

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3386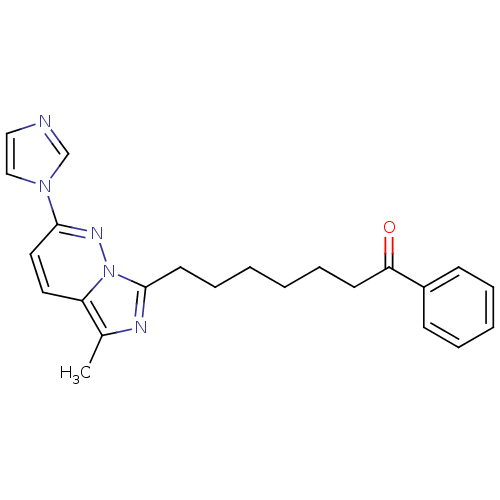 (7-[2-(1H-Imidazol-l-yl)-5-methylimidazo[1,5-b]pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042631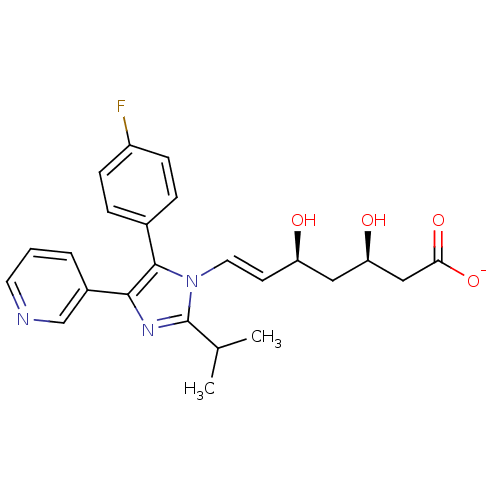 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042625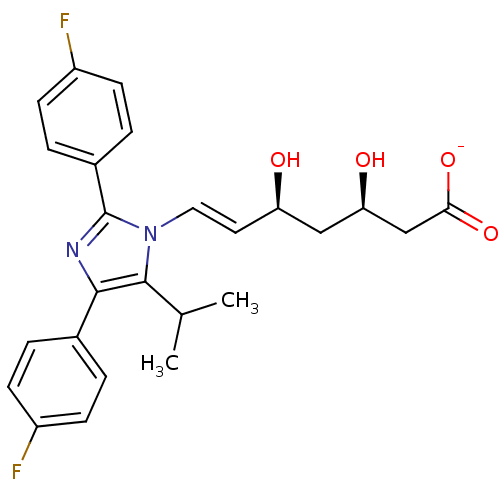 ((E)-(3R,5S)-7-[2,4-Bis-(4-fluoro-phenyl)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042607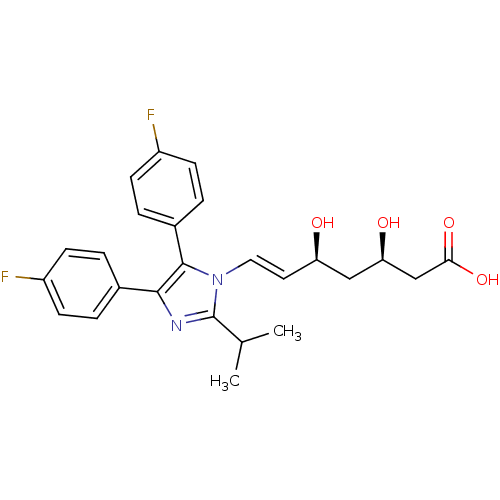 ((E)-(3R,5S)-7-[4,5-Bis-(4-fluoro-phenyl)-2-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042620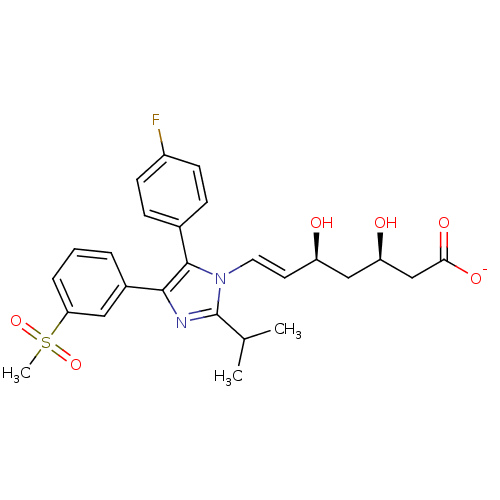 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042614 (CHEMBL120932 | Sodium; 7-[4,5-bis-(4-fluoro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042615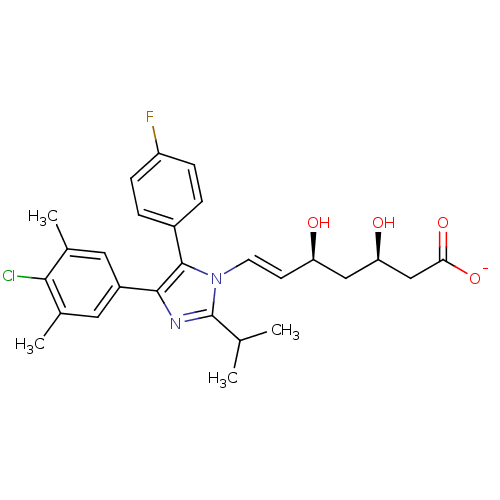 ((E)-(3R,5S)-7-[4-(4-Chloro-3,5-dimethyl-phenyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042629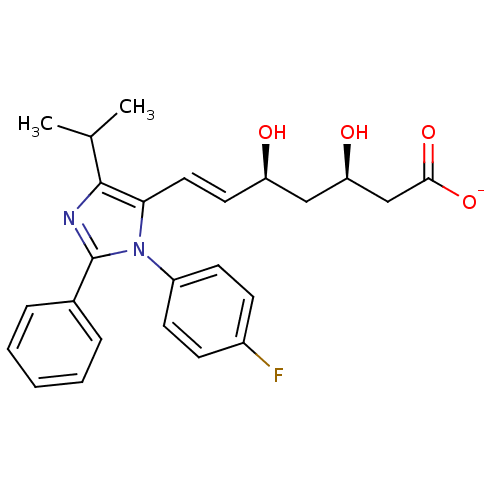 (CHEMBL121309 | Sodium; 7-[3-(4-fluoro-phenyl)-5-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042601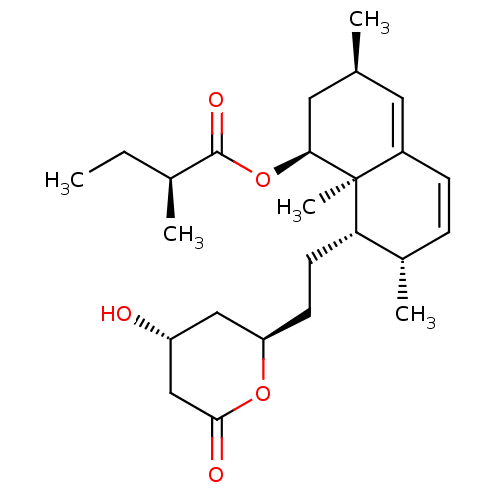 (2-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042622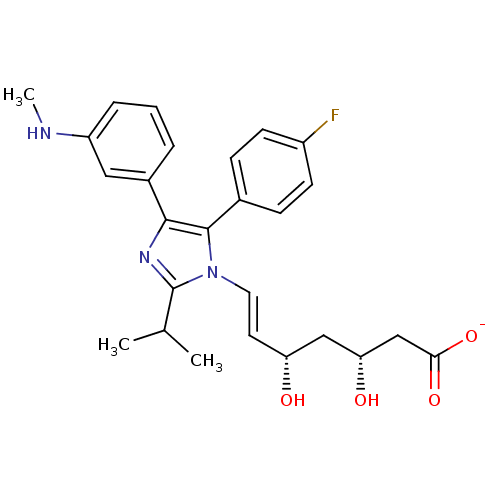 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042612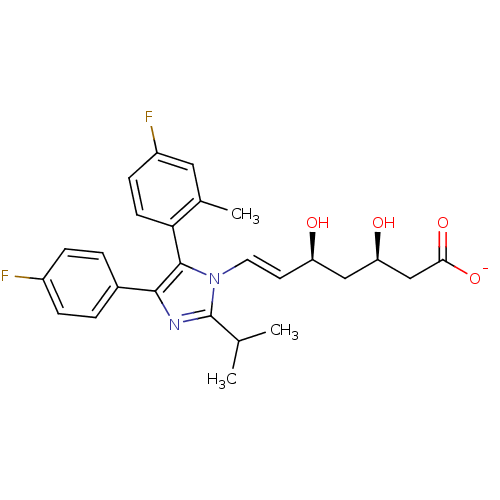 (CHEMBL121610 | Sodium; 7-[5-(4-fluoro-2-methyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042611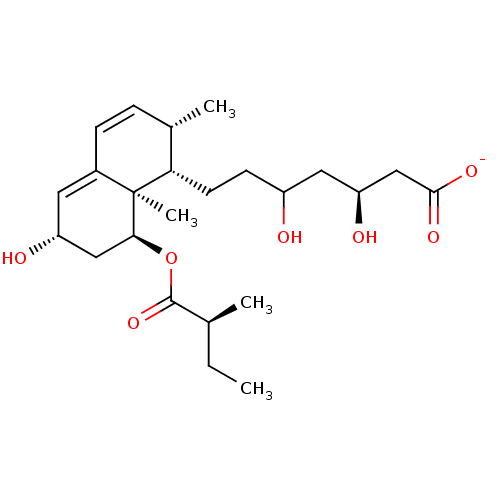 (CHEMBL333003 | Sodium; 3,5-dihydroxy-7-[6-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042616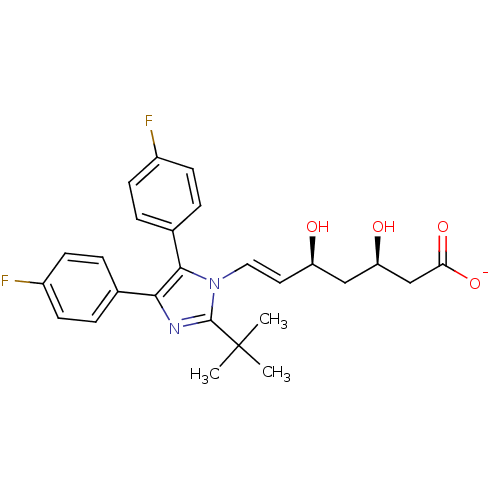 ((E)-(3R,5S)-7-[2-tert-Butyl-4,5-bis-(4-fluoro-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042643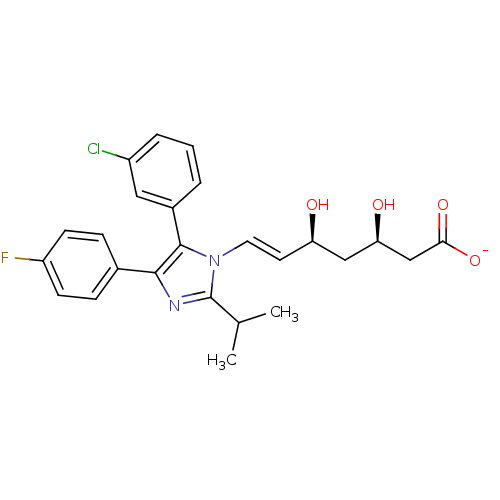 ((E)-(3R,5S)-7-[5-(3-Chloro-phenyl)-4-(4-fluoro-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042634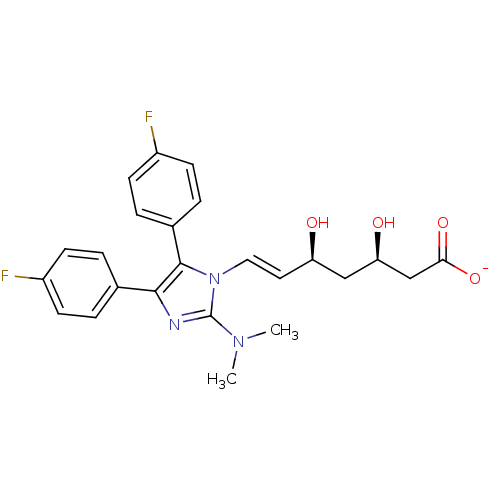 ((E)-(3R,5S)-7-[2-Dimethylamino-4,5-bis-(4-fluoro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042628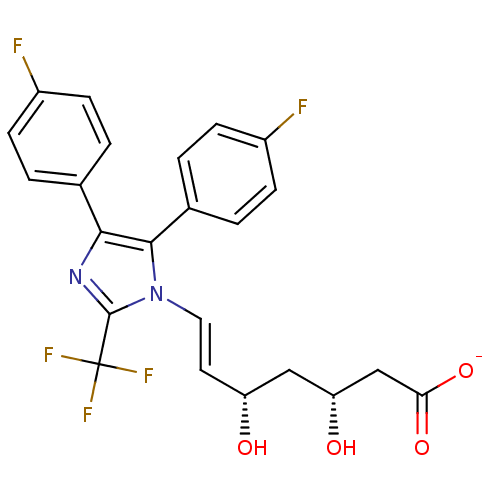 ((E)-(3R,5S)-7-[4,5-Bis-(4-fluoro-phenyl)-2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042640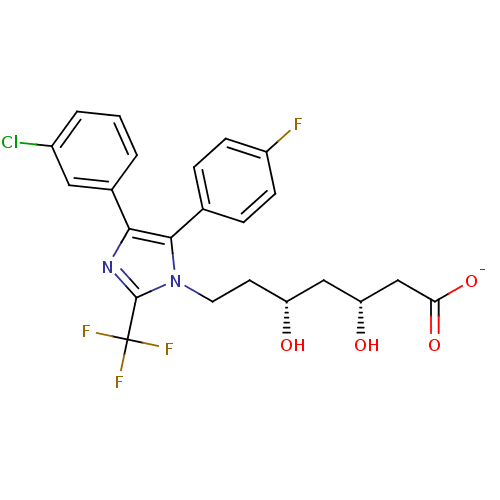 (CHEMBL332580 | Sodium; 7-[4-(3-chloro-phenyl)-5-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042608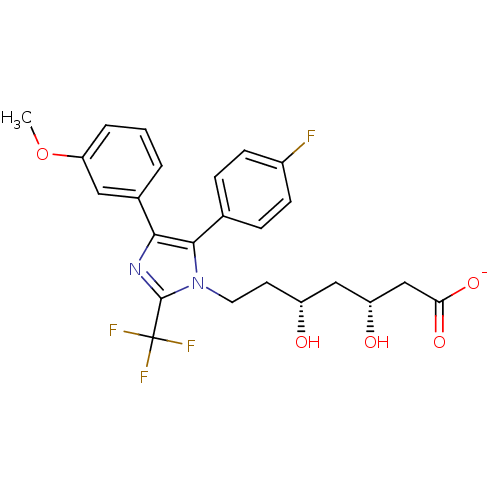 (CHEMBL431842 | Sodium; 7-[5-(4-fluoro-phenyl)-4-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042603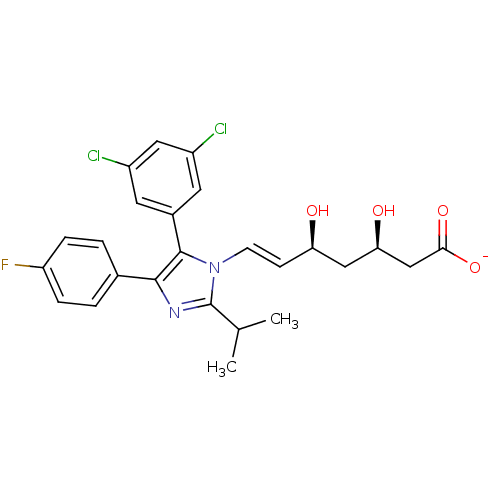 ((E)-(3R,5S)-7-[5-(3,5-Dichloro-phenyl)-4-(4-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042641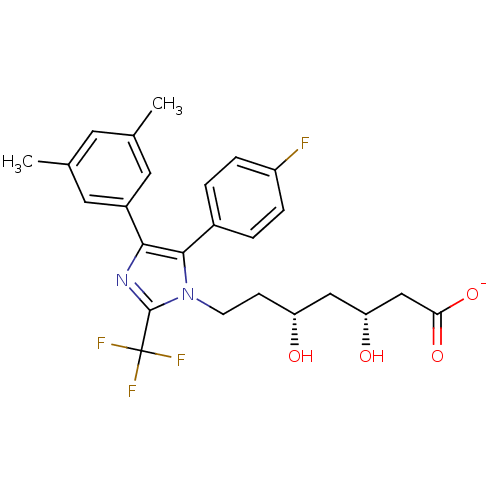 (CHEMBL333479 | Sodium; 7-[4-(3,5-dimethyl-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042604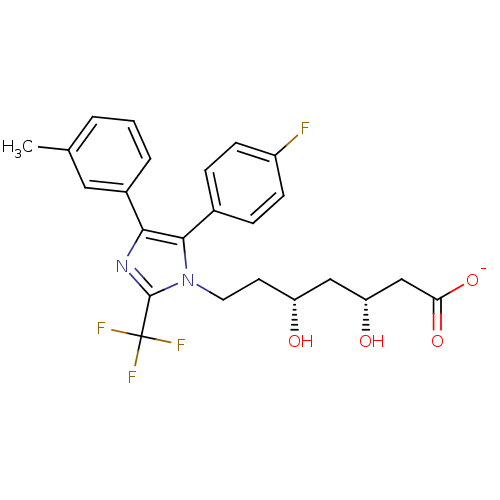 (CHEMBL120860 | Sodium; 7-[5-(4-fluoro-phenyl)-4-m-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042624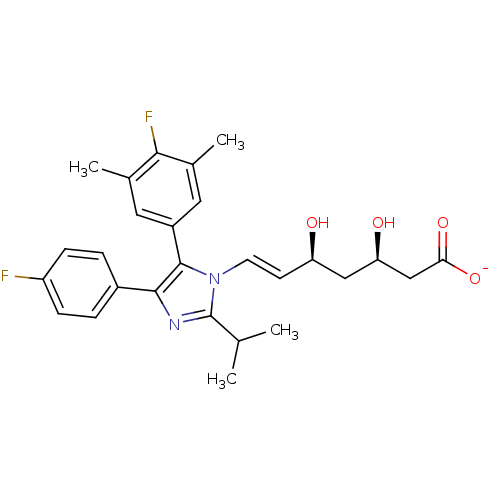 ((E)-(3R,5S)-7-[5-(4-Fluoro-3,5-dimethyl-phenyl)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042644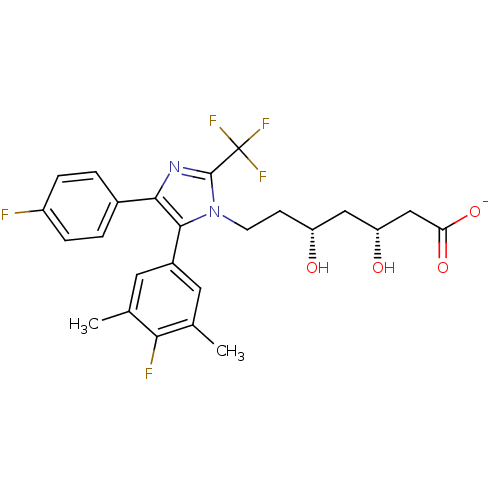 (CHEMBL333717 | Sodium; 7-[5-(4-fluoro-3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042618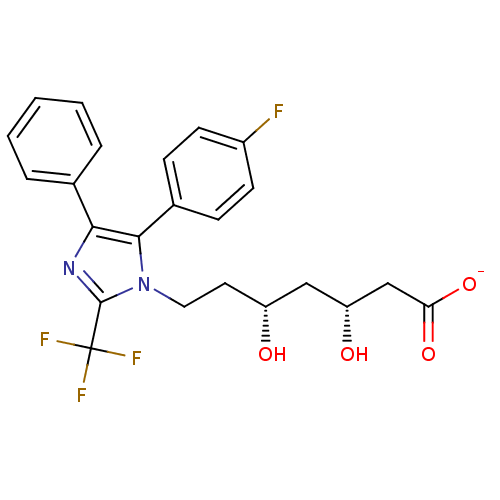 (CHEMBL121122 | Sodium; 7-[5-(4-fluoro-phenyl)-4-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042610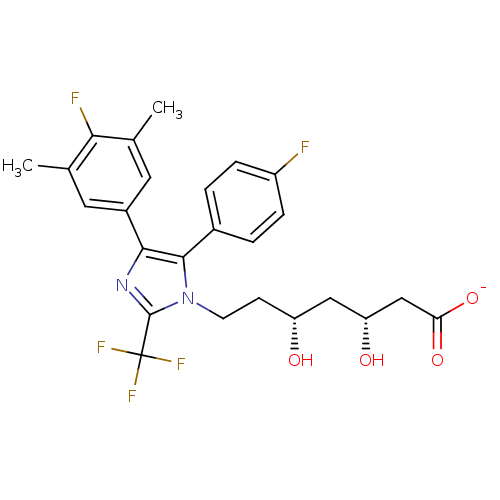 (CHEMBL332202 | Sodium; 7-[4-(4-fluoro-3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042605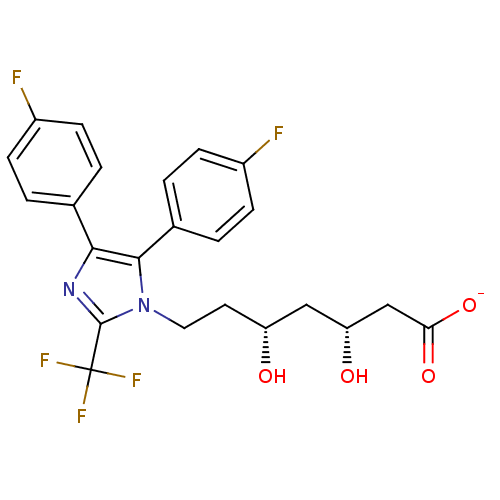 (CHEMBL120908 | Sodium; 7-[4,5-bis-(4-fluoro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042636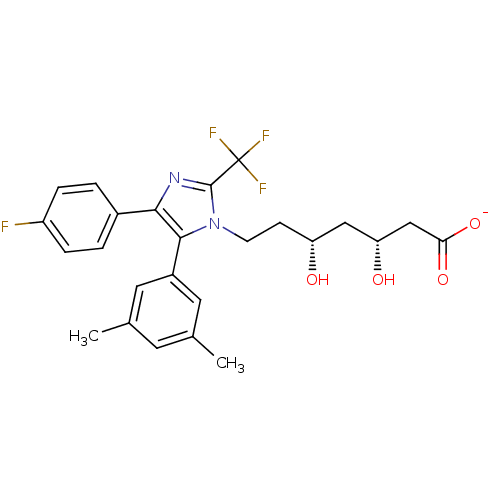 (CHEMBL121230 | Sodium; 7-[5-(3,5-dimethyl-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042621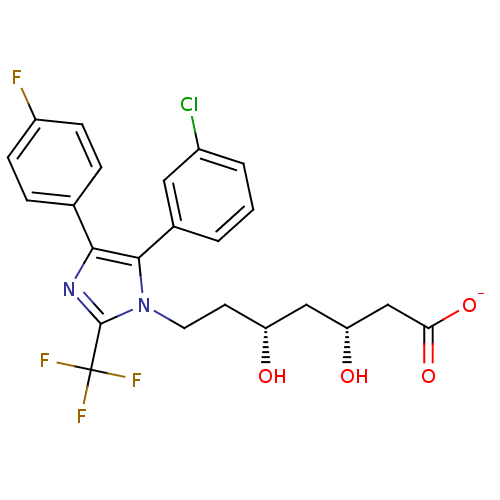 (CHEMBL123253 | Sodium; 7-[5-(3-chloro-phenyl)-4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042635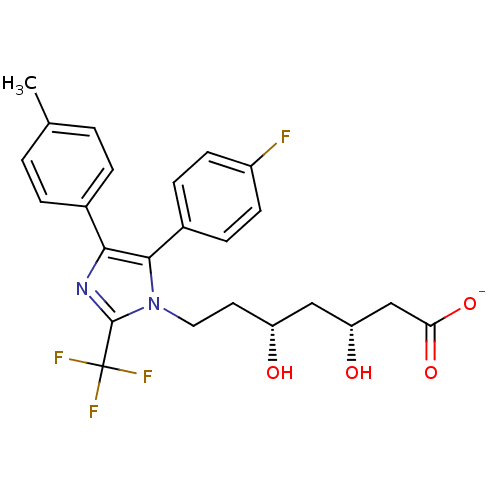 (CHEMBL123354 | Sodium; 7-[5-(4-fluoro-phenyl)-4-p-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3383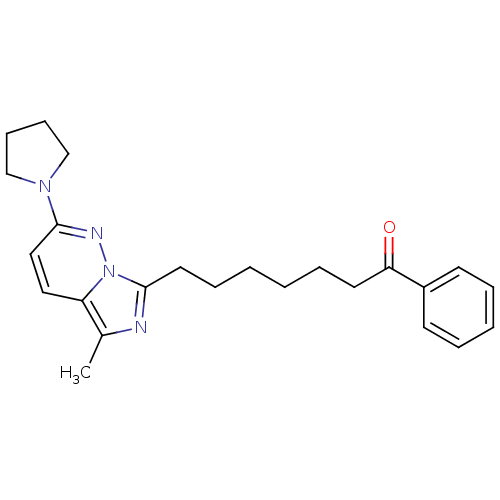 (7-[5-Methyl-2-(1-pyrrolidinyl)imidazo[1,5-b]pyrida...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042638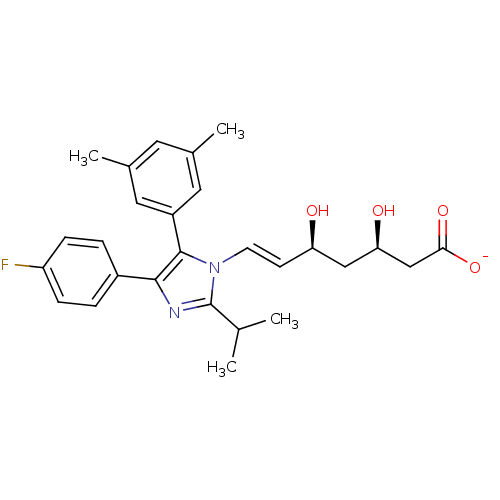 ((E)-(3R,5S)-7-[5-(3,5-Dimethyl-phenyl)-4-(4-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042617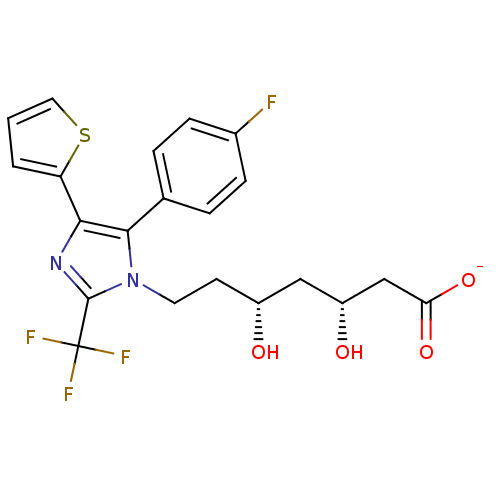 (CHEMBL330995 | Sodium; 7-[5-(4-fluoro-phenyl)-4-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042619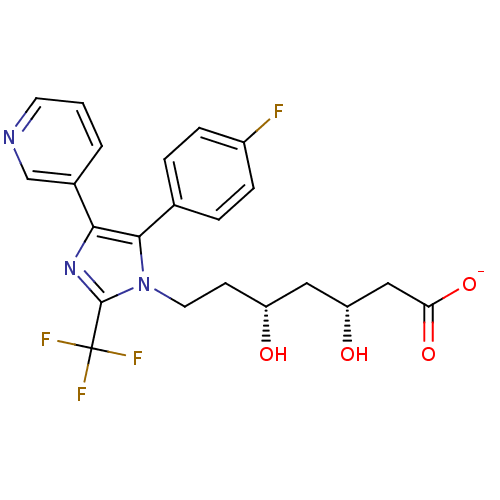 (CHEMBL420899 | Sodium; 7-[5-(4-fluoro-phenyl)-4-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042602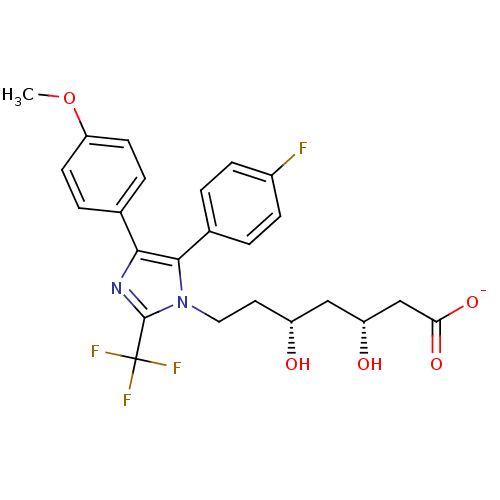 (CHEMBL120869 | Sodium; 7-[5-(4-fluoro-phenyl)-4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042630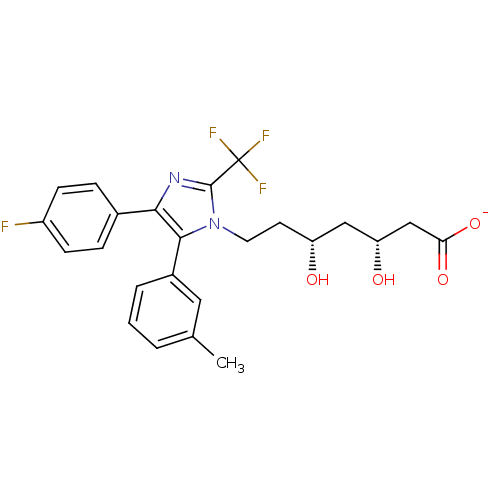 (CHEMBL123487 | Sodium; 7-[4-(4-fluoro-phenyl)-5-m-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3385 (7-[5-methyl-2-(1H-pyrrol-1-yl)imidazo[1,5-a]pyrida...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3379 (7-[5-Methyl-2-(methylthio)imidazo[1,5-b]pyridazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042626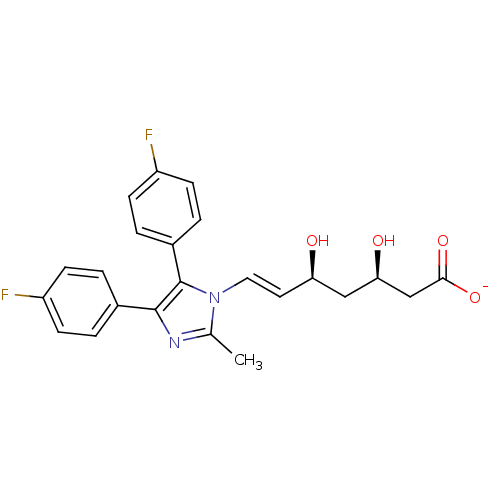 (CHEMBL434224 | Sodium; 7-[4,5-bis-(4-fluoro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3388 (7-[5-methyl-2-(1H-1,2,4-triazol-1-yl)imidazo[1,5-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3392 (7-[5-methyl-2-(5-methyl-1H-imidazol-1-yl)imidazo[1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042633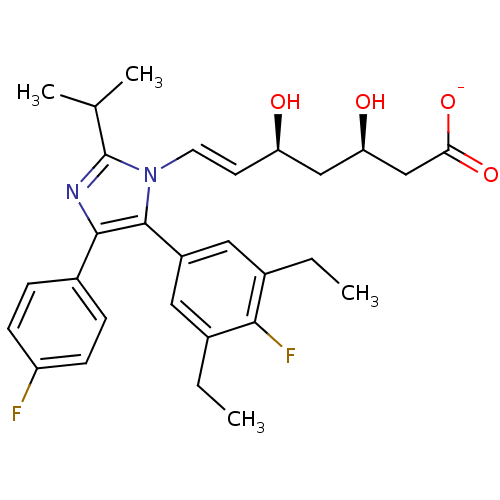 ((E)-(3R,5S)-7-[5-(3,5-Diethyl-4-fluoro-phenyl)-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042632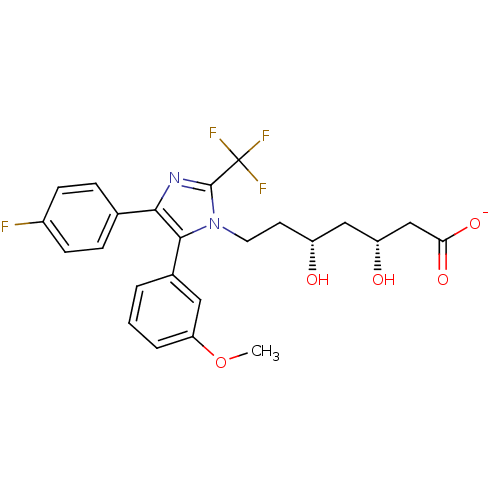 (CHEMBL332125 | Sodium; 7-[4-(4-fluoro-phenyl)-5-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042642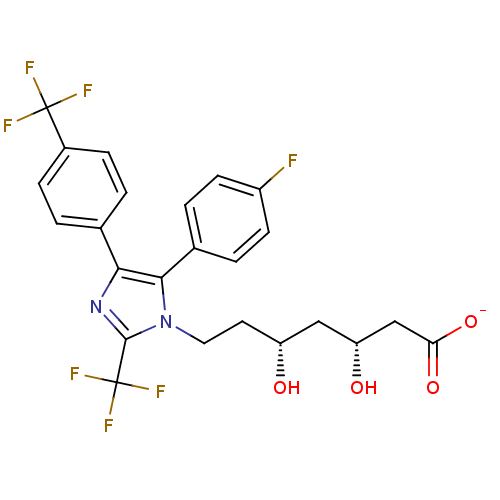 (CHEMBL332244 | Sodium; 7-[5-(4-fluoro-phenyl)-2-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042609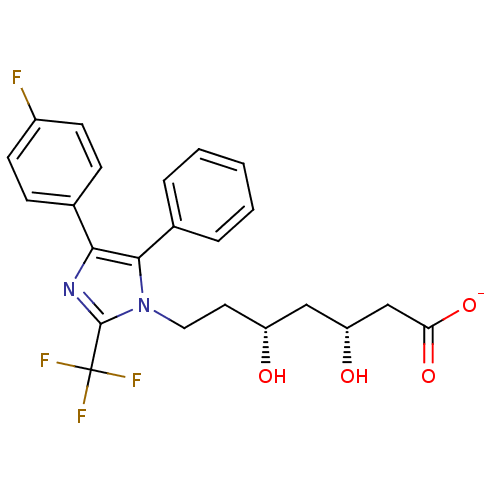 (CHEMBL331923 | Sodium; 7-[4-(4-fluoro-phenyl)-5-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042623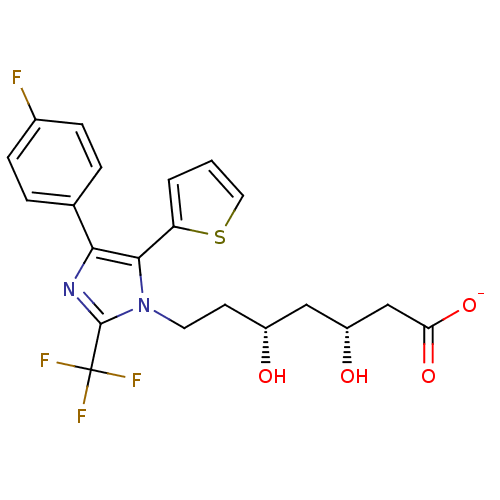 (CHEMBL121052 | Sodium; 7-[4-(4-fluoro-phenyl)-5-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3395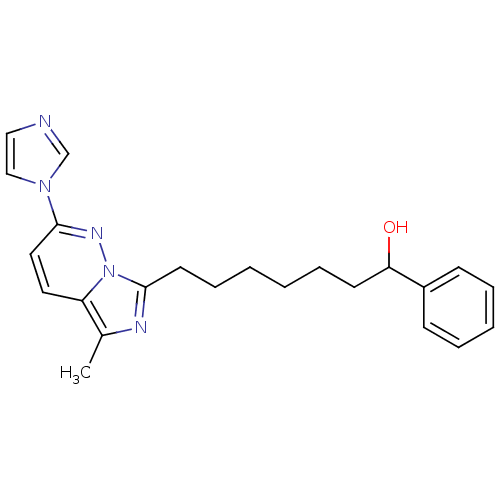 (7-[2-(1H-imidazol-1-yl)-5-methylimidazo[1,5-a]pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3382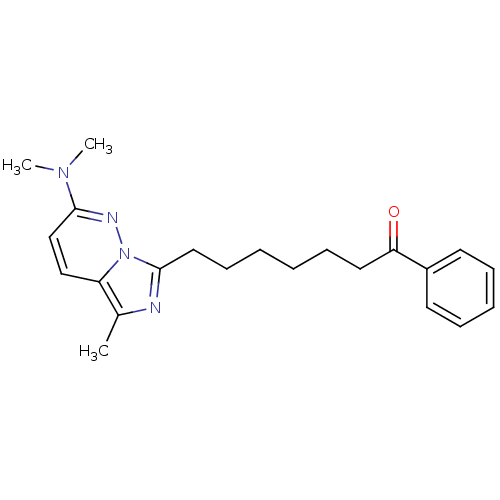 (7-[2-(Dimethylamino)-5-methylimidazo[1,5-b]pyridaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042639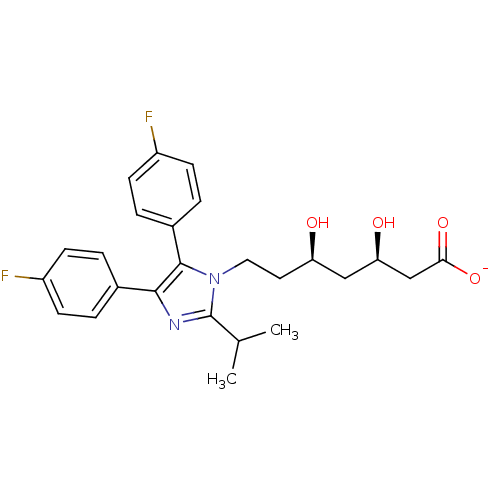 (CHEMBL331675 | Sodium; 7-[4,5-bis-(4-fluoro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3374 (7-{2-chloroimidazo[1,5-a]pyridazin-7-yl}-1-phenylh...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3373 (( 1RS)-2-Chloro-5-methyl-alpha-phenyl-7-imidazo[1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |