 Found 6182 hits with Last Name = 'sparks' and Initial = 'rb'
Found 6182 hits with Last Name = 'sparks' and Initial = 'rb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13467
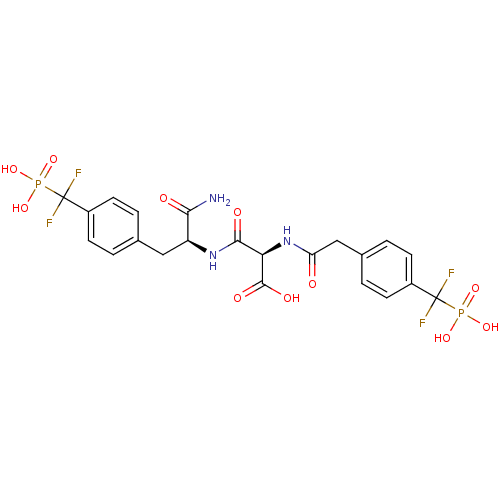
((2R)-2-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@@H](NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C22H23F4N3O11P2/c23-21(24,41(35,36)37)13-5-1-11(2-6-13)9-15(18(27)31)28-19(32)17(20(33)34)29-16(30)10-12-3-7-14(8-4-12)22(25,26)42(38,39)40/h1-8,15,17H,9-10H2,(H2,27,31)(H,28,32)(H,29,30)(H,33,34)(H2,35,36,37)(H2,38,39,40)/t15-,17+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 38013-21 (2006)
Article DOI: 10.1074/jbc.M607913200
BindingDB Entry DOI: 10.7270/Q2JW8C4X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529386
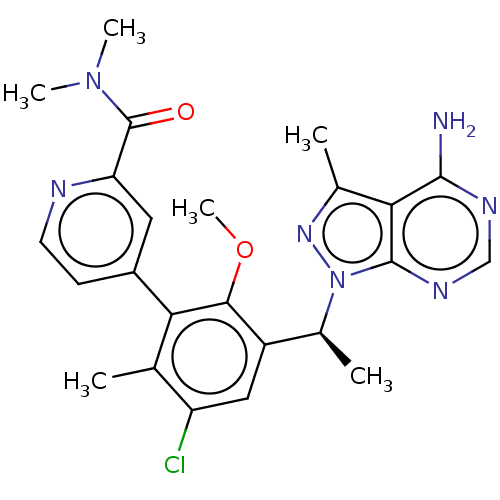
(CHEMBL4458634)Show SMILES COc1c(cc(Cl)c(C)c1-c1ccnc(c1)C(=O)N(C)C)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H26ClN7O2/c1-12-17(25)10-16(14(3)32-23-20(13(2)30-32)22(26)28-11-29-23)21(34-6)19(12)15-7-8-27-18(9-15)24(33)31(4)5/h7-11,14H,1-6H3,(H2,26,28,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529384
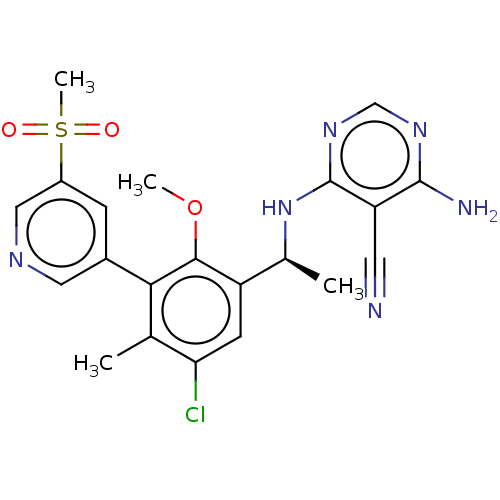
(CHEMBL4438376)Show SMILES COc1c(cc(Cl)c(C)c1-c1cncc(c1)S(C)(=O)=O)[C@H](C)Nc1ncnc(N)c1C#N |r| Show InChI InChI=1S/C21H21ClN6O3S/c1-11-17(22)6-15(12(2)28-21-16(7-23)20(24)26-10-27-21)19(31-3)18(11)13-5-14(9-25-8-13)32(4,29)30/h5-6,8-10,12H,1-4H3,(H3,24,26,27,28)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261330
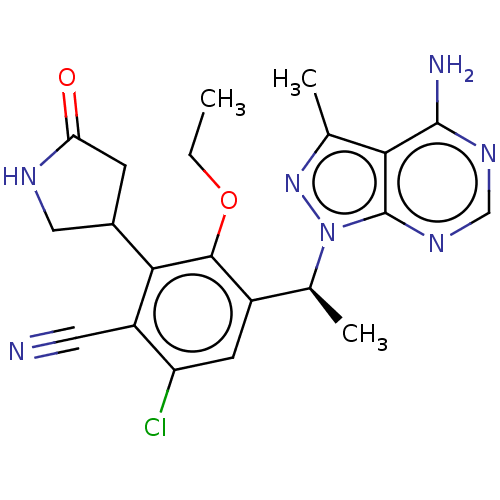
(US10092570, Example 352 | US9707233, 350)Show SMILES CCOc1c(cc(Cl)c(C#N)c1C1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C21H22ClN7O2/c1-4-31-19-13(6-15(22)14(7-23)18(19)12-5-16(30)25-8-12)11(3)29-21-17(10(2)28-29)20(24)26-9-27-21/h6,9,11-12H,4-5,8H2,1-3H3,(H,25,30)(H2,24,26,27)/t11-,12?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM272573
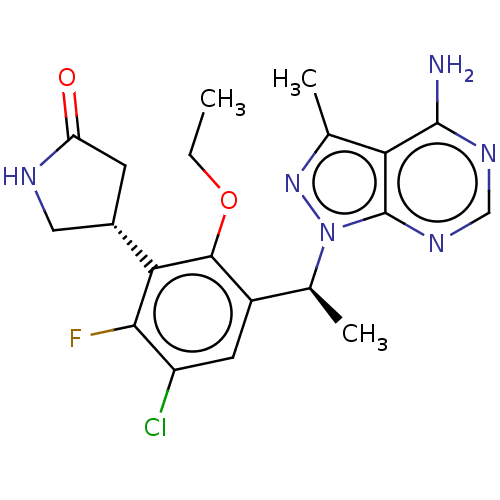
(US10065963, 32c | US10376513, Example 346 | US1064...)Show SMILES CCOc1c(cc(Cl)c(F)c1[C@@H]1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22ClFN6O2/c1-4-30-18-12(6-13(21)17(22)16(18)11-5-14(29)24-7-11)10(3)28-20-15(9(2)27-28)19(23)25-8-26-20/h6,8,10-11H,4-5,7H2,1-3H3,(H,24,29)(H2,23,25,26)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM272573

(US10065963, 32c | US10376513, Example 346 | US1064...)Show SMILES CCOc1c(cc(Cl)c(F)c1[C@@H]1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22ClFN6O2/c1-4-30-18-12(6-13(21)17(22)16(18)11-5-14(29)24-7-11)10(3)28-20-15(9(2)27-28)19(23)25-8-26-20/h6,8,10-11H,4-5,7H2,1-3H3,(H,24,29)(H2,23,25,26)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human Ramos cells assessed as reduction in AKT phosphorylation incubated for 2 hrs by Alexa flour 488 based FACS analysis |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM272573

(US10065963, 32c | US10376513, Example 346 | US1064...)Show SMILES CCOc1c(cc(Cl)c(F)c1[C@@H]1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22ClFN6O2/c1-4-30-18-12(6-13(21)17(22)16(18)11-5-14(29)24-7-11)10(3)28-20-15(9(2)27-28)19(23)25-8-26-20/h6,8,10-11H,4-5,7H2,1-3H3,(H,24,29)(H2,23,25,26)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]-ATP base... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261317
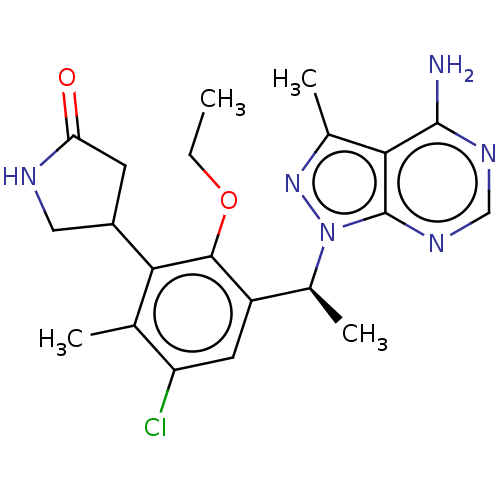
(US10376513, Example 310 | US11433071, Example 311 ...)Show SMILES CCOc1c(cc(Cl)c(C)c1C1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C21H25ClN6O2/c1-5-30-19-14(7-15(22)10(2)17(19)13-6-16(29)24-8-13)12(4)28-21-18(11(3)27-28)20(23)25-9-26-21/h7,9,12-13H,5-6,8H2,1-4H3,(H,24,29)(H2,23,25,26)/t12-,13?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13469
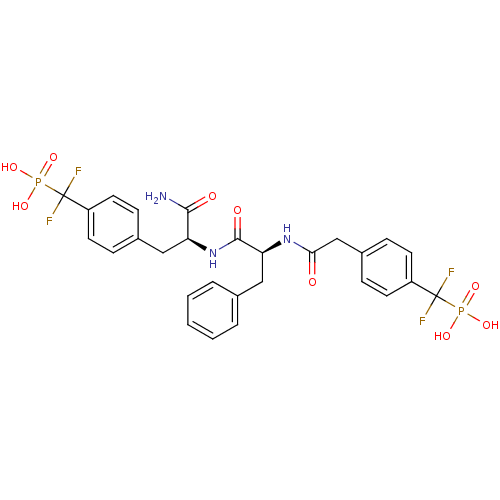
(({4-[(2S)-2-carbamoyl-2-[(2S)-2-(1-{4-[difluoro(ph...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C28H29F4N3O9P2/c29-27(30,45(39,40)41)20-10-6-18(7-11-20)14-22(25(33)37)35-26(38)23(15-17-4-2-1-3-5-17)34-24(36)16-19-8-12-21(13-9-19)28(31,32)46(42,43)44/h1-13,22-23H,14-16H2,(H2,33,37)(H,34,36)(H,35,38)(H2,39,40,41)(H2,42,43,44)/t22-,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 38013-21 (2006)
Article DOI: 10.1074/jbc.M607913200
BindingDB Entry DOI: 10.7270/Q2JW8C4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529381
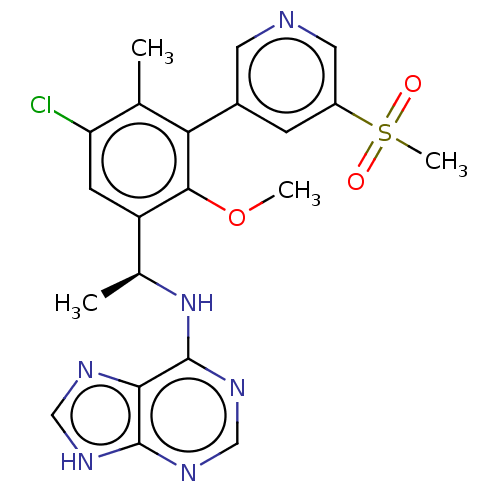
(CHEMBL4446120)Show SMILES COc1c(cc(Cl)c(C)c1-c1cncc(c1)S(C)(=O)=O)[C@H](C)Nc1ncnc2[nH]cnc12 |r| Show InChI InChI=1S/C21H21ClN6O3S/c1-11-16(22)6-15(12(2)28-21-18-20(25-9-24-18)26-10-27-21)19(31-3)17(11)13-5-14(8-23-7-13)32(4,29)30/h5-10,12H,1-4H3,(H2,24,25,26,27,28)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529385
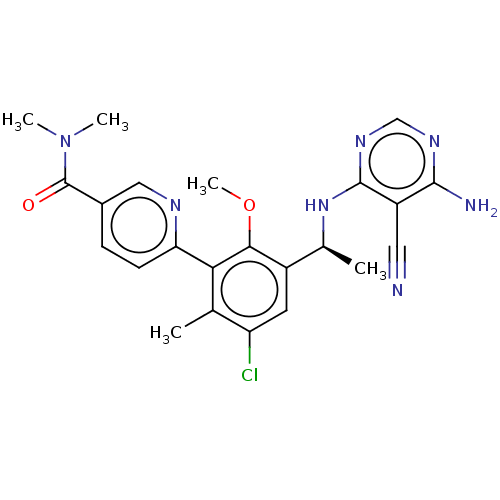
(CHEMBL4444112)Show SMILES COc1c(cc(Cl)c(C)c1-c1ccc(cn1)C(=O)N(C)C)[C@H](C)Nc1ncnc(N)c1C#N |r| Show InChI InChI=1S/C23H24ClN7O2/c1-12-17(24)8-15(13(2)30-22-16(9-25)21(26)28-11-29-22)20(33-5)19(12)18-7-6-14(10-27-18)23(32)31(3)4/h6-8,10-11,13H,1-5H3,(H3,26,28,29,30)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM125902
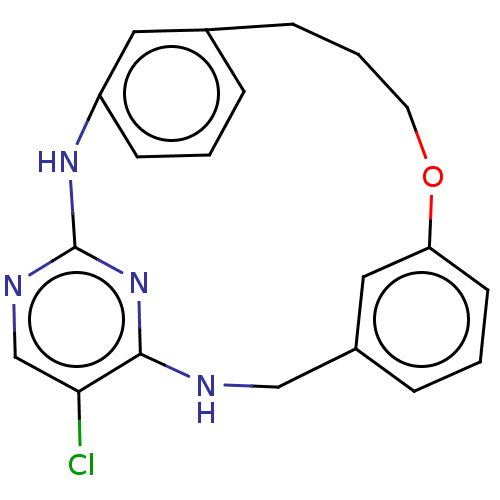
(US8765727, 5)Show InChI InChI=1S/C20H19ClN4O/c21-18-13-23-20-24-16-7-1-4-14(10-16)6-3-9-26-17-8-2-5-15(11-17)12-22-19(18)25-20/h1-2,4-5,7-8,10-11,13H,3,6,9,12H2,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM384664
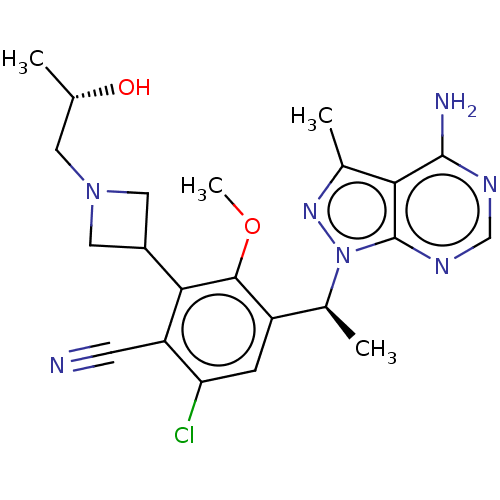
(4-((5)-1-(4-amino-3-methyl-1H-pyrazolo[3,4-d]pyrim...)Show SMILES COc1c(cc(Cl)c(C#N)c1C1CN(C[C@H](C)O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C22H26ClN7O2/c1-11(31)7-29-8-14(9-29)19-16(6-24)17(23)5-15(20(19)32-4)13(3)30-22-18(12(2)28-30)21(25)26-10-27-22/h5,10-11,13-14,31H,7-9H2,1-4H3,(H2,25,26,27)/t11-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM272573

(US10065963, 32c | US10376513, Example 346 | US1064...)Show SMILES CCOc1c(cc(Cl)c(F)c1[C@@H]1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22ClFN6O2/c1-4-30-18-12(6-13(21)17(22)16(18)11-5-14(29)24-7-11)10(3)28-20-15(9(2)27-28)19(23)25-8-26-20/h6,8,10-11H,4-5,7H2,1-3H3,(H,24,29)(H2,23,25,26)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human whole blood assessed as reduction in anti-IgE antibody-induced CD63 expression by flow cast kit method |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM125902

(US8765727, 5)Show InChI InChI=1S/C20H19ClN4O/c21-18-13-23-20-24-16-7-1-4-14(10-16)6-3-9-26-17-8-2-5-15(11-17)12-22-19(18)25-20/h1-2,4-5,7-8,10-11,13H,3,6,9,12H2,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529388
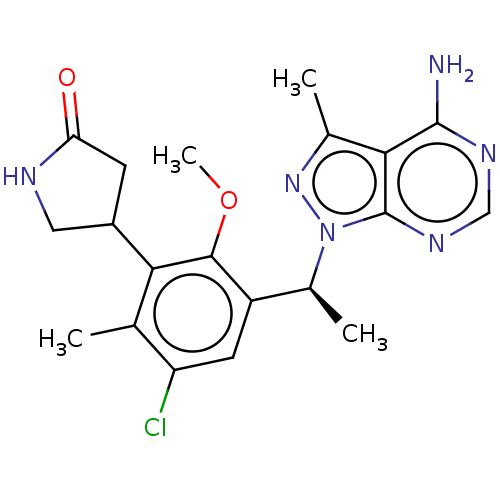
(CHEMBL4443149)Show SMILES COc1c(cc(Cl)c(C)c1C1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C20H23ClN6O2/c1-9-14(21)6-13(18(29-4)16(9)12-5-15(28)23-7-12)11(3)27-20-17(10(2)26-27)19(22)24-8-25-20/h6,8,11-12H,5,7H2,1-4H3,(H,23,28)(H2,22,24,25)/t11-,12?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529391

(CHEMBL4513857)Show SMILES COc1c(cc(Cl)c(C)c1-c1cnn(C)c1)[C@H](C)Nc1ncnc2[nH]cnc12 |r| Show InChI InChI=1S/C19H20ClN7O/c1-10-14(20)5-13(17(28-4)15(10)12-6-25-27(3)7-12)11(2)26-19-16-18(22-8-21-16)23-9-24-19/h5-9,11H,1-4H3,(H2,21,22,23,24,26)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50004547

(CHEMBL2216863 | US10065963, Compound 28 | US104280...)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2scc(C)n2c(=O)c1-c1cccc(F)c1 |r| Show InChI InChI=1S/C20H16FN7OS/c1-10-7-30-20-27-15(11(2)26-18-16-17(23-8-22-16)24-9-25-18)14(19(29)28(10)20)12-4-3-5-13(21)6-12/h3-9,11H,1-2H3,(H2,22,23,24,25,26)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529390
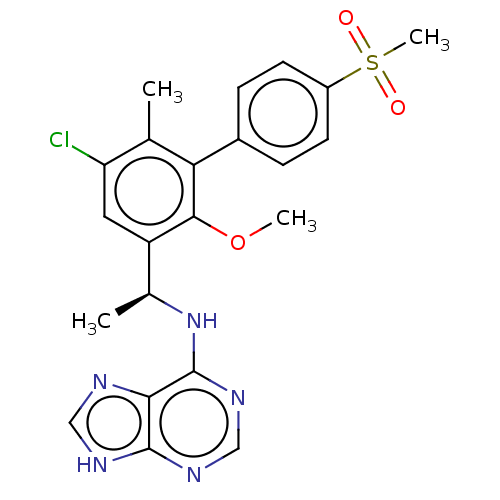
(CHEMBL4470672)Show SMILES COc1c(cc(Cl)c(C)c1-c1ccc(cc1)S(C)(=O)=O)[C@H](C)Nc1ncnc2[nH]cnc12 |r| Show InChI InChI=1S/C22H22ClN5O3S/c1-12-17(23)9-16(13(2)28-22-19-21(25-10-24-19)26-11-27-22)20(31-3)18(12)14-5-7-15(8-6-14)32(4,29)30/h5-11,13H,1-4H3,(H2,24,25,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM125902

(US8765727, 5)Show InChI InChI=1S/C20H19ClN4O/c21-18-13-23-20-24-16-7-1-4-14(10-16)6-3-9-26-17-8-2-5-15(11-17)12-22-19(18)25-20/h1-2,4-5,7-8,10-11,13H,3,6,9,12H2,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM125913
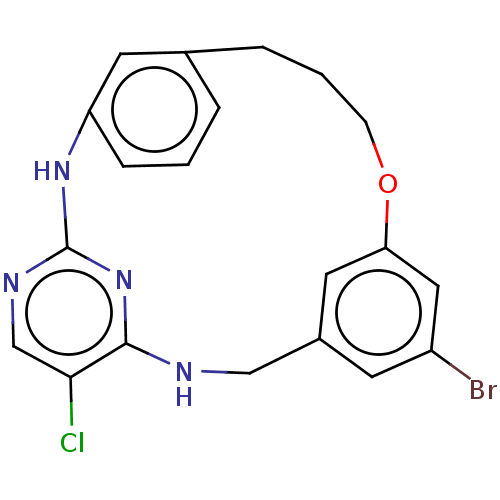
(US8765727, 16)Show SMILES Clc1cnc2Nc3cccc(CCCOc4cc(Br)cc(CNc1n2)c4)c3 Show InChI InChI=1S/C20H18BrClN4O/c21-15-7-14-9-17(10-15)27-6-2-4-13-3-1-5-16(8-13)25-20-24-12-18(22)19(26-20)23-11-14/h1,3,5,7-10,12H,2,4,6,11H2,(H2,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529383

(CHEMBL4570334)Show SMILES CC(Nc1ncnc2[nH]cnc12)c1cc(Cl)c(C)c(-c2cnn(C)c2)c1-c1cccc(F)c1 Show InChI InChI=1S/C24H21ClFN7/c1-13-19(25)8-18(14(2)32-24-22-23(28-11-27-22)29-12-30-24)21(15-5-4-6-17(26)7-15)20(13)16-9-31-33(3)10-16/h4-12,14H,1-3H3,(H2,27,28,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM125904
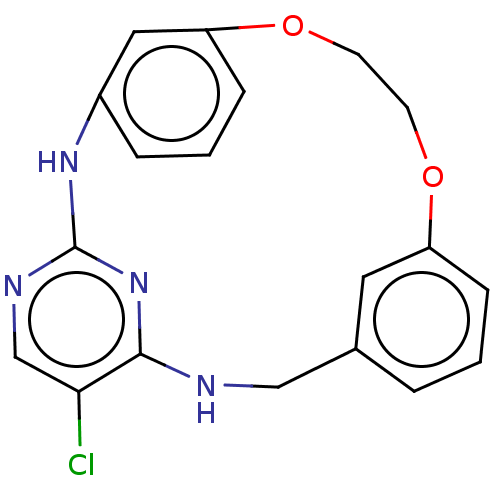
(US8765727, 7)Show InChI InChI=1S/C19H17ClN4O2/c20-17-12-22-19-23-14-4-2-6-16(10-14)26-8-7-25-15-5-1-3-13(9-15)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM125904

(US8765727, 7)Show InChI InChI=1S/C19H17ClN4O2/c20-17-12-22-19-23-14-4-2-6-16(10-14)26-8-7-25-15-5-1-3-13(9-15)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529389
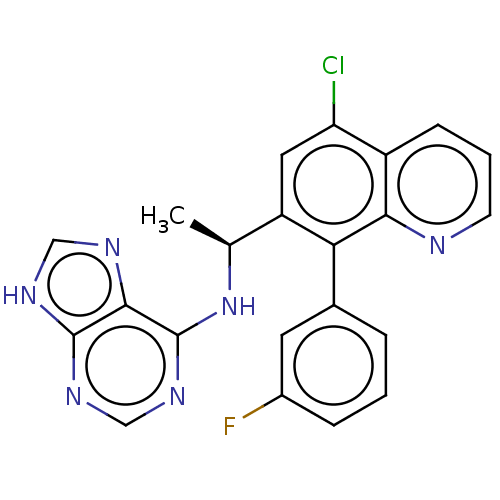
(CHEMBL4440146)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1cc(Cl)c2cccnc2c1-c1cccc(F)c1 |r| Show InChI InChI=1S/C22H16ClFN6/c1-12(30-22-20-21(27-10-26-20)28-11-29-22)16-9-17(23)15-6-3-7-25-19(15)18(16)13-4-2-5-14(24)8-13/h2-12H,1H3,(H2,26,27,28,29,30)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM125899
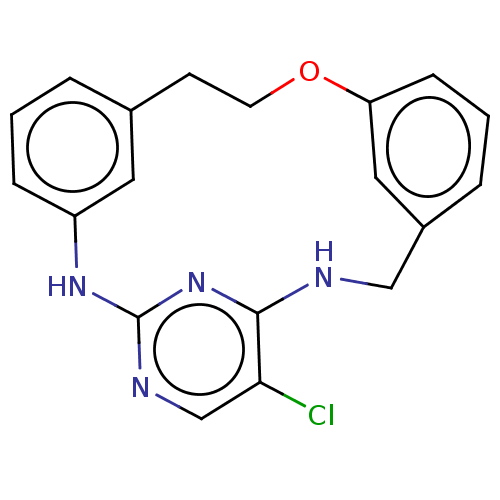
(US8765727, 2)Show InChI InChI=1S/C19H17ClN4O/c20-17-12-22-19-23-15-5-1-3-13(9-15)7-8-25-16-6-2-4-14(10-16)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255856
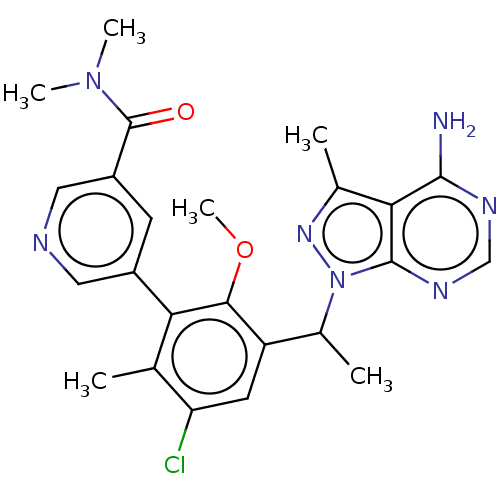
(5-{3-[1-(4-amino-3-methyl-1H-pyrazolo[3,4-d]pyrimi...)Show SMILES COc1c(cc(Cl)c(C)c1-c1cncc(c1)C(=O)N(C)C)C(C)n1nc(C)c2c(N)ncnc12 Show InChI InChI=1S/C24H26ClN7O2/c1-12-18(25)8-17(14(3)32-23-20(13(2)30-32)22(26)28-11-29-23)21(34-6)19(12)15-7-16(10-27-9-15)24(33)31(4)5/h7-11,14H,1-6H3,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255800
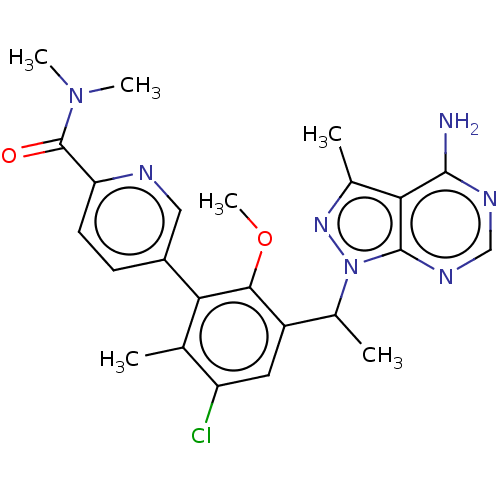
(5-{3-[1-(4-Amino-3-methyl-1H-pyrazolo[3,4-d]pyrimi...)Show SMILES COc1c(cc(Cl)c(C)c1-c1ccc(nc1)C(=O)N(C)C)C(C)n1nc(C)c2c(N)ncnc12 Show InChI InChI=1S/C24H26ClN7O2/c1-12-17(25)9-16(14(3)32-23-20(13(2)30-32)22(26)28-11-29-23)21(34-6)19(12)15-7-8-18(27-10-15)24(33)31(4)5/h7-11,14H,1-6H3,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255858

(US11433071, Example 67-1 | US9707233, 67 (1st peak...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(C1)C(C)C)[C@@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C21H26ClFN6O/c1-10(2)28-7-13(8-28)17-18(23)15(22)6-14(19(17)30-5)12(4)29-21-16(11(3)27-29)20(24)25-9-26-21/h6,9-10,12-13H,7-8H2,1-5H3,(H2,24,25,26)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM441880
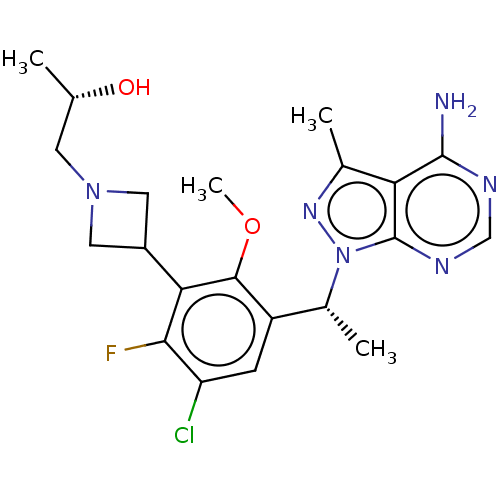
((2R)-1-(3-{3-[1-(4-Amino-3- methyl-1H-pyrazolo[3,4...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(C[C@H](C)O)C1)[C@@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C21H26ClFN6O2/c1-10(30)6-28-7-13(8-28)17-18(23)15(22)5-14(19(17)31-4)12(3)29-21-16(11(2)27-29)20(24)25-9-26-21/h5,9-10,12-13,30H,6-8H2,1-4H3,(H2,24,25,26)/t10-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM570418
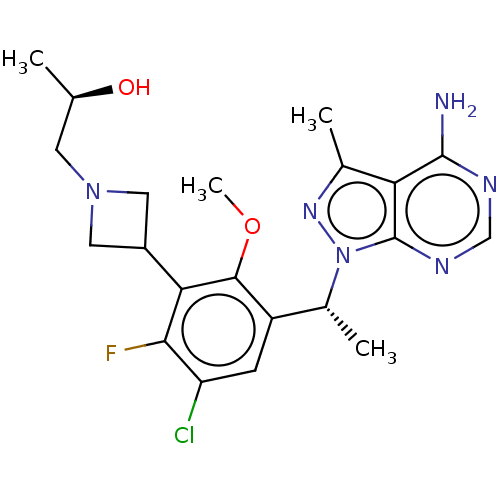
((2R)-1-(3-{3-[1-(4-Amino-3- methyl-1H-pyrazolo[3,4...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(C[C@@H](C)O)C1)[C@@H](C)n1nc(C)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
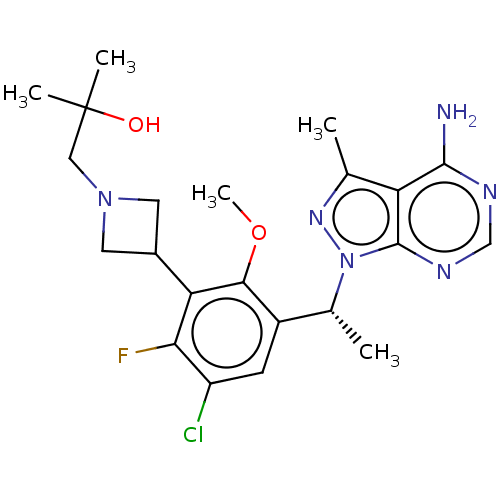
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM570419
(1-(3-{3-[1-(4-Amino-3-methyl-1H- pyrazolo[3,4-d]py...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(CC(C)(C)O)C1)[C@@H](C)n1nc(C)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
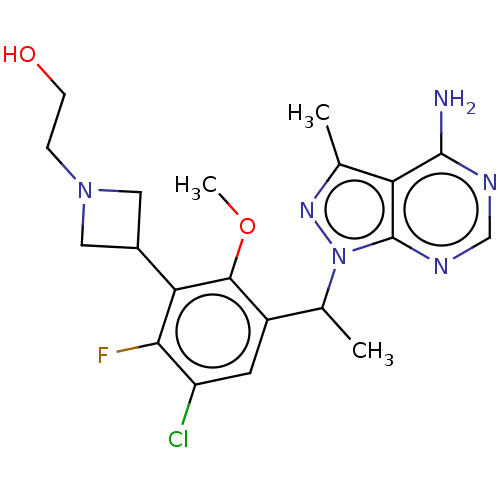
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255862
(2-(3-{3-[1-(4-Amino-3-methyl-1H-pyrazolo[3,4-d]pyr...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(CCO)C1)C(C)n1nc(C)c2c(N)ncnc12 Show InChI InChI=1S/C20H24ClFN6O2/c1-10-15-19(23)24-9-25-20(15)28(26-10)11(2)13-6-14(21)17(22)16(18(13)30-3)12-7-27(8-12)4-5-29/h6,9,11-12,29H,4-5,7-8H2,1-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
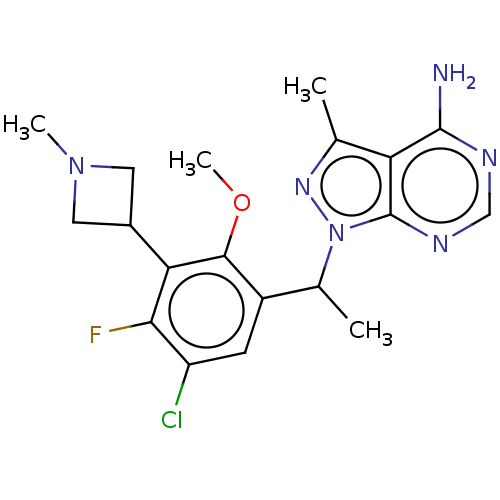
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255863
(1-{1-[5-Chloro-4-fluoro-2-methoxy-3-(1-oxetan-3-yl...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(C)C1)C(C)n1nc(C)c2c(N)ncnc12 Show InChI InChI=1S/C19H22ClFN6O/c1-9-14-18(22)23-8-24-19(14)27(25-9)10(2)12-5-13(20)16(21)15(17(12)28-4)11-6-26(3)7-11/h5,8,10-11H,6-7H2,1-4H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255864

(1-{1-[5-Chloro-4-fluoro-3-(1-isopropylazetidin-3-y...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(C1)C(C)C)C(C)n1nc(C(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C21H24ClF3N6O/c1-9(2)30-6-11(7-30)14-16(23)13(22)5-12(18(14)32-4)10(3)31-21-15(17(29-31)19(24)25)20(26)27-8-28-21/h5,8-11,19H,6-7H2,1-4H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM289073

(US10092570, Example 74 | US10376513, Example 74 | ...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(CCO)C1)C(C)n1nc(C(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C20H22ClF3N6O2/c1-9(30-20-14(16(28-30)18(23)24)19(25)26-8-27-20)11-5-12(21)15(22)13(17(11)32-2)10-6-29(7-10)3-4-31/h5,8-10,18,31H,3-4,6-7H2,1-2H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM289074
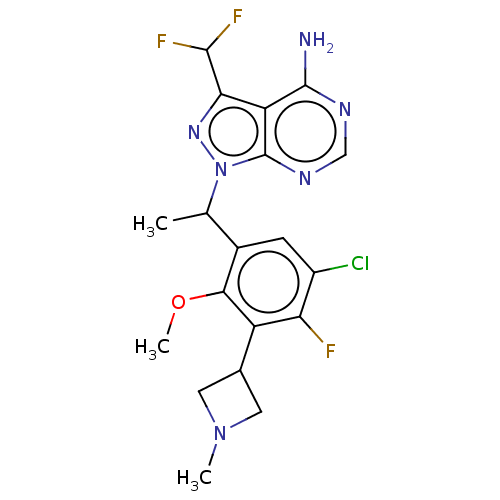
(1-{1-[5-Chloro-4-fluoro-2- methoxy-3-(1-methylazet...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(C)C1)C(C)n1nc(C(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C19H20ClF3N6O/c1-8(29-19-13(15(27-29)17(22)23)18(24)25-7-26-19)10-4-11(20)14(21)12(16(10)30-3)9-5-28(2)6-9/h4,7-9,17H,5-6H2,1-3H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM256122
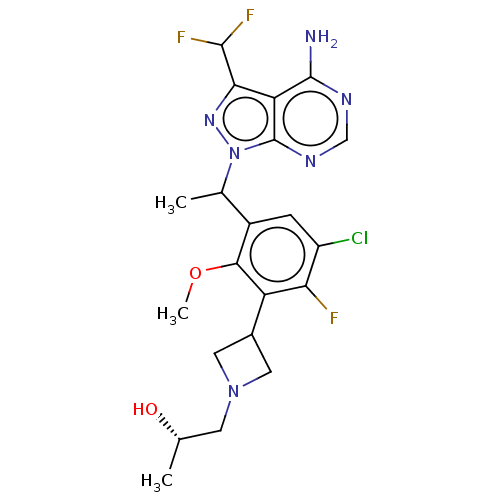
((2S)-1-[3-(3-{1-[4-amino-3-(difluoromethyl)-1H-pyr...)Show SMILES COc1c(cc(Cl)c(F)c1C1CN(C[C@H](C)O)C1)C(C)n1nc(C(F)F)c2c(N)ncnc12 |r| Show InChI InChI=1S/C21H24ClF3N6O2/c1-9(32)5-30-6-11(7-30)14-16(23)13(22)4-12(18(14)33-3)10(2)31-21-15(17(29-31)19(24)25)20(26)27-8-28-21/h4,8-11,19,32H,5-7H2,1-3H3,(H2,26,27,28)/t9-,10?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM256123
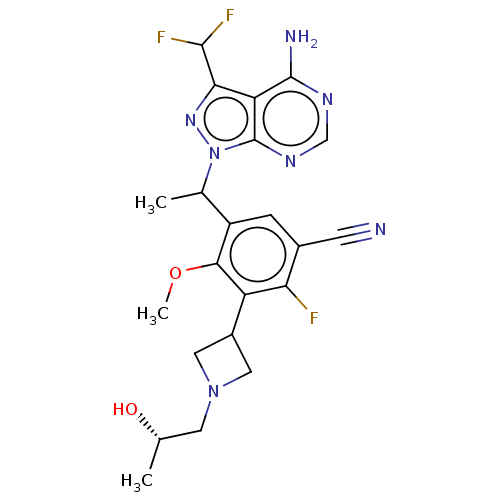
(5-(1-(4-Amino-3-(difluoromethyl)-1H-pyrazolo[3,4-d...)Show SMILES COc1c(cc(C#N)c(F)c1C1CN(C[C@H](C)O)C1)C(C)n1nc(C(F)F)c2c(N)ncnc12 |r| Show InChI InChI=1S/C22H24F3N7O2/c1-10(33)6-31-7-13(8-31)15-17(23)12(5-26)4-14(19(15)34-3)11(2)32-22-16(18(30-32)20(24)25)21(27)28-9-29-22/h4,9-11,13,20,33H,6-8H2,1-3H3,(H2,27,28,29)/t10-,11?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM289077
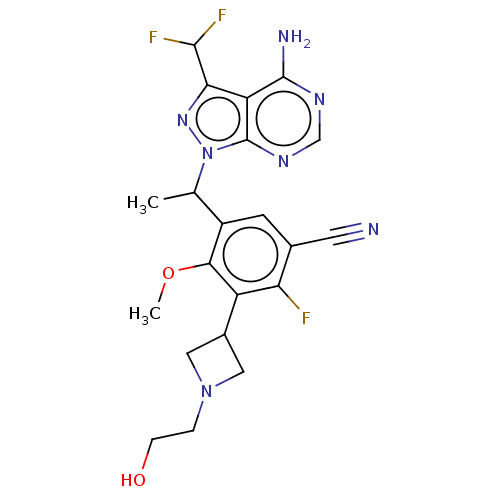
(5-{1-[4-Amino-3-(difluoromethyl)- 1H-pyrazolo[3,4-...)Show SMILES COc1c(cc(C#N)c(F)c1C1CN(CCO)C1)C(C)n1nc(C(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C21H22F3N7O2/c1-10(31-21-15(17(29-31)19(23)24)20(26)27-9-28-21)13-5-11(6-25)16(22)14(18(13)33-2)12-7-30(8-12)3-4-32/h5,9-10,12,19,32H,3-4,7-8H2,1-2H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM259101
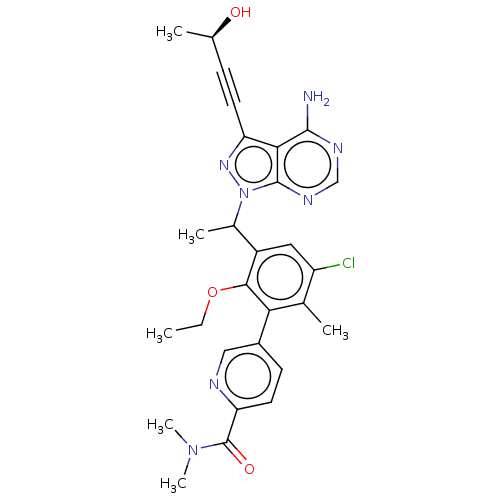
(5-[3-(1-{4-Amino-3-[(3R)-3-hydroxybut-1-yn-1-yl]-1...)Show SMILES CCOc1c(cc(Cl)c(C)c1-c1ccc(nc1)C(=O)N(C)C)C(C)n1nc(C#C[C@@H](C)O)c2c(N)ncnc12 |r| Show InChI InChI=1S/C28H30ClN7O3/c1-7-39-25-19(12-20(29)16(3)23(25)18-9-11-22(31-13-18)28(38)35(5)6)17(4)36-27-24(26(30)32-14-33-27)21(34-36)10-8-15(2)37/h9,11-15,17,37H,7H2,1-6H3,(H2,30,32,33)/t15-,17?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261057
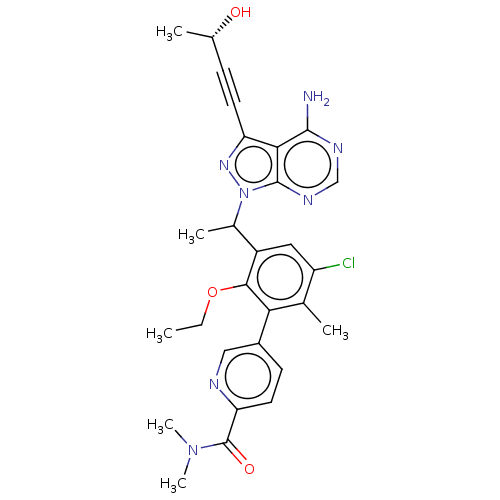
(5-[3-(1-{4-Amino-3-[(3S)-3-hydroxybut-1-yn-1-yl]-1...)Show SMILES CCOc1c(cc(Cl)c(C)c1-c1ccc(nc1)C(=O)N(C)C)C(C)n1nc(C#C[C@H](C)O)c2c(N)ncnc12 |r| Show InChI InChI=1S/C28H30ClN7O3/c1-7-39-25-19(12-20(29)16(3)23(25)18-9-11-22(31-13-18)28(38)35(5)6)17(4)36-27-24(26(30)32-14-33-27)21(34-36)10-8-15(2)37/h9,11-15,17,37H,7H2,1-6H3,(H2,30,32,33)/t15-,17?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261059
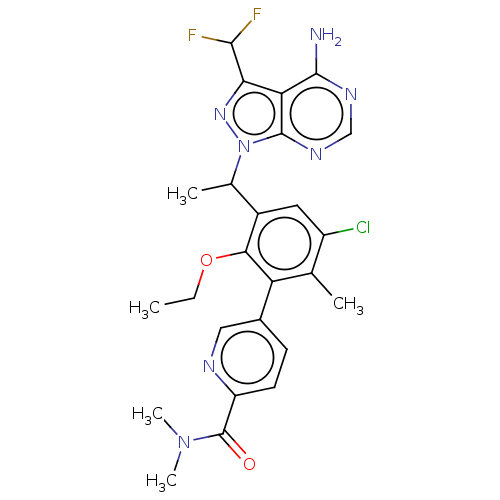
(5-(3-{1-[4-Amino-3-(difluoromethyl)-1H-pyrazolo[3,...)Show SMILES CCOc1c(cc(Cl)c(C)c1-c1ccc(nc1)C(=O)N(C)C)C(C)n1nc(C(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C25H26ClF2N7O2/c1-6-37-21-15(13(3)35-24-19(20(33-35)22(27)28)23(29)31-11-32-24)9-16(26)12(2)18(21)14-7-8-17(30-10-14)25(36)34(4)5/h7-11,13,22H,6H2,1-5H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261060
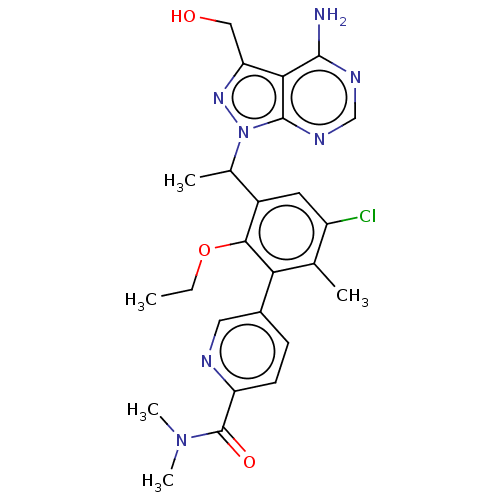
(5-(3-{1-[4-Amino-3-(hydroxymethyl)-1H-pyrazolo[3,4...)Show SMILES CCOc1c(cc(Cl)c(C)c1-c1ccc(nc1)C(=O)N(C)C)C(C)n1nc(CO)c2c(N)ncnc12 Show InChI InChI=1S/C25H28ClN7O3/c1-6-36-22-16(14(3)33-24-21(19(11-34)31-33)23(27)29-12-30-24)9-17(26)13(2)20(22)15-7-8-18(28-10-15)25(35)32(4)5/h7-10,12,14,34H,6,11H2,1-5H3,(H2,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261063
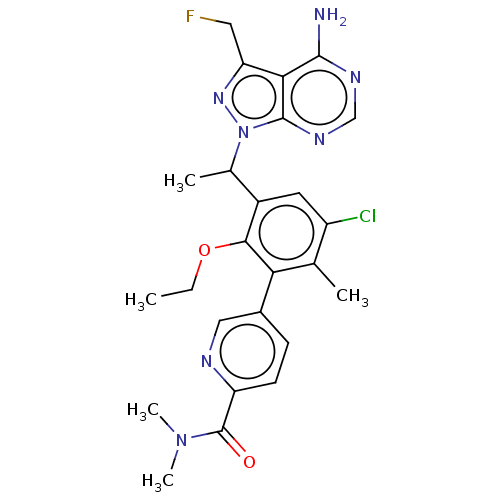
(5-(3-{1-[4-Amino-3-(fluoromethyl)-1H-pyrazolo[3,4-...)Show SMILES CCOc1c(cc(Cl)c(C)c1-c1ccc(nc1)C(=O)N(C)C)C(C)n1nc(CF)c2c(N)ncnc12 Show InChI InChI=1S/C25H27ClFN7O2/c1-6-36-22-16(14(3)34-24-21(19(10-27)32-34)23(28)30-12-31-24)9-17(26)13(2)20(22)15-7-8-18(29-11-15)25(35)33(4)5/h7-9,11-12,14H,6,10H2,1-5H3,(H2,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261064
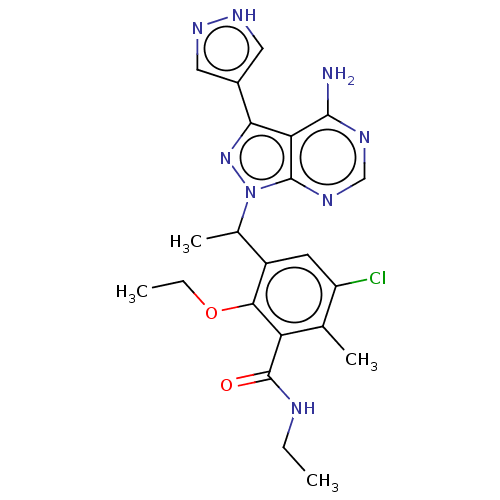
(3-{1-[4-Amino-3-(1H-pyrazol-4-yl)-1H-pyrazolo[3,4-...)Show SMILES CCNC(=O)c1c(C)c(Cl)cc(C(C)n2nc(-c3cn[nH]c3)c3c(N)ncnc23)c1OCC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261065
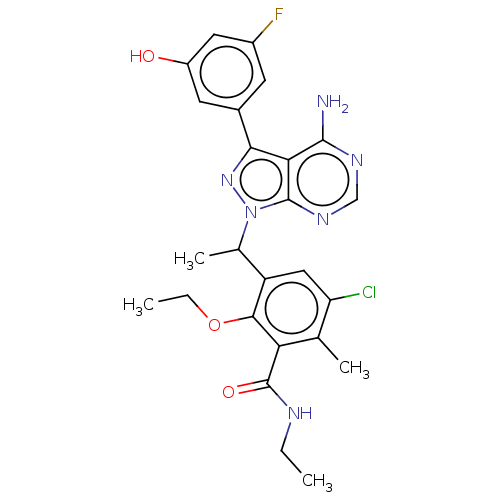
(3-{1-[4-Amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyra...)Show SMILES CCNC(=O)c1c(C)c(Cl)cc(C(C)n2nc(-c3cc(O)cc(F)c3)c3c(N)ncnc23)c1OCC Show InChI InChI=1S/C25H26ClFN6O3/c1-5-29-25(35)19-12(3)18(26)10-17(22(19)36-6-2)13(4)33-24-20(23(28)30-11-31-24)21(32-33)14-7-15(27)9-16(34)8-14/h7-11,13,34H,5-6H2,1-4H3,(H,29,35)(H2,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261066
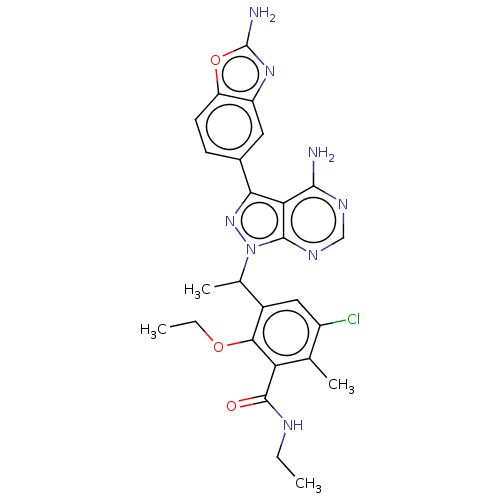
(3-(1-(4-Amino-3-(2-aminobenzo[d]oxazol-5-yl)-1H-py...)Show SMILES CCNC(=O)c1c(C)c(Cl)cc(C(C)n2nc(-c3ccc4oc(N)nc4c3)c3c(N)ncnc23)c1OCC Show InChI InChI=1S/C26H27ClN8O3/c1-5-30-25(36)19-12(3)16(27)10-15(22(19)37-6-2)13(4)35-24-20(23(28)31-11-32-24)21(34-35)14-7-8-18-17(9-14)33-26(29)38-18/h7-11,13H,5-6H2,1-4H3,(H2,29,33)(H,30,36)(H2,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261067
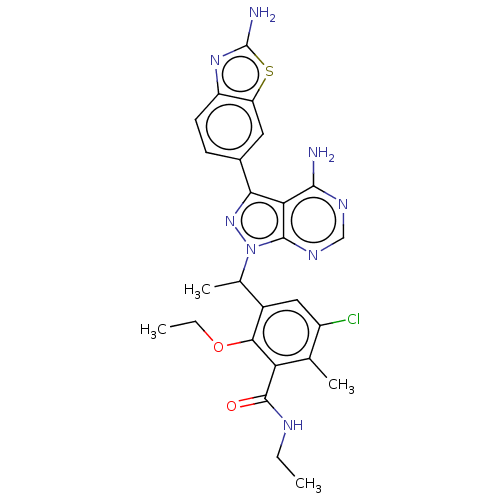
(3-{1-[4-Amino-3-(2-amino-1,3-benzothiazol-6-yl)-1H...)Show SMILES CCNC(=O)c1c(C)c(Cl)cc(C(C)n2nc(-c3ccc4nc(N)sc4c3)c3c(N)ncnc23)c1OCC Show InChI InChI=1S/C26H27ClN8O2S/c1-5-30-25(36)19-12(3)16(27)10-15(22(19)37-6-2)13(4)35-24-20(23(28)31-11-32-24)21(34-35)14-7-8-17-18(9-14)38-26(29)33-17/h7-11,13H,5-6H2,1-4H3,(H2,29,33)(H,30,36)(H2,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261068
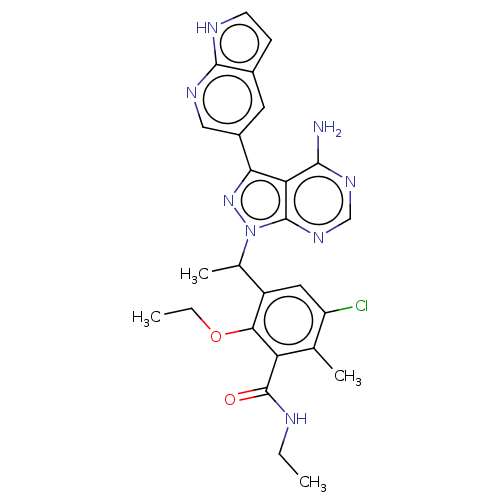
(3-{1-[4-Amino-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)-1H...)Show SMILES CCNC(=O)c1c(C)c(Cl)cc(C(C)n2nc(-c3cnc4[nH]ccc4c3)c3c(N)ncnc23)c1OCC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The kinase reaction was conducted in polystyrene 384-well matrix white plate from Thermo Fisher Scientific in a final volume of 25 μL. Inhibitor... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2417199 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data