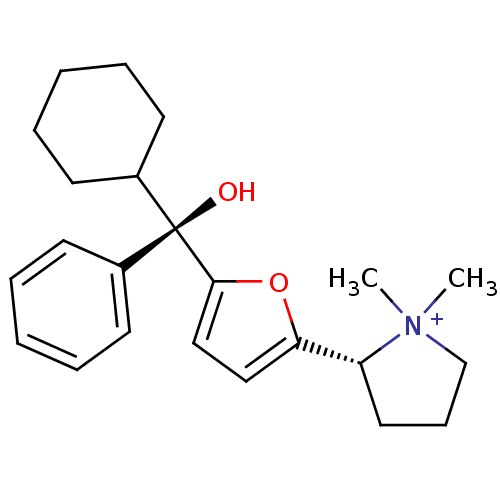 (CHEMBL569307) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239279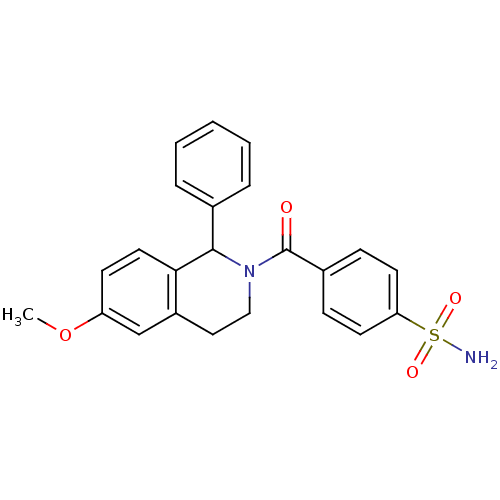 (CHEMBL4070634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239272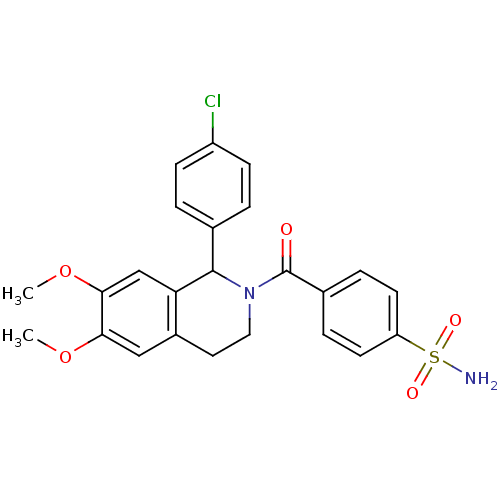 (CHEMBL4098299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50528166 (CHEMBL4519039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated with enzyme for 1 hr prior to testing by phenol red-based stopped-flow CO2 hydratio... | J Med Chem 63: 5185-5200 (2020) Article DOI: 10.1021/acs.jmedchem.9b02135 BindingDB Entry DOI: 10.7270/Q2W380S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239274 (CHEMBL4079453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50239280 (CHEMBL4071251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM11014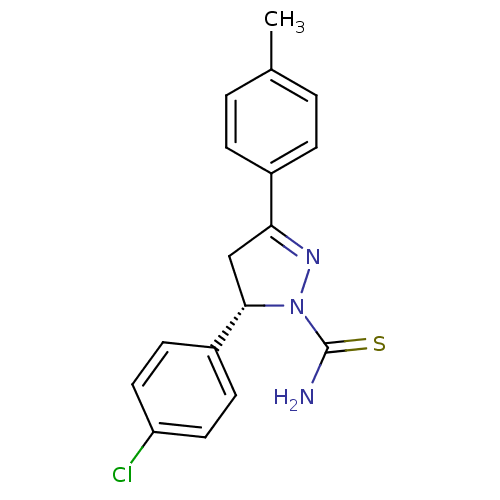 ((-)-(S)1 | (5S)-5-(4-chlorophenyl)-3-(4-methylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50514576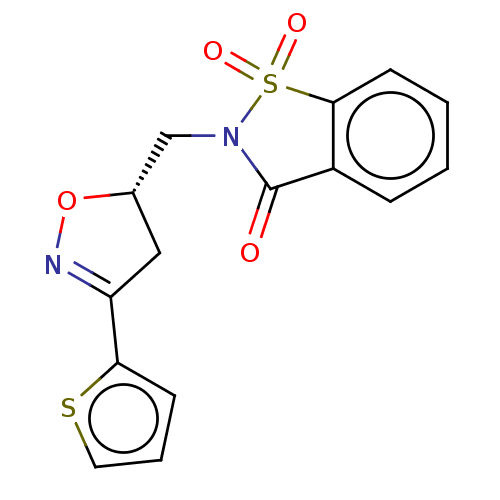 (CHEMBL4543790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 12 incubated for 15 mins prior to testing measured for 10 to 100 secs by phenol red-based stopped-... | J Med Chem 63: 2470-2488 (2020) Article DOI: 10.1021/acs.jmedchem.9b01434 BindingDB Entry DOI: 10.7270/Q27S7S4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50528166 (CHEMBL4519039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 preincubated with enzyme for 1 hr prior to testing by phenol red-based stopped-flow CO2 hydratio... | J Med Chem 63: 5185-5200 (2020) Article DOI: 10.1021/acs.jmedchem.9b02135 BindingDB Entry DOI: 10.7270/Q2W380S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50415144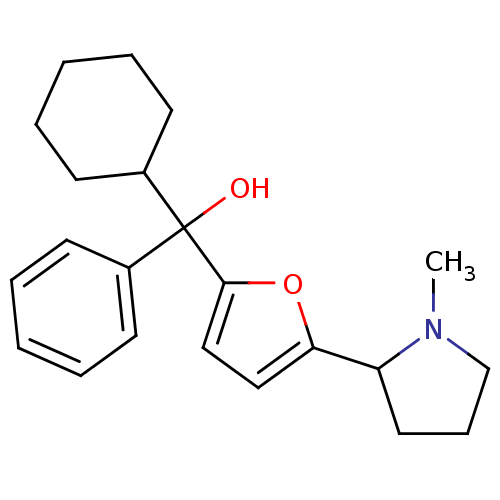 (CHEMBL583051) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50415145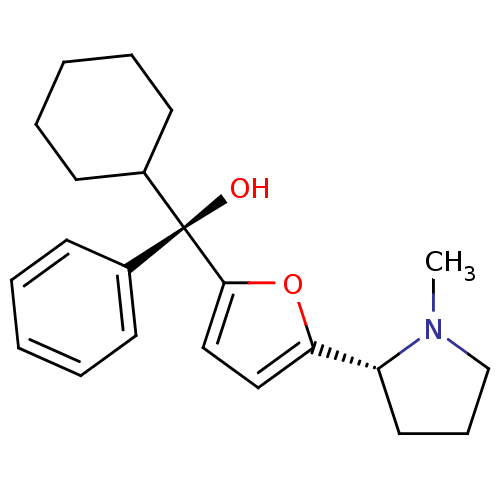 (CHEMBL571121) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50239279 (CHEMBL4070634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM11004 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50415153 (CHEMBL569307) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50415153 (CHEMBL569307) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M5 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50415145 (CHEMBL571121) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50528172 (CHEMBL4465406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated with enzyme for 1 hr prior to testing by phenol red-based stopped-flow CO2 hydratio... | J Med Chem 63: 5185-5200 (2020) Article DOI: 10.1021/acs.jmedchem.9b02135 BindingDB Entry DOI: 10.7270/Q2W380S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50239274 (CHEMBL4079453) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 14 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50292171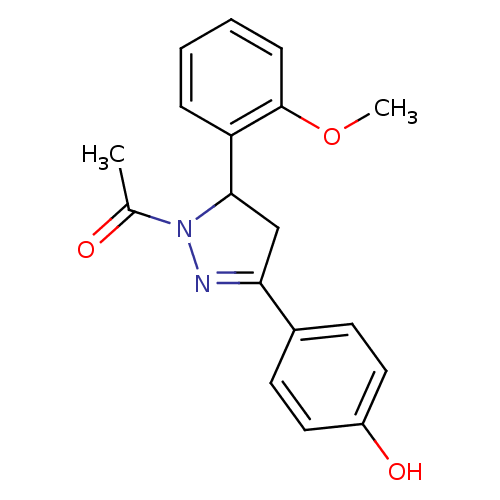 (1-[3-(4-Hydroxy-phenyl)-5-(2-methoxy-phenyl)-4,5-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Binding affinity was evaluated against human monoamino oxidase A | J Med Chem 47: 2071-4 (2004) Article DOI: 10.1021/jm031042b BindingDB Entry DOI: 10.7270/Q28053BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50292171 (1-[3-(4-Hydroxy-phenyl)-5-(2-methoxy-phenyl)-4,5-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Binding affinity was evaluated against human monoamino oxidase A | J Med Chem 47: 2071-4 (2004) Article DOI: 10.1021/jm031042b BindingDB Entry DOI: 10.7270/Q28053BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50415152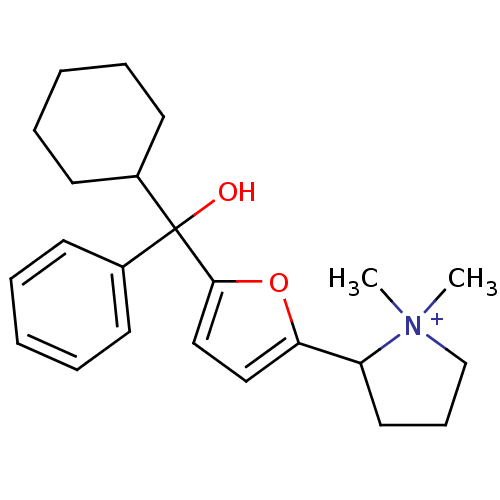 (CHEMBL569760) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50239275 (CHEMBL4073364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50415153 (CHEMBL569307) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M2 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50514576 (CHEMBL4543790) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 incubated for 15 mins prior to testing measured for 10 to 100 secs by phenol red-based stopped-f... | J Med Chem 63: 2470-2488 (2020) Article DOI: 10.1021/acs.jmedchem.9b01434 BindingDB Entry DOI: 10.7270/Q27S7S4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50239279 (CHEMBL4070634) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 14 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50528164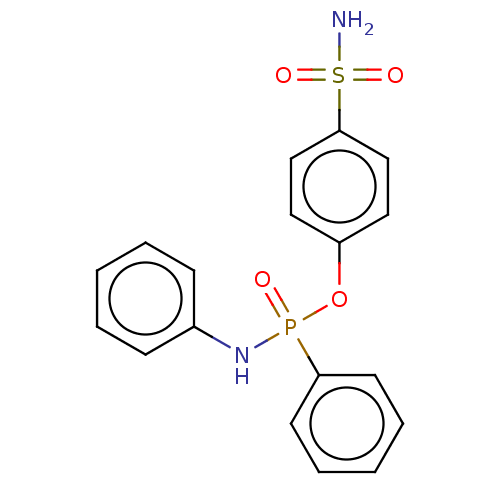 (CHEMBL4453128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated with enzyme for 1 hr prior to testing by phenol red-based stopped-flow CO2 hydratio... | J Med Chem 63: 5185-5200 (2020) Article DOI: 10.1021/acs.jmedchem.9b02135 BindingDB Entry DOI: 10.7270/Q2W380S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 preincubated with enzyme for 1 hr prior to testing by phenol red-based stopped-flow CO2 hydratio... | J Med Chem 63: 5185-5200 (2020) Article DOI: 10.1021/acs.jmedchem.9b02135 BindingDB Entry DOI: 10.7270/Q2W380S2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50528177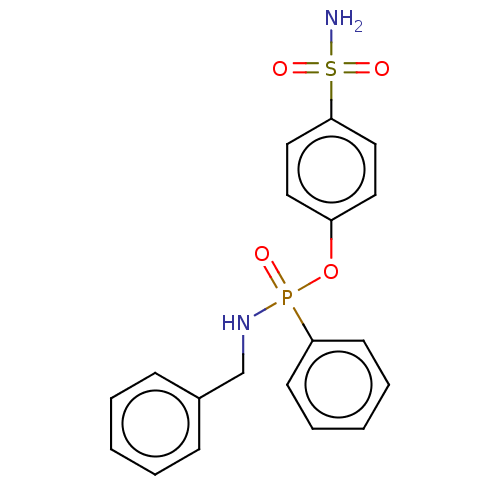 (CHEMBL4449772) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated with enzyme for 1 hr prior to testing by phenol red-based stopped-flow CO2 hydratio... | J Med Chem 63: 5185-5200 (2020) Article DOI: 10.1021/acs.jmedchem.9b02135 BindingDB Entry DOI: 10.7270/Q2W380S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM11015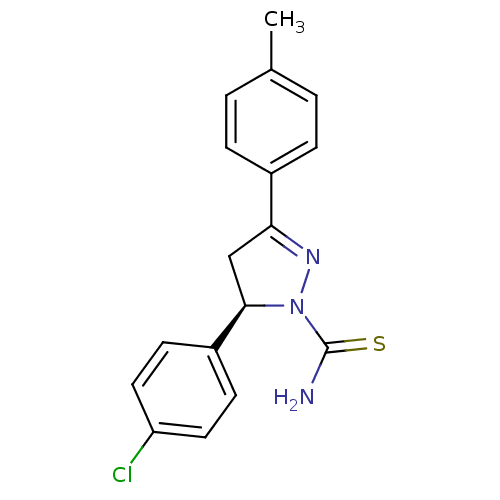 ((+)-(R)1 | (5R)-5-(4-chlorophenyl)-3-(4-methylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.70 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50239274 (CHEMBL4079453) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 14 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50239274 (CHEMBL4079453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | J Med Chem 60: 4316-4326 (2017) Article DOI: 10.1021/acs.jmedchem.7b00264 BindingDB Entry DOI: 10.7270/Q2RB76RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50528162 (CHEMBL4476108) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated with enzyme for 1 hr prior to testing by phenol red-based stopped-flow CO2 hydratio... | J Med Chem 63: 5185-5200 (2020) Article DOI: 10.1021/acs.jmedchem.9b02135 BindingDB Entry DOI: 10.7270/Q2W380S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50528169 (CHEMBL4579472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated with enzyme for 1 hr prior to testing by phenol red-based stopped-flow CO2 hydratio... | J Med Chem 63: 5185-5200 (2020) Article DOI: 10.1021/acs.jmedchem.9b02135 BindingDB Entry DOI: 10.7270/Q2W380S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1454 total ) | Next | Last >> |