

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23232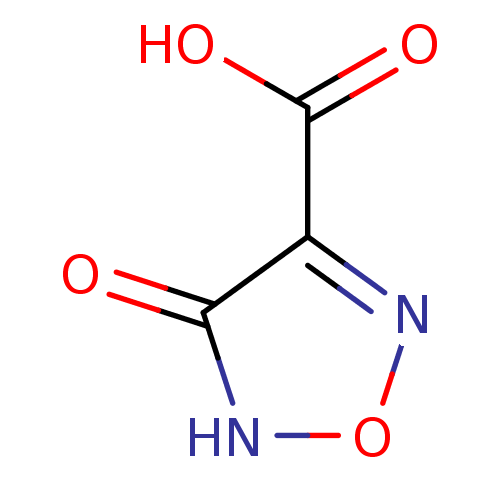 (1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 210 | -38.1 | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23251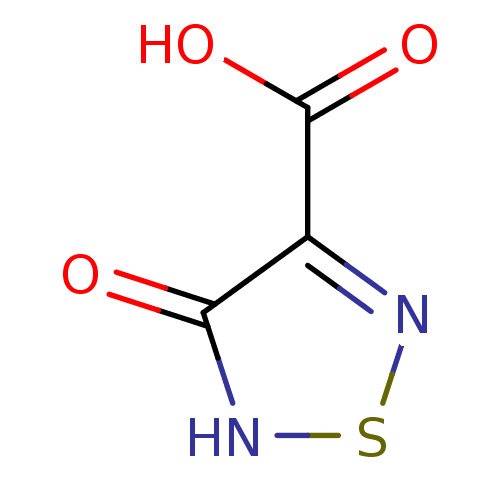 (1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 290 | -37.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23242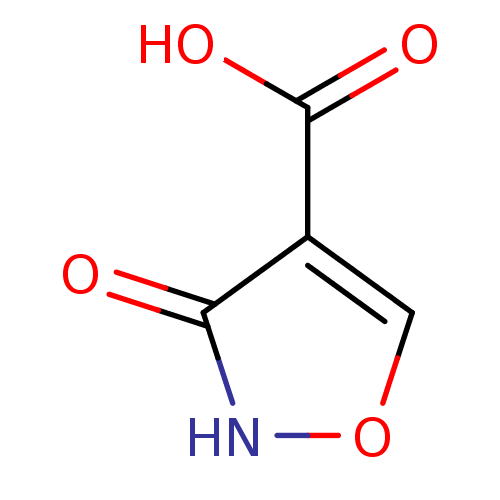 (1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 470 | -36.1 | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23251 (1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23243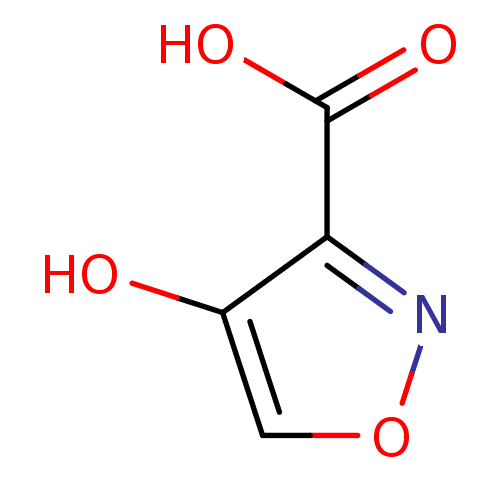 (1,2(1,5)-Isoxazole, IOA2 | 4-hydroxy-1,2-oxazole-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional enhancer factor TEF-4 (Homo sapiens) | BDBM50531838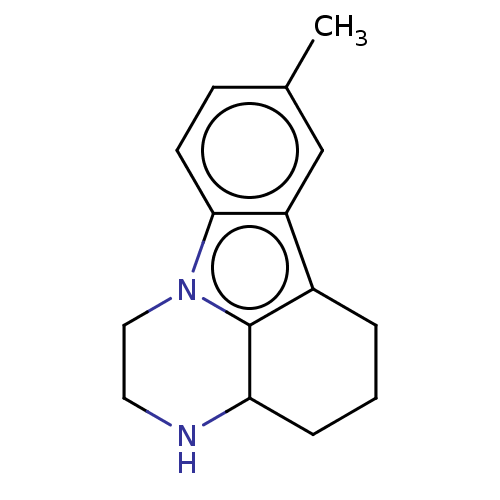 (Pirazidol | Pirlindole) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Inhibition of Gal4-fused TEAD2 (unknown origin) interaction with YAP expressed in human HeLa cells assessed as basal transcriptional activity level a... | J Med Chem 62: 1291-1305 (2019) Article DOI: 10.1021/acs.jmedchem.8b01402 BindingDB Entry DOI: 10.7270/Q2DV1PB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional enhancer factor TEF-3 (Homo sapiens) | BDBM50531838 (Pirazidol | Pirlindole) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Inhibition of Gal4-fused TEAD4 (unknown origin) interaction with YAP expressed in human HeLa cells assessed as basal transcriptional activity level a... | J Med Chem 62: 1291-1305 (2019) Article DOI: 10.1021/acs.jmedchem.8b01402 BindingDB Entry DOI: 10.7270/Q2DV1PB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional enhancer factor TEF-1 (Homo sapiens) | BDBM50531838 (Pirazidol | Pirlindole) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Inhibition of Gal4-fused TEAD1 (unknown origin) interaction with YAP expressed in human HeLa cells assessed as basal transcriptional activity level a... | J Med Chem 62: 1291-1305 (2019) Article DOI: 10.1021/acs.jmedchem.8b01402 BindingDB Entry DOI: 10.7270/Q2DV1PB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional enhancer factor TEF-5 (Homo sapiens) | BDBM50531838 (Pirazidol | Pirlindole) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Inhibition of Gal4-fused TEAD3 (unknown origin) interaction with YAP expressed in human HeLa cells assessed as basal transcriptional activity level a... | J Med Chem 62: 1291-1305 (2019) Article DOI: 10.1021/acs.jmedchem.8b01402 BindingDB Entry DOI: 10.7270/Q2DV1PB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional enhancer factor TEF-1 (Homo sapiens) | BDBM50531837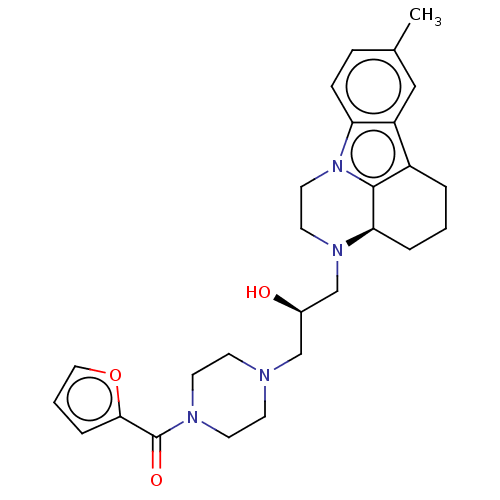 (CHEMBL4578591) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Inhibition of NL-tagged YAP (unknown origin) interaction with myc-tagged human TEAD1 preincubated for 1 hr followed by NL-tagged YAP addition by Nano... | J Med Chem 62: 1291-1305 (2019) Article DOI: 10.1021/acs.jmedchem.8b01402 BindingDB Entry DOI: 10.7270/Q2DV1PB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23242 (1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50020712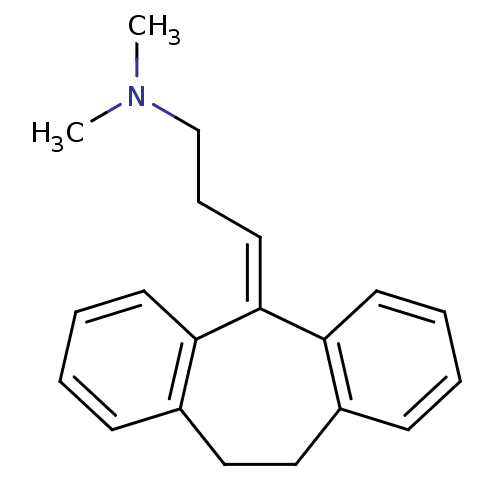 (10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southmead Hospital Curated by ChEMBL | Assay Description Displacement of [125I]NGF from full length human TrkA expressed in HEKN3S cells cells by gamma counting based radioligand competition assay | J Med Chem 58: 767-77 (2015) Article DOI: 10.1021/jm501307e BindingDB Entry DOI: 10.7270/Q25B045H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23232 (1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 7.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23250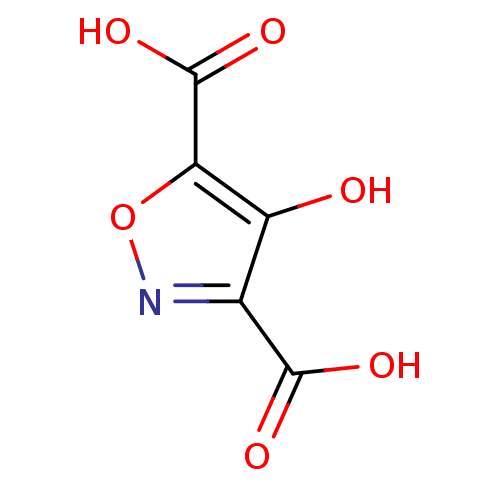 (1,2(1,5)-Isoxazole, IOA9 | 4-hydroxy-1,2-oxazole-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23258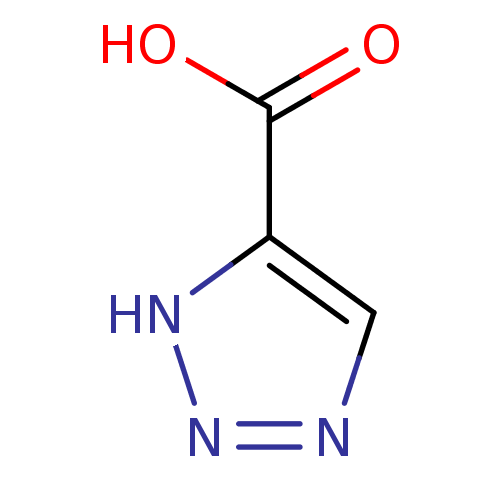 (1H-1,2,3-triazole-5-carboxylic acid | Triazole, TR...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23256 (1-benzyl-4-hydroxy-1H-1,2,3-triazole-5-carboxylic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23257 (2-benzyl-5-hydroxy-2H-1,2,3-triazole-4-carboxylic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23244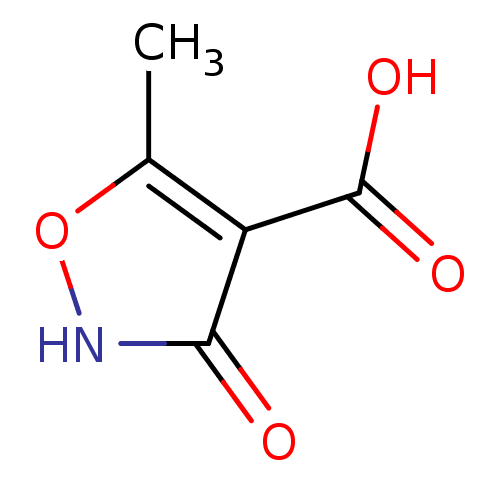 (1,2(1,5)-Isoxazole, IOA3 | 3-hydroxy-5-methyl-1,2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23239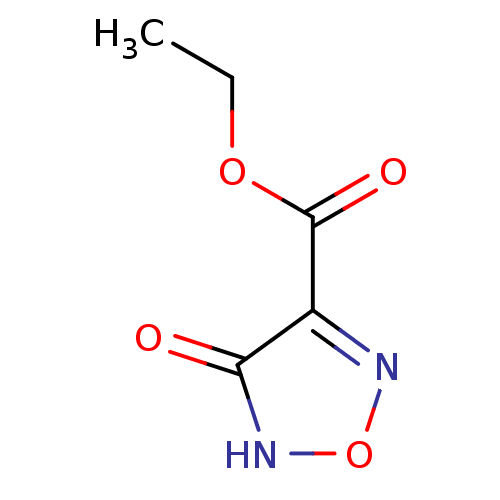 (1,2,5-oxadiazole, OXD7 | ethyl 4-hydroxy-1,2,5-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23245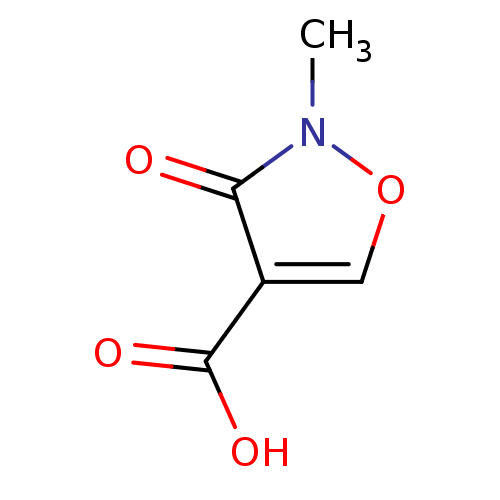 (1,2(1,5)-Isoxazole, IOA4 | 2-methyl-3-oxo-2,3-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23246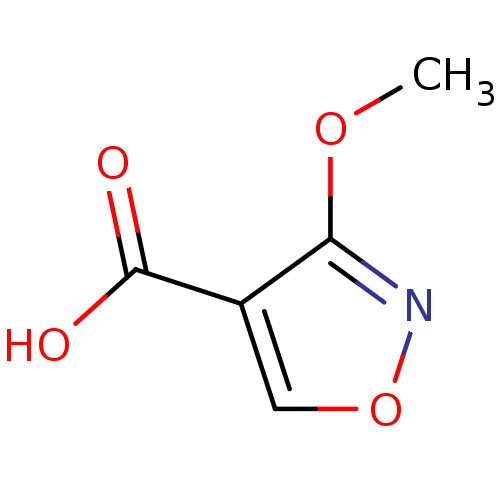 (1,2(1,5)-Isoxazole, IOA5 | 3-methoxy-1,2-oxazole-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23243 (1,2(1,5)-Isoxazole, IOA2 | 4-hydroxy-1,2-oxazole-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23244 (1,2(1,5)-Isoxazole, IOA3 | 3-hydroxy-5-methyl-1,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23245 (1,2(1,5)-Isoxazole, IOA4 | 2-methyl-3-oxo-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23246 (1,2(1,5)-Isoxazole, IOA5 | 3-methoxy-1,2-oxazole-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23247 (1,2(1,5)-Isoxazole, IOA6 | ethyl 3-hydroxy-1,2-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23255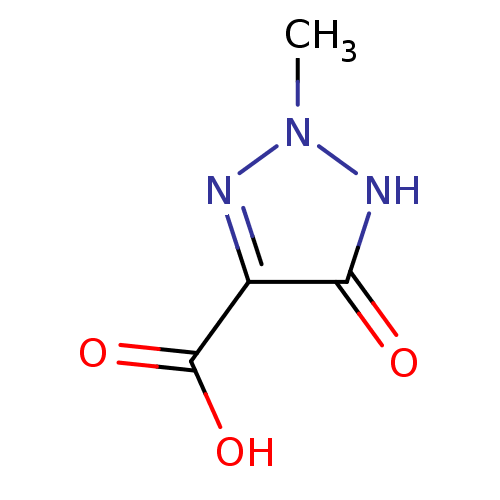 (5-hydroxy-2-methyl-2H-1,2,3-triazole-4-carboxylic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23254 (4-hydroxy-1-methyl-1H-1,2,3-triazole-5-carboxylic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23248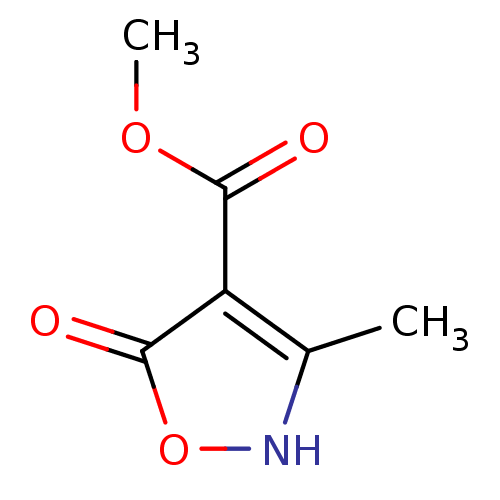 (1,2(1,5)-Isoxazole, IOA7 | methyl 5-hydroxy-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23252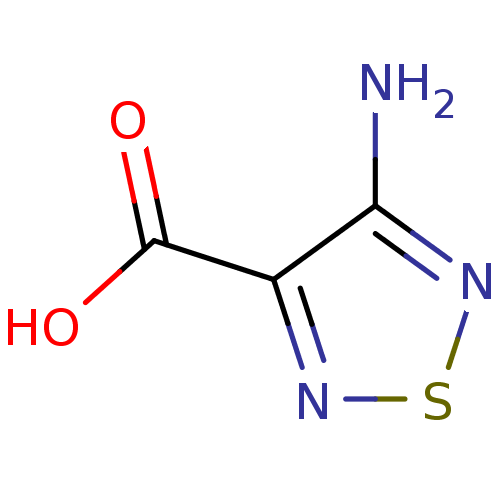 (1,2,5-Thiadiazole, TDA2 | 4-amino-1,2,5-thiadiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23249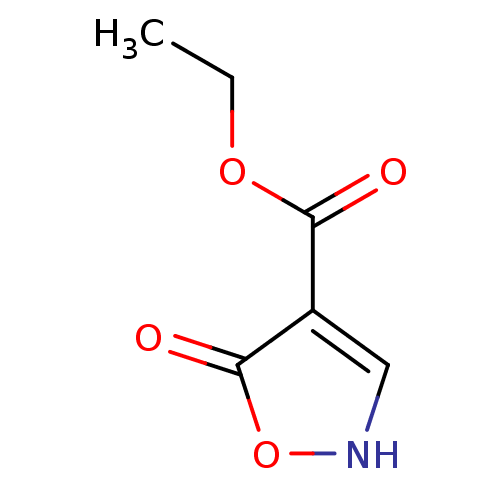 (1,2(1,5)-Isoxazole, IOA8 | ethyl 5-hydroxy-1,2-oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23248 (1,2(1,5)-Isoxazole, IOA7 | methyl 5-hydroxy-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23249 (1,2(1,5)-Isoxazole, IOA8 | ethyl 5-hydroxy-1,2-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23250 (1,2(1,5)-Isoxazole, IOA9 | 4-hydroxy-1,2-oxazole-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23252 (1,2,5-Thiadiazole, TDA2 | 4-amino-1,2,5-thiadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23253 (1,2,5-Thiadiazole, TDA3 | 4-methoxy-1,2,5-thiadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23254 (4-hydroxy-1-methyl-1H-1,2,3-triazole-5-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23255 (5-hydroxy-2-methyl-2H-1,2,3-triazole-4-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23256 (1-benzyl-4-hydroxy-1H-1,2,3-triazole-5-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23257 (2-benzyl-5-hydroxy-2H-1,2,3-triazole-4-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23247 (1,2(1,5)-Isoxazole, IOA6 | ethyl 3-hydroxy-1,2-oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23253 (1,2,5-Thiadiazole, TDA3 | 4-methoxy-1,2,5-thiadiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23258 (1H-1,2,3-triazole-5-carboxylic acid | Triazole, TR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23241 (1,2,5-oxadiazole, OXD9 | ethyl 4-[(4-methoxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23240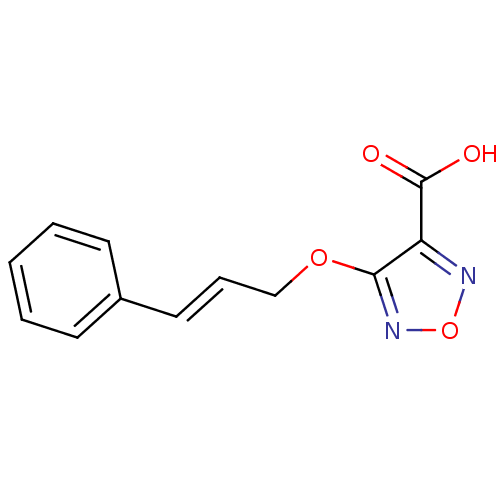 (1,2,5-oxadiazole, OXD8 | 4-{[(2E)-3-phenylprop-2-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23238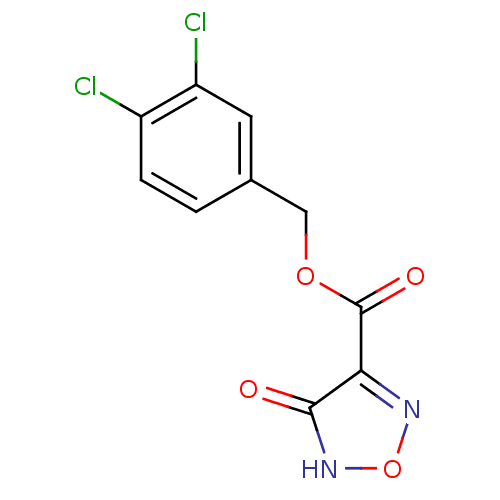 ((3,4-dichlorophenyl)methyl 4-hydroxy-1,2,5-oxadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23237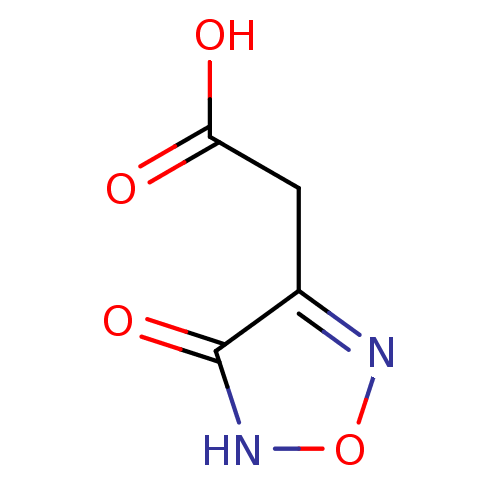 (1,2,5-oxadiazole, OXD5 | 2-(4-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23236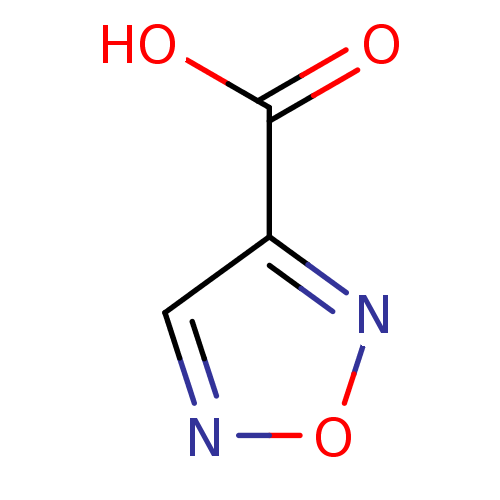 (1,2,5-oxadiazole, OXD4 | 1,2,5-oxadiazole-3-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23235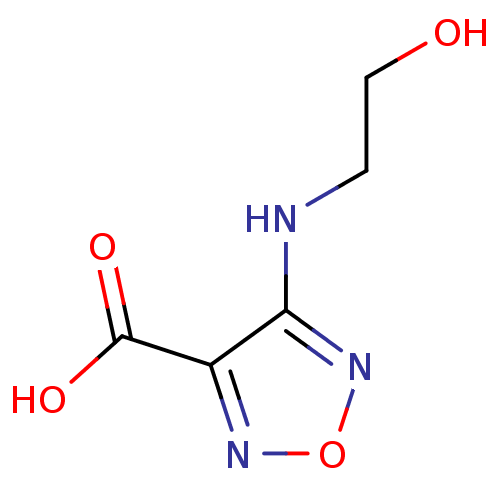 (1,2,5-oxadiazole, OXD3 | 4-[(2-hydroxyethyl)amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM23234 (1,2,5-oxadiazole, OXD2 | 4-amino-1,2,5-oxadiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |