

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369432 (CHEMBL1627465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Irreversible inhibition of human steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50545255 (CHEMBL4643348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Irreversible inhibition of human steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50168219 (CHEMBL3805209) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center (CHUL, T4) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells assessed as reduction in transformation of [3H]-E1S to E1 after 2 hrs by l... | Eur J Med Chem 119: 169-82 (2016) Article DOI: 10.1016/j.ejmech.2016.04.044 BindingDB Entry DOI: 10.7270/Q2QR503Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369432 (CHEMBL1627465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50545254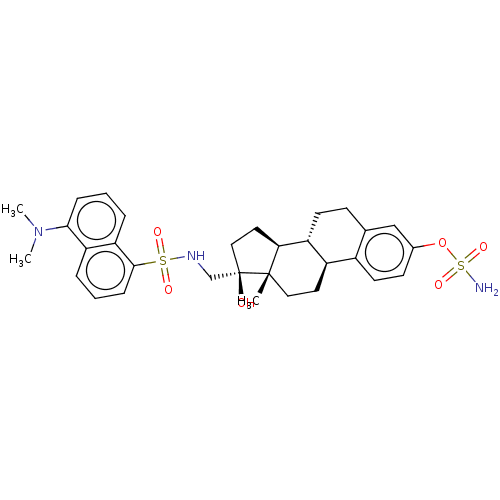 (CHEMBL4639657) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Irreversible inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation coun... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50193084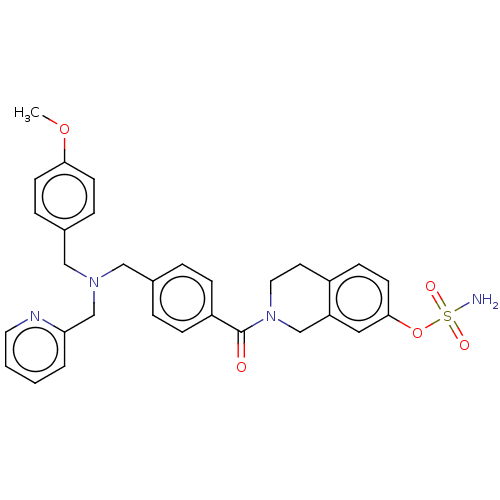 (CHEMBL3910182) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center (CHUL, T4) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells assessed as reduction in transformation of [3H]-E1S to E1 after 2 hrs by l... | Eur J Med Chem 119: 169-82 (2016) Article DOI: 10.1016/j.ejmech.2016.04.044 BindingDB Entry DOI: 10.7270/Q2QR503Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50350421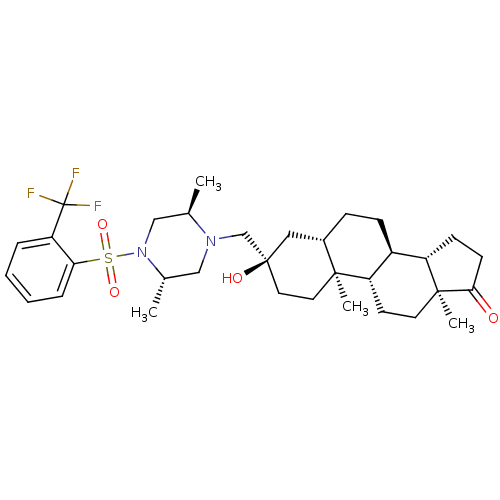 (CHEMBL1813731) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center Curated by ChEMBL | Assay Description Inhibition of 17betaHSD3 (unknown origin) transfected in HEK293 cells | Bioorg Med Chem Lett 26: 2179-83 (2016) Article DOI: 10.1016/j.bmcl.2016.03.069 BindingDB Entry DOI: 10.7270/Q2FT8Q2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50350421 (CHEMBL1813731) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ (CHUL)-Research Center Curated by ChEMBL | Assay Description Inhibition of 17Beta-HSD3 expressed in intact HEK293 cells assessed as transformation of [14C]-4-androstene-3,17-dione into [14C]-testosterone in pre... | Bioorg Med Chem 19: 4652-68 (2011) Article DOI: 10.1016/j.bmc.2011.06.003 BindingDB Entry DOI: 10.7270/Q2222V4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50193082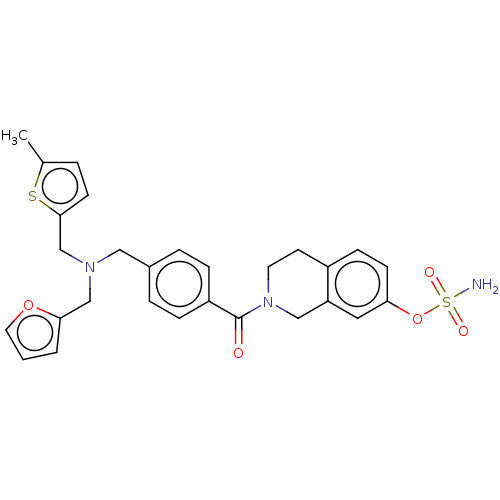 (CHEMBL3953180) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center (CHUL, T4) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells assessed as reduction in transformation of [3H]-E1S to E1 after 2 hrs by l... | Eur J Med Chem 119: 169-82 (2016) Article DOI: 10.1016/j.ejmech.2016.04.044 BindingDB Entry DOI: 10.7270/Q2QR503Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM50350421 (CHEMBL1813731) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in rat testes microsomes using [14C]-4-androstene-3,17-dione as substrate after 2 hrs | Bioorg Med Chem 23: 5433-51 (2015) Article DOI: 10.1016/j.bmc.2015.07.049 BindingDB Entry DOI: 10.7270/Q20C4XHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50193083 (CHEMBL3981927) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center (CHUL, T4) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells assessed as reduction in transformation of [3H]-E1S to E1 after 2 hrs by l... | Eur J Med Chem 119: 169-82 (2016) Article DOI: 10.1016/j.ejmech.2016.04.044 BindingDB Entry DOI: 10.7270/Q2QR503Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) by fluorescence assay | J Med Chem 57: 204-22 (2014) Article DOI: 10.1021/jm401639v BindingDB Entry DOI: 10.7270/Q2V98C2Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using AMMC by fluorescence assay | Bioorg Med Chem 22: 5847-59 (2014) Article DOI: 10.1016/j.bmc.2014.09.026 BindingDB Entry DOI: 10.7270/Q2BG2S0T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50373700 (CHEMBL410242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Inhibition of 17-beta HSD1 in human T47D cells assessed as transformation of [14C]E1 to [14C]E2 after 24 hrs by thin layer chromatography | J Med Chem 57: 204-22 (2014) Article DOI: 10.1021/jm401639v BindingDB Entry DOI: 10.7270/Q2V98C2Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50373700 (CHEMBL410242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human T47D cells assessed as decrease in transformation of [14C]estrone to [14C]-estradiol after 24 hrs by thin layer ch... | ACS Med Chem Lett 2: 678-681 (2011) Article DOI: 10.1021/ml200093v BindingDB Entry DOI: 10.7270/Q23T9JCS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Reversible inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate by scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM50111763 (CHEMBL3605211) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in rat testes microsomes using [14C]-4-androstene-3,17-dione as substrate after 2 hrs | Bioorg Med Chem 23: 5433-51 (2015) Article DOI: 10.1016/j.bmc.2015.07.049 BindingDB Entry DOI: 10.7270/Q20C4XHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50108810 (CHEMBL347019 | N-Adamantan-2-ylmethyl-N-(3-hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description Concentration to inhibit 50% activity of the Type-3 17-beta- hydroxysteroid dehydrogenase | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50350422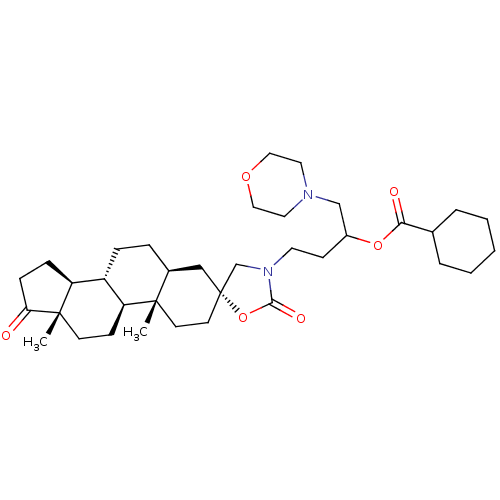 (CHEMBL1813912) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ (CHUL)-Research Center Curated by ChEMBL | Assay Description Inhibition of 17Beta-HSD3 expressed in intact HEK293 cells assessed as transformation of [14C]-4-androstene-3,17-dione into [14C]-testosterone in pre... | Bioorg Med Chem 19: 4652-68 (2011) Article DOI: 10.1016/j.bmc.2011.06.003 BindingDB Entry DOI: 10.7270/Q2222V4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50108814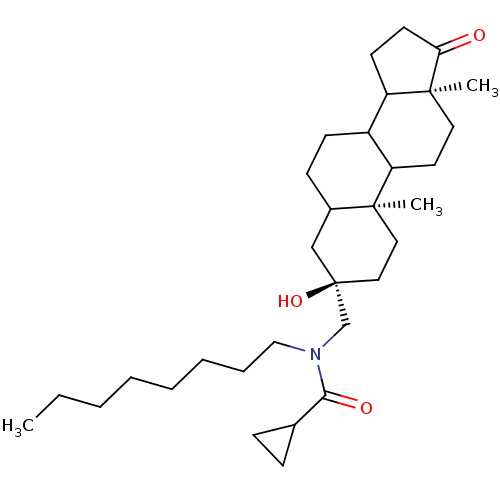 (CHEMBL158995 | Cyclopropanecarboxylic acid (3-hydr...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description The ability to inhibit the Type-3 17-beta- hydroxysteroid dehydrogenase activity in transfected human embryonic kidney (HEK)-293 cells experiment 2 | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50093902 ((3R,10S,13S)-3-Benzyl-3-hydroxy-10,13-dimethyl-hex...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description The ability to inhibit the Type-3 17-beta- hydroxysteroid dehydrogenase activity in transfected human embryonic kidney (HEK)-293 cells experiment 1 | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50108810 (CHEMBL347019 | N-Adamantan-2-ylmethyl-N-(3-hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description Concentration to inhibit 50% activity of the Type-3 17-beta- hydroxysteroid dehydrogenase | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM50528377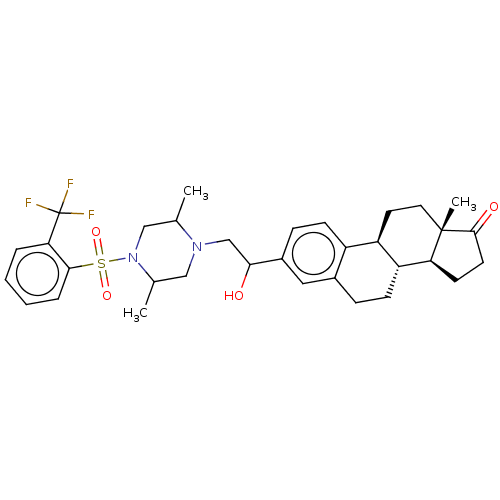 (CHEMBL4451878) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in Sprague-Dawley rat testes microsomal fraction assessed as reduction in [14C]-testosterone formation from [14C]-4-androst... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50400509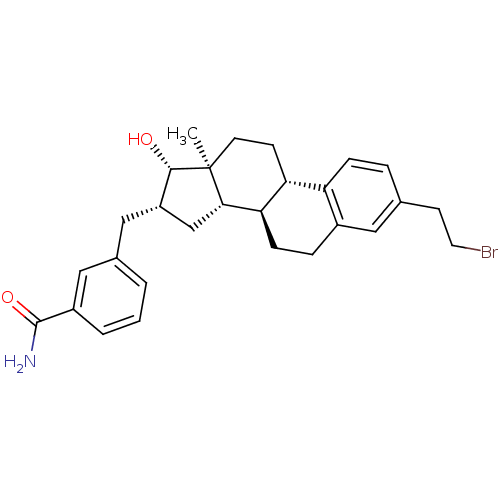 (CHEMBL2203397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Inhibition of 17-beta HSD1 in human T47D cells assessed as transformation of [14C]E1 to [14C]E2 after 24 hrs by thin layer chromatography | J Med Chem 57: 204-22 (2014) Article DOI: 10.1021/jm401639v BindingDB Entry DOI: 10.7270/Q2V98C2Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50400509 (CHEMBL2203397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human T47D cells assessed as decrease in transformation of [14C]estrone to [14C]-estradiol after 24 hrs by thin layer ch... | ACS Med Chem Lett 2: 678-681 (2011) Article DOI: 10.1021/ml200093v BindingDB Entry DOI: 10.7270/Q23T9JCS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50545253 (CHEMBL4632735) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Reversible inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counti... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50108813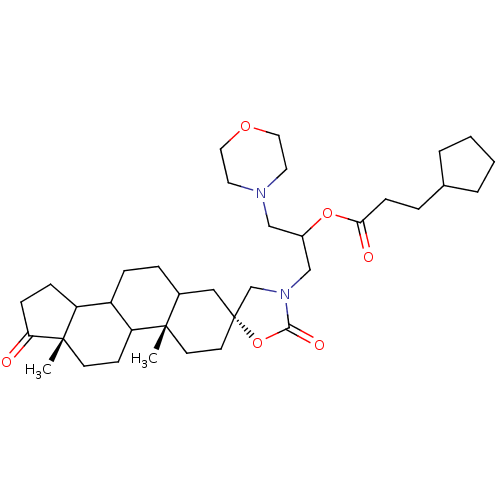 (2-[9a,11a-dimethyl-1,2'-dioxospiro[perhydrocyclope...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description Concentration to inhibit 50% activity of the Type-3 17-beta- hydroxysteroid dehydrogenase | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50108811 (3-[(Adamantan-2-ylmethyl-butyl-amino)-methyl]-3-hy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description Concentration to inhibit 50% activity of the Type-3 17-beta- hydroxysteroid dehydrogenase | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM50443289 (CHEMBL3088217) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in Sprague-Dawley rat testes microsomal fraction assessed as reduction in [14C]-testosterone formation from [14C]-4-androst... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50108812 (CHEMBL160184 | Cyclopropanecarboxylic acid cyclohe...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description Concentration to inhibit 50% activity of the Type-3 17-beta- hydroxysteroid dehydrogenase | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50350421 (CHEMBL1813731) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center Curated by ChEMBL | Assay Description Inhibition of 17betaHSD3 (unknown origin) transfected in human LNCAP cells assessed as conversion of [14C]-4-androstene-3,17-dione into [14C]-testost... | Bioorg Med Chem Lett 26: 2179-83 (2016) Article DOI: 10.1016/j.bmcl.2016.03.069 BindingDB Entry DOI: 10.7270/Q2FT8Q2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM50111764 (CHEMBL3605212) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in rat testes microsomes using [14C]-4-androstene-3,17-dione as substrate after 2 hrs | Bioorg Med Chem 23: 5433-51 (2015) Article DOI: 10.1016/j.bmc.2015.07.049 BindingDB Entry DOI: 10.7270/Q20C4XHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM17639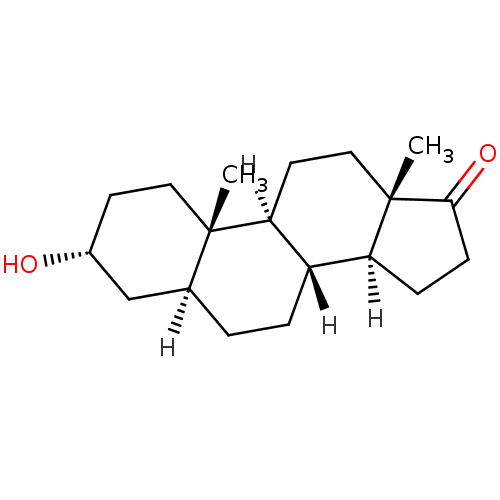 ((1S,2S,5R,7S,10R,11S,15S)-5-hydroxy-2,15-dimethylt...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in rat testes microsomes using [14C]-4-androstene-3,17-dione as substrate after 2 hrs | Bioorg Med Chem 23: 5433-51 (2015) Article DOI: 10.1016/j.bmc.2015.07.049 BindingDB Entry DOI: 10.7270/Q20C4XHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50443289 (CHEMBL3088217) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 (unknown origin) expressed in human LNCAP cells assessed as reduction in [14C]-testosterone formation from [14C]-4-androste... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50400509 (CHEMBL2203397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Inhibition of 17-beta HSD1 in human T47D cells | J Med Chem 57: 204-22 (2014) Article DOI: 10.1021/jm401639v BindingDB Entry DOI: 10.7270/Q2V98C2Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50093902 ((3R,10S,13S)-3-Benzyl-3-hydroxy-10,13-dimethyl-hex...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description The ability to inhibit the Type-3 17-beta- hydroxysteroid dehydrogenase activity in transfected human embryonic kidney (HEK)-293 cells experiment 3 | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50528381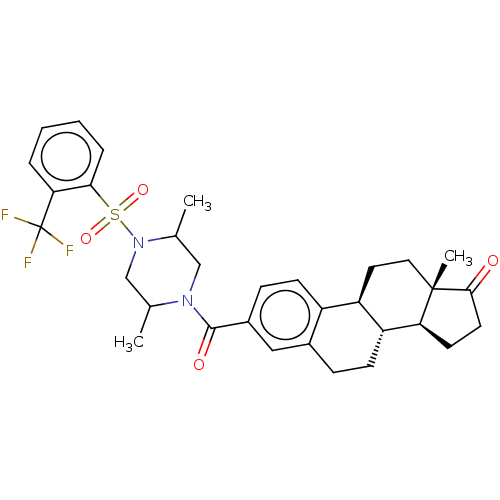 (CHEMBL4468509) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 (unknown origin) expressed in human LNCAP cells assessed as reduction in [14C]-testosterone formation from [14C]-4-androste... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM50528383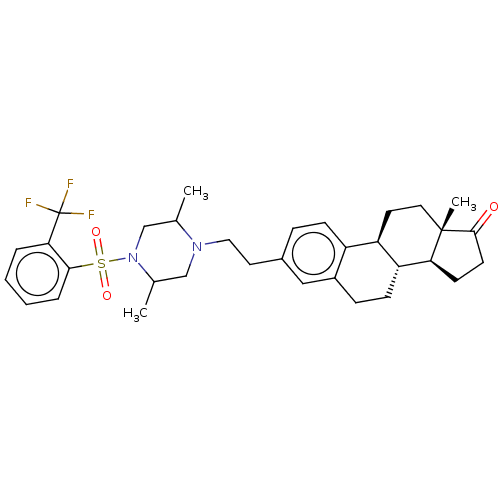 (CHEMBL4445467) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in Sprague-Dawley rat testes microsomal fraction assessed as reduction in [14C]-testosterone formation from [14C]-4-androst... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM50528381 (CHEMBL4468509) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in Sprague-Dawley rat testes microsomal fraction assessed as reduction in [14C]-testosterone formation from [14C]-4-androst... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50350421 (CHEMBL1813731) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Inhibition of 17-beta-HSD3 (unknown origin) expressed in human LNCaP cells using [14C]-4-androstene-3,17-dione as substrate assessed as reduction of ... | Bioorg Med Chem 25: 2065-2073 (2017) Article DOI: 10.1016/j.bmc.2017.02.008 BindingDB Entry DOI: 10.7270/Q2668GF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM50528382 (CHEMBL4515514) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in Sprague-Dawley rat testes microsomal fraction assessed as reduction in [14C]-testosterone formation from [14C]-4-androst... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) using AMMC by fluorescence assay | Bioorg Med Chem 22: 5847-59 (2014) Article DOI: 10.1016/j.bmc.2014.09.026 BindingDB Entry DOI: 10.7270/Q2BG2S0T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50528383 (CHEMBL4445467) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 (unknown origin) expressed in human LNCAP cells assessed as reduction in [14C]-testosterone formation from [14C]-4-androste... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50528377 (CHEMBL4451878) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 (unknown origin) expressed in human LNCAP cells assessed as reduction in [14C]-testosterone formation from [14C]-4-androste... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50528377 (CHEMBL4451878) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 (unknown origin) expressed in human LNCAP cells assessed as reduction in [14C]-testosterone formation from [14C]-4-androste... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50235625 (CHEMBL4076831) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Inhibition of 17-beta-HSD3 (unknown origin) expressed in human LNCaP cells using [14C]-4-androstene-3,17-dione as substrate assessed as reduction of ... | Bioorg Med Chem 25: 2065-2073 (2017) Article DOI: 10.1016/j.bmc.2017.02.008 BindingDB Entry DOI: 10.7270/Q2668GF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50495475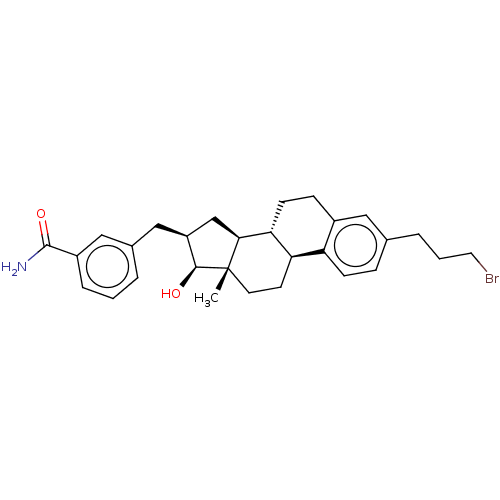 (CHEMBL3108979) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Inhibition of 17-beta HSD1 in human T47D cells assessed as transformation of [14C]E1 to [14C]E2 after 24 hrs by thin layer chromatography | J Med Chem 57: 204-22 (2014) Article DOI: 10.1021/jm401639v BindingDB Entry DOI: 10.7270/Q2V98C2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Rattus norvegicus) | BDBM91713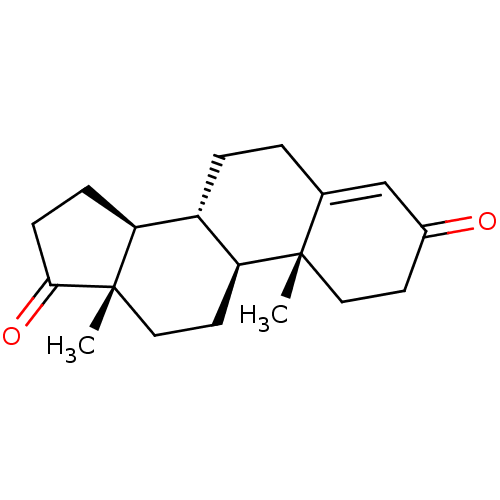 (Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 in rat testes microsomes using [14C]-4-androstene-3,17-dione as substrate after 2 hrs | Bioorg Med Chem 23: 5433-51 (2015) Article DOI: 10.1016/j.bmc.2015.07.049 BindingDB Entry DOI: 10.7270/Q20C4XHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50528382 (CHEMBL4515514) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Queb£c-Research Center Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 (unknown origin) expressed in human LNCAP cells assessed as reduction in [14C]-testosterone formation from [14C]-4-androste... | J Med Chem 62: 7070-7088 (2019) Article DOI: 10.1021/acs.jmedchem.9b00624 BindingDB Entry DOI: 10.7270/Q2B56P6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |