

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50287720 (6,7-Dibromo-3-[(2S,5R)-5-(6-bromo-1H-indol-3-yl)-4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against human Alpha-1b adrenergic receptor | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50287720 (6,7-Dibromo-3-[(2S,5R)-5-(6-bromo-1H-indol-3-yl)-4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50287718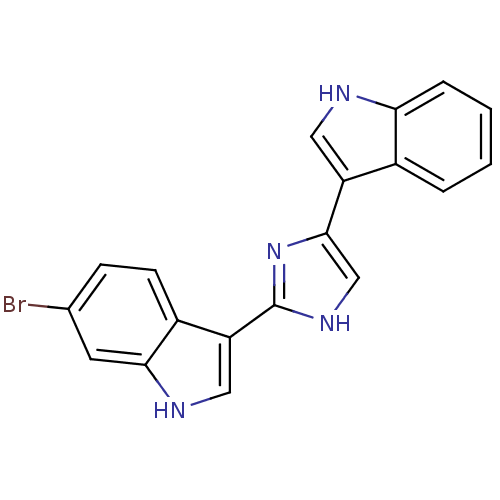 (6-Bromo-3-[4-(1H-indol-3-yl)-1H-imidazol-2-yl]-1H-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50287720 (6,7-Dibromo-3-[(2S,5R)-5-(6-bromo-1H-indol-3-yl)-4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined against Alpha-1a adrenergic receptor | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50287720 (6,7-Dibromo-3-[(2S,5R)-5-(6-bromo-1H-indol-3-yl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50287719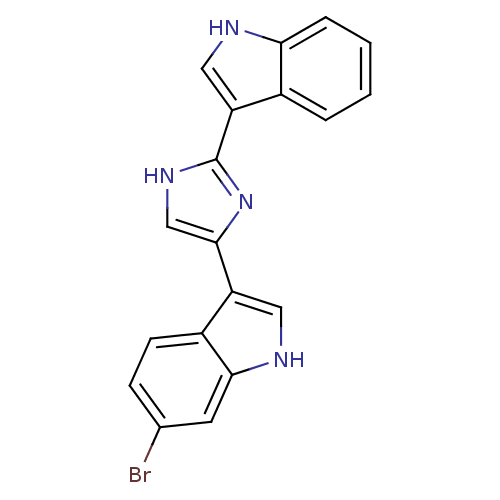 (6-Bromo-3-[2-(1H-indol-3-yl)-1H-imidazol-4-yl]-1H-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50287723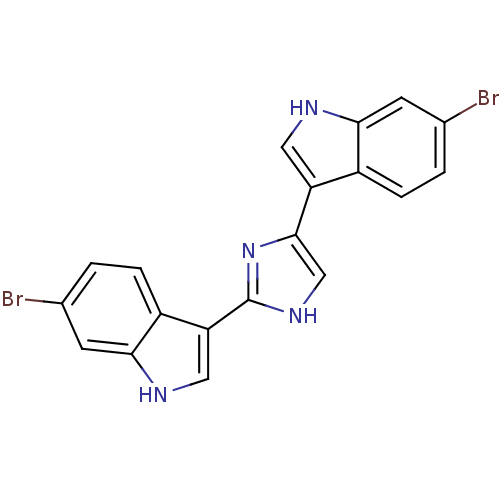 (6-bromo-3-[4-(6-bromo-1H-3-indolyl)-1H-2-imidazoly...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50287719 (6-Bromo-3-[2-(1H-indol-3-yl)-1H-imidazol-4-yl]-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50287722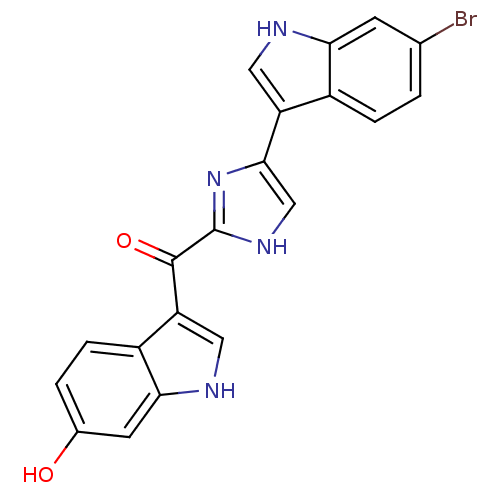 (CHEMBL436760 | [4-(6-Bromo-1H-indol-3-yl)-1H-imida...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50287718 (6-Bromo-3-[4-(1H-indol-3-yl)-1H-imidazol-2-yl]-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50287721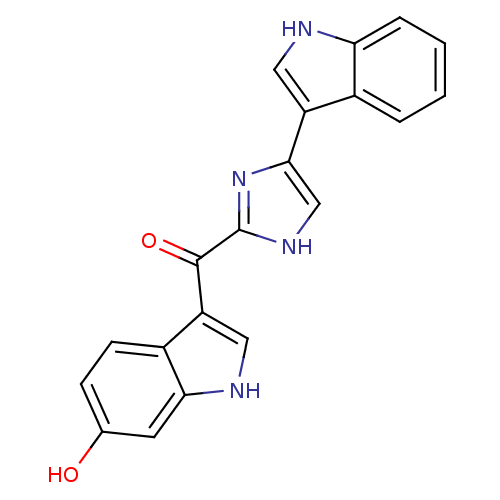 ((6-Hydroxy-1H-indol-3-yl)-[5-(1H-indol-3-yl)-1H-im...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50287723 (6-bromo-3-[4-(6-bromo-1H-3-indolyl)-1H-2-imidazoly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50287721 ((6-Hydroxy-1H-indol-3-yl)-[5-(1H-indol-3-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster alpha1b adrenergic receptor by displacing [125I]-HEAT (2-b... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50287722 (CHEMBL436760 | [4-(6-Bromo-1H-indol-3-yl)-1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... | Bioorg Med Chem Lett 6: 2103-2106 (1996) Article DOI: 10.1016/0960-894X(96)00376-9 BindingDB Entry DOI: 10.7270/Q2SJ1KMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Piau£ Curated by ChEMBL | Assay Description Inhibition of Wistar rat plasma angiotensin 1-converting enzyme using H-hippuryl-His-Leu-OH as substrate after 20 mins by fluorescence assay | Eur J Med Chem 139: 401-411 (2017) Article DOI: 10.1016/j.ejmech.2017.08.019 BindingDB Entry DOI: 10.7270/Q2T43WMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451769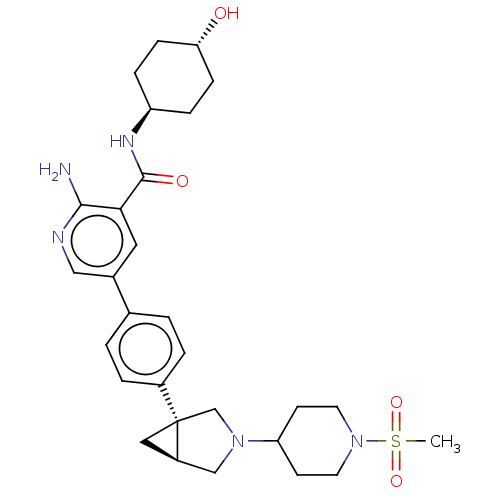 (US10710980, Example 1 | US10947218, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451772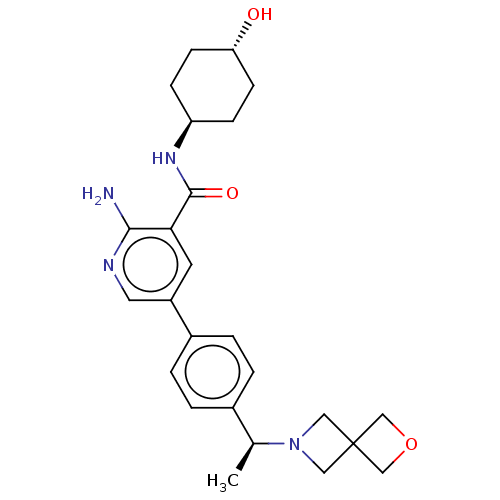 (US10710980, Example 4 | US10947218, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451769 (US10710980, Example 1 | US10947218, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451772 (US10710980, Example 4 | US10947218, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451789 (US10710980, Example 21 | US10947218, Example 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451789 (US10710980, Example 21 | US10947218, Example 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451798 (US10710980, Example 30 | US10947218, Example 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451806 (US10710980, Example 38 | US10947218, Example 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451772 (US10710980, Example 4 | US10947218, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451788 (US10710980, Example 20 | US10947218, Example 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451806 (US10710980, Example 38 | US10947218, Example 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451806 (US10710980, Example 38 | US10947218, Example 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451798 (US10710980, Example 30 | US10947218, Example 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451806 (US10710980, Example 38 | US10947218, Example 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451772 (US10710980, Example 4 | US10947218, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | 80 | n/a | n/a | n/a | n/a |
GPC Biotech Incorporated | Assay Description CAII assays were performed at CEREP (paris). All other kinase assays were performed at Upstate (Dundee, UK). | Chem Biol 13: 711-22 (2006) Article DOI: 10.1016/j.chembiol.2006.05.008 BindingDB Entry DOI: 10.7270/Q2WM1BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451773 (US10710980, Example 5 | US10947218, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451773 (US10710980, Example 5 | US10947218, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451788 (US10710980, Example 20 | US10947218, Example 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451805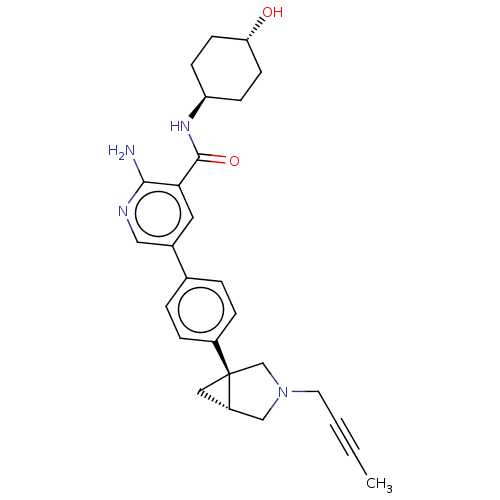 (US10710980, Example 37 | US10947218, Example 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451819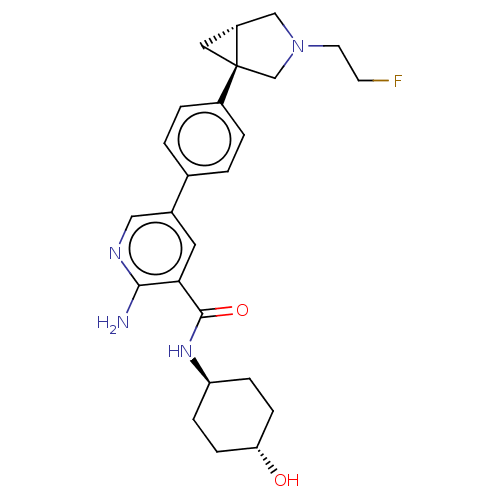 (US10710980, Example 48 | US10947218, Example 48) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451769 (US10710980, Example 1 | US10947218, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451775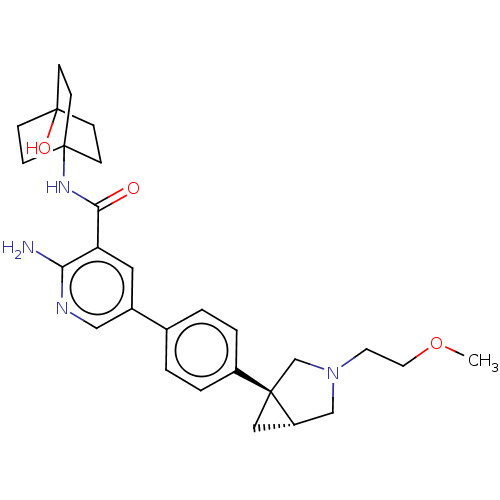 (US10710980, Example 7 | US10947218, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451781 (US10710980, Example 13 | US10947218, Example 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451805 (US10710980, Example 37 | US10947218, Example 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451805 (US10710980, Example 37 | US10947218, Example 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451805 (US10710980, Example 37 | US10947218, Example 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451819 (US10710980, Example 48 | US10947218, Example 48) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451769 (US10710980, Example 1 | US10947218, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499,R206H] (Homo sapiens (Human)) | BDBM451775 (US10710980, Example 7 | US10947218, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451781 (US10710980, Example 13 | US10947218, Example 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 6 ul. Dose-response curves were generated by ... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451769 (US10710980, Example 1 | US10947218, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451779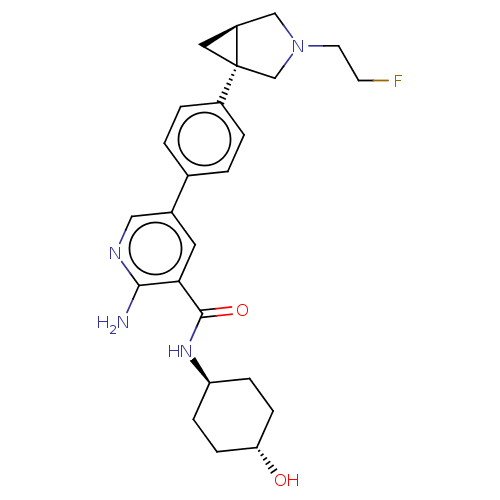 (US10710980, Example 11 | US10947218, Example 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451790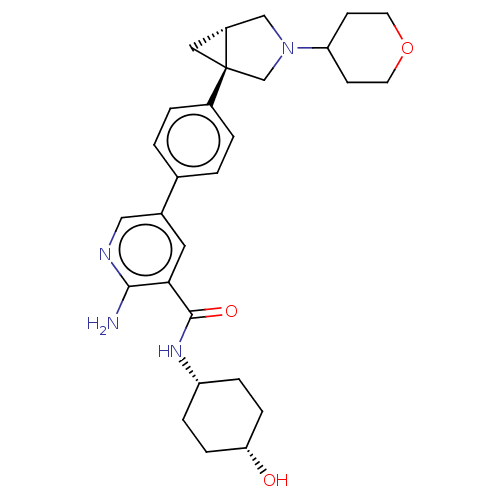 (US10710980, Example 22 | US10947218, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [172-499] (Homo sapiens (Human)) | BDBM451791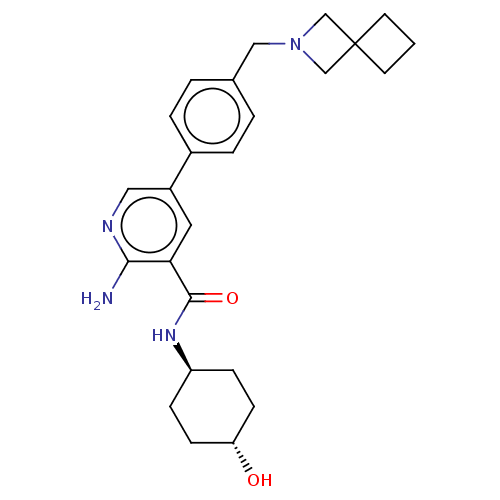 (US10710980, Example 23 | US10947218, Example 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures autophosphorylation using the ADP-Glo Kinase Assay (Promega, V9101) was set-up for wild-type ALK2 (aa172-49... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1273 total ) | Next | Last >> |