 Found 515 hits with Last Name = 'lewis' and Initial = 'rj'
Found 515 hits with Last Name = 'lewis' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613517
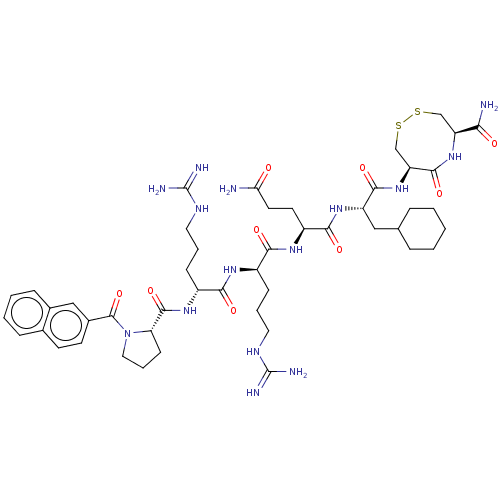
(CHEMBL5273346)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccc2ccccc2c1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21995
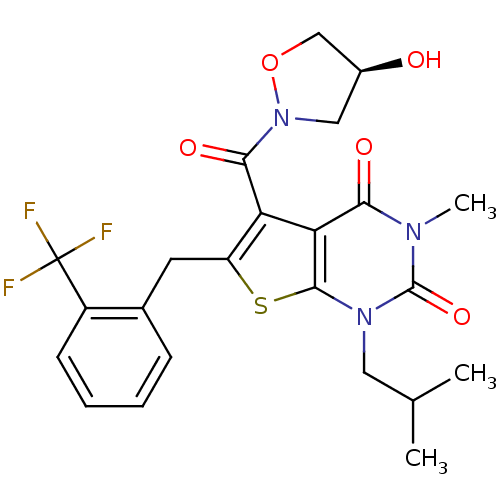
(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C23H24F3N3O5S/c1-12(2)9-28-21-18(19(31)27(3)22(28)33)17(20(32)29-10-14(30)11-34-29)16(35-21)8-13-6-4-5-7-15(13)23(24,25)26/h4-7,12,14,30H,8-11H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613518

(CHEMBL5289754)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613552

(CHEMBL5281808)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)NCC(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613546
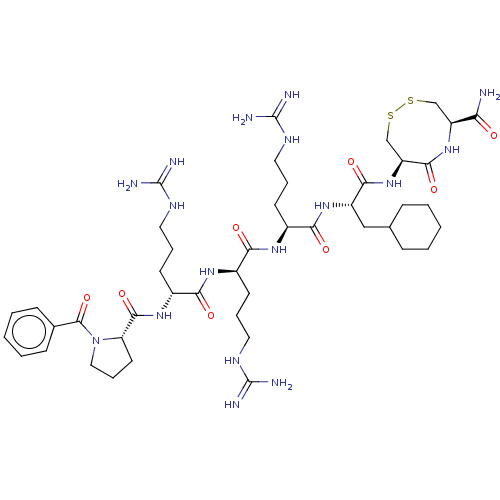
(CHEMBL5273114)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613519
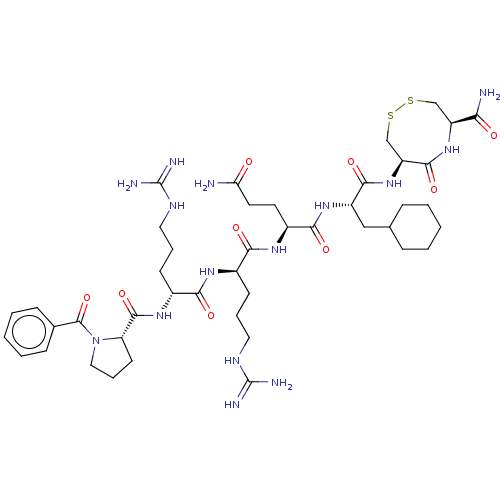
(CHEMBL5281912)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613478
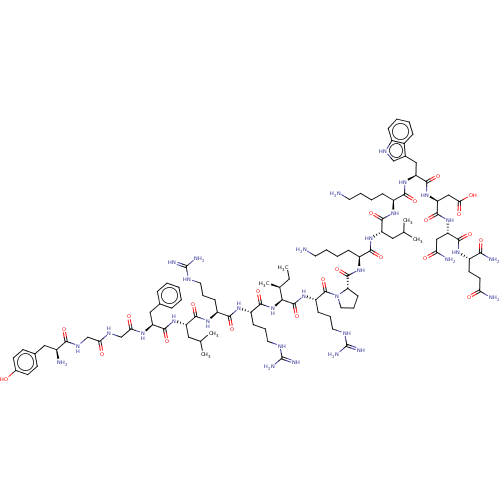
(CHEMBL5273273)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613548

(CHEMBL5286081)Show SMILES C[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50332720
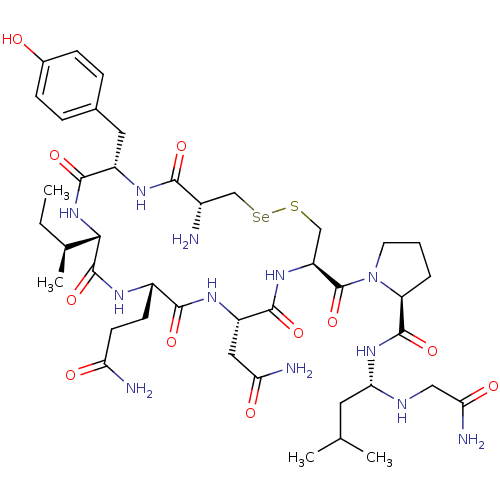
((S)-1-((4R,7S,10S,13S,16S,19R)-4-amino-16-(2-amino...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)C[Se]SC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)NCC(N)=O |r| Show InChI InChI=1S/C42H66N12O11SSe/c1-5-22(4)35-41(64)48-26(12-13-31(44)56)37(60)50-28(17-32(45)57)38(61)51-29(42(65)54-14-6-7-30(54)40(63)52-34(15-21(2)3)47-18-33(46)58)19-66-67-20-25(43)36(59)49-27(39(62)53-35)16-23-8-10-24(55)11-9-23/h8-11,21-22,25-30,34-35,47,55H,5-7,12-20,43H2,1-4H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,48,64)(H,49,59)(H,50,60)(H,51,61)(H,52,63)(H,53,62)/t22-,25-,26-,27-,28-,29-,30-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from oxytocin receptor expressed in COS1 cells |
J Med Chem 53: 8585-96 (2010)
Article DOI: 10.1021/jm100989w
BindingDB Entry DOI: 10.7270/Q2CC10X9 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21992
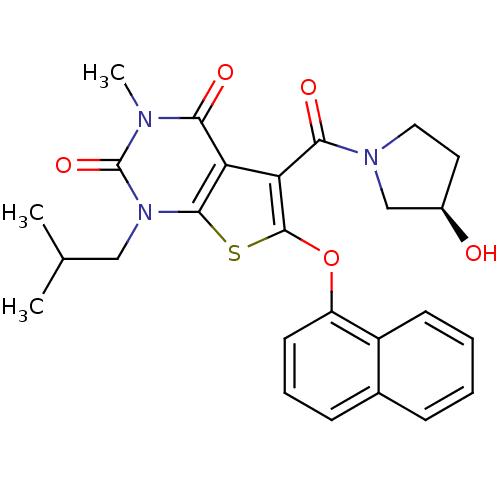
(5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...)Show SMILES CC(C)Cn1c2sc(Oc3cccc4ccccc34)c(C(=O)N3CC[C@@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H27N3O5S/c1-15(2)13-29-24-20(22(31)27(3)26(29)33)21(23(32)28-12-11-17(30)14-28)25(35-24)34-19-10-6-8-16-7-4-5-9-18(16)19/h4-10,15,17,30H,11-14H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50002184

(CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C75H127N25O14/c1-7-45(6)61(71(113)96-54(25-17-35-88-75(84)85)72(114)100-36-18-26-58(100)70(112)95-51(22-12-14-32-77)65(107)97-55(37-43(2)3)67(109)92-50(62(79)104)21-11-13-31-76)99-66(108)53(24-16-34-87-74(82)83)93-64(106)52(23-15-33-86-73(80)81)94-68(110)56(38-44(4)5)98-69(111)57(40-46-19-9-8-10-20-46)91-60(103)42-89-59(102)41-90-63(105)49(78)39-47-27-29-48(101)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,101H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H2,79,104)(H,89,102)(H,90,105)(H,91,103)(H,92,109)(H,93,106)(H,94,110)(H,95,112)(H,96,113)(H,97,107)(H,98,111)(H,99,108)(H4,80,81,86)(H4,82,83,87)(H4,84,85,88)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613550
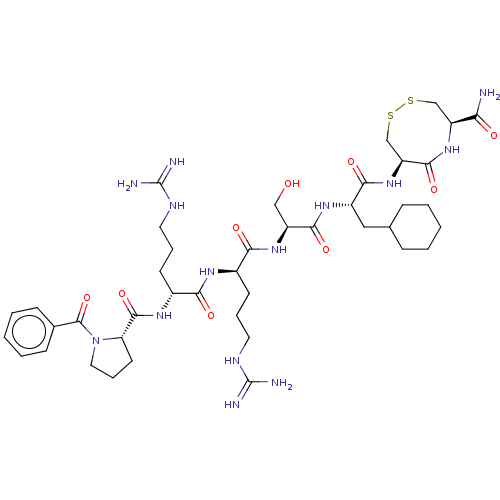
(CHEMBL5279208)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21996
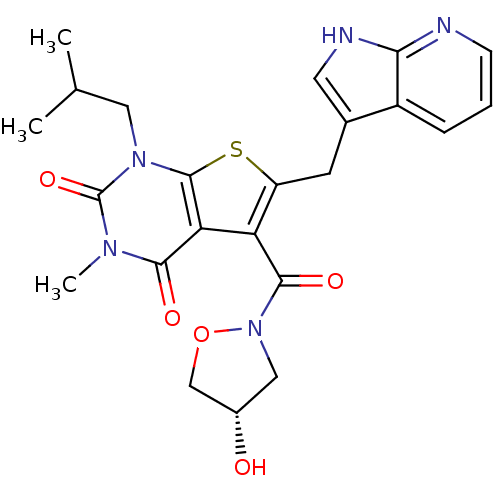
(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3c[nH]c4ncccc34)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C23H25N5O5S/c1-12(2)9-27-22-18(20(30)26(3)23(27)32)17(21(31)28-10-14(29)11-33-28)16(34-22)7-13-8-25-19-15(13)5-4-6-24-19/h4-6,8,12,14,29H,7,9-11H2,1-3H3,(H,24,25)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613528
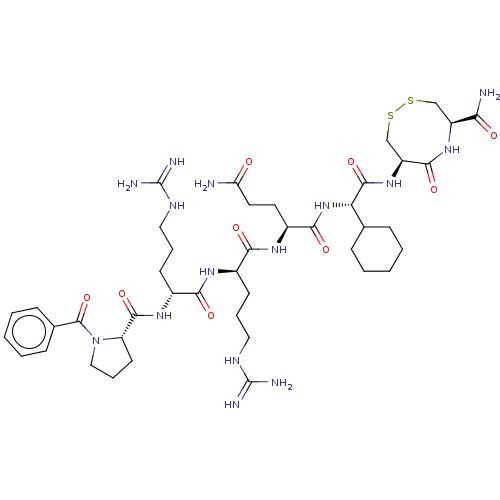
(CHEMBL5282326)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21994

(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccccc34)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C25H26N4O5S/c1-14(2)11-28-24-21(22(31)27(3)25(28)33)20(23(32)29-12-16(30)13-34-29)19(35-24)10-15-8-9-26-18-7-5-4-6-17(15)18/h4-9,14,16,30H,10-13H2,1-3H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613520
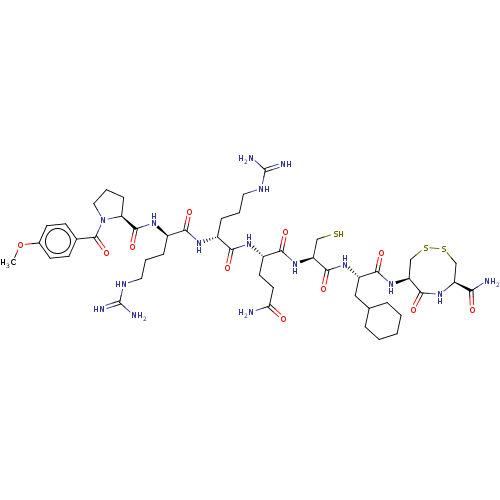
(CHEMBL5283811)Show SMILES COc1ccc(cc1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613547
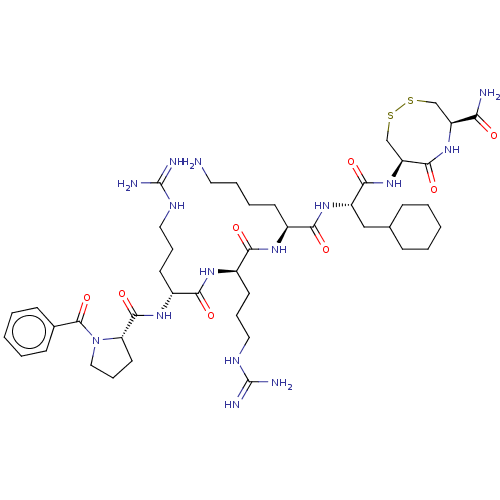
(CHEMBL5278935)Show SMILES NCCCC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613551
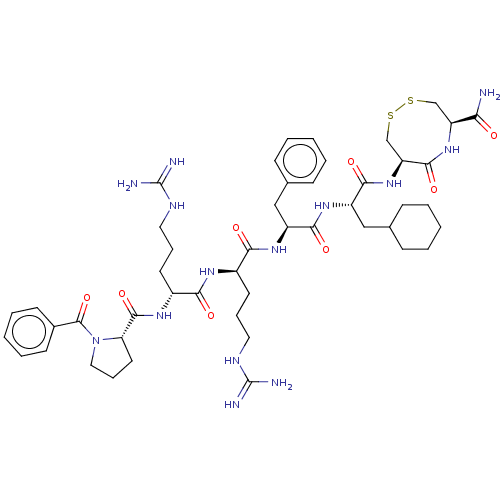
(CHEMBL5283035)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613545
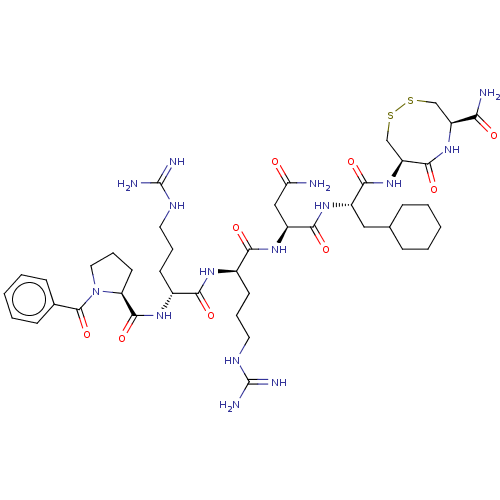
(CHEMBL5288658)Show SMILES NC(=O)C[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613513
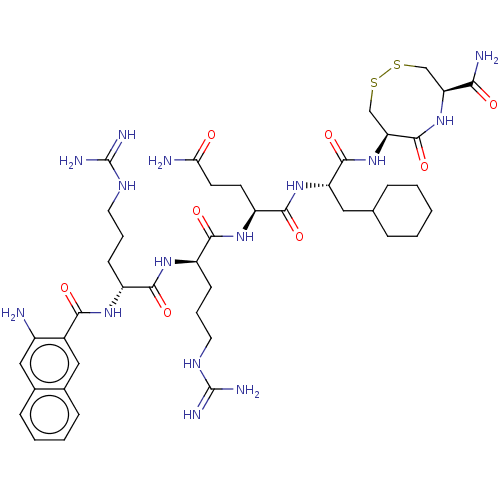
(CHEMBL5280997)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)c1cc2ccccc2cc1N)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613514
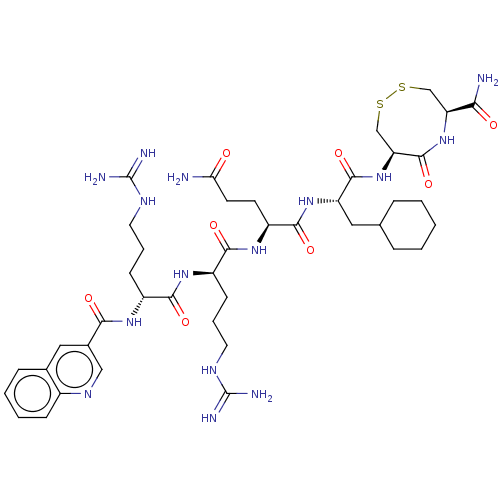
(CHEMBL5284775)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)c1cnc2ccccc2c1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50332721
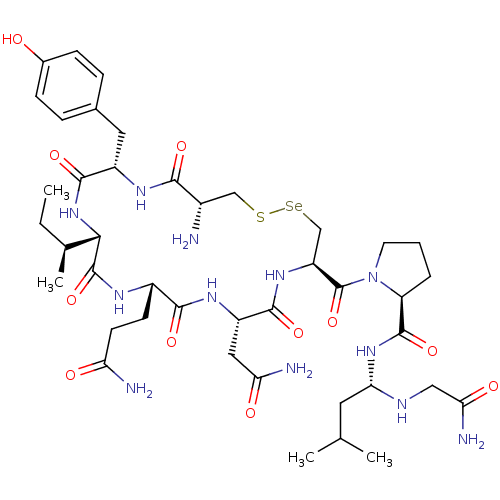
((S)-N-((S)-1-(2-amino-2-oxoethylamino)-3-methylbut...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CS[Se]C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)NCC(N)=O |r| Show InChI InChI=1S/C42H66N12O11SSe/c1-5-22(4)35-41(64)48-26(12-13-31(44)56)37(60)50-28(17-32(45)57)38(61)51-29(42(65)54-14-6-7-30(54)40(63)52-34(15-21(2)3)47-18-33(46)58)20-67-66-19-25(43)36(59)49-27(39(62)53-35)16-23-8-10-24(55)11-9-23/h8-11,21-22,25-30,34-35,47,55H,5-7,12-20,43H2,1-4H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,48,64)(H,49,59)(H,50,60)(H,51,61)(H,52,63)(H,53,62)/t22-,25-,26-,27-,28-,29-,30-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from oxytocin receptor expressed in COS1 cells |
J Med Chem 53: 8585-96 (2010)
Article DOI: 10.1021/jm100989w
BindingDB Entry DOI: 10.7270/Q2CC10X9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from oxytocin receptor expressed in COS1 cells |
J Med Chem 53: 8585-96 (2010)
Article DOI: 10.1021/jm100989w
BindingDB Entry DOI: 10.7270/Q2CC10X9 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21987

(6-[(6-fluoroquinolin-4-yl)methyl]-5-{[(3R)-3-hydro...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccc(F)cc34)c(C(=O)N3CC[C@@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H27FN4O4S/c1-14(2)12-31-25-22(23(33)29(3)26(31)35)21(24(34)30-9-7-17(32)13-30)20(36-25)10-15-6-8-28-19-5-4-16(27)11-18(15)19/h4-6,8,11,14,17,32H,7,9-10,12-13H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613515
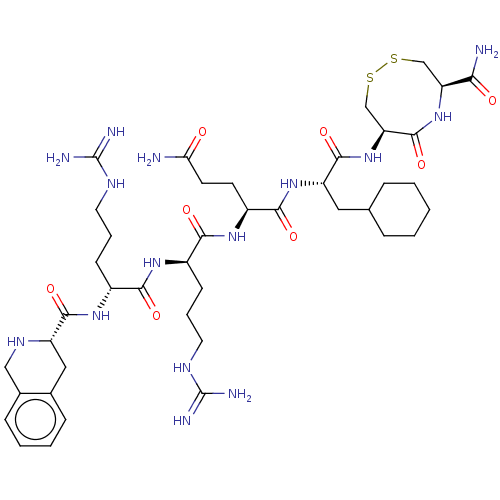
(CHEMBL5276702)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1Cc2ccccc2CN1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613521
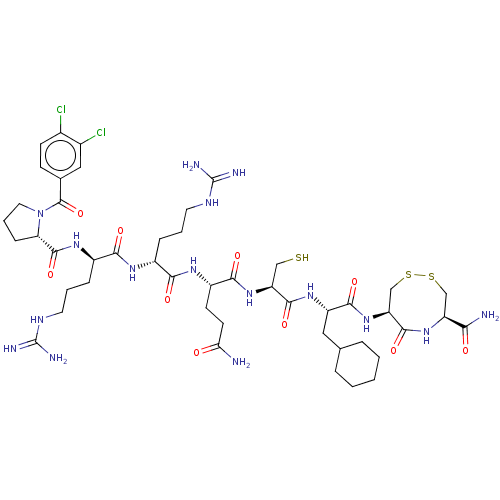
(CHEMBL5285730)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccc(Cl)c(Cl)c1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus) | BDBM50031347
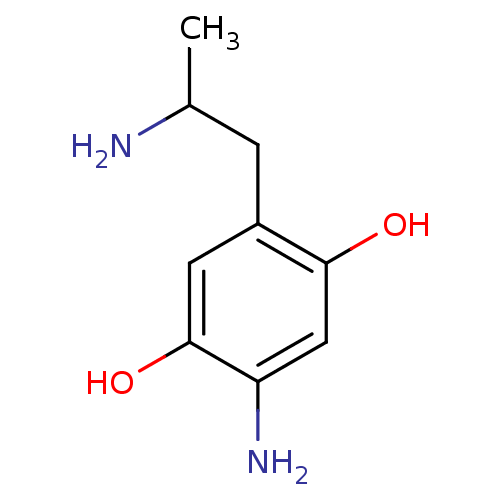
(2-Amino-5-(2-amino-propyl)-benzene-1,4-diol | CHEM...)Show InChI InChI=1S/C9H14N2O2/c1-5(10)2-6-3-9(13)7(11)4-8(6)12/h3-5,12-13H,2,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oklahoma
Curated by ChEMBL
| Assay Description
Evaluated for the Non competitive inhibition of uptake of norepinephrine |
J Med Chem 38: 4087-97 (1995)
BindingDB Entry DOI: 10.7270/Q2930S73 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613522
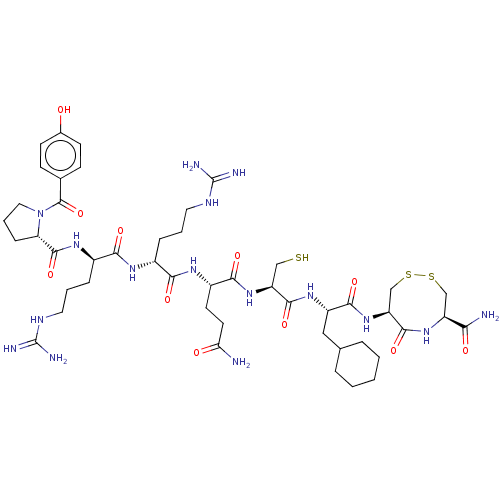
(CHEMBL5270523)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccc(O)cc1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613511
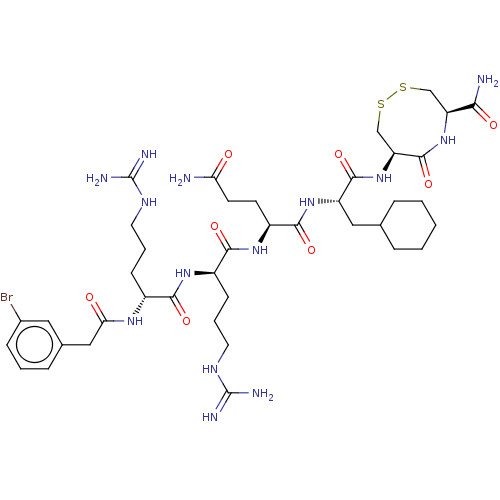
(CHEMBL5278992)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)Cc1cccc(Br)c1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613516
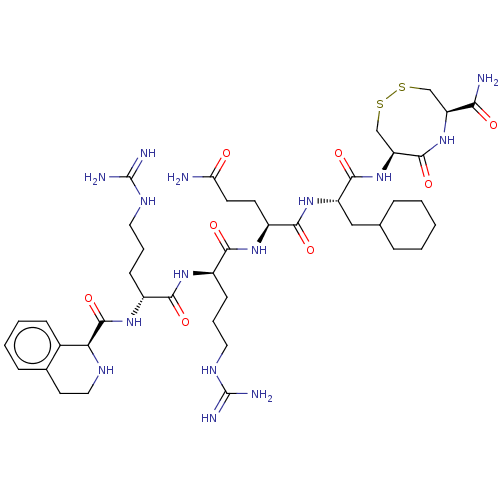
(CHEMBL5275165)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H]1NCCc2ccccc12)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21998
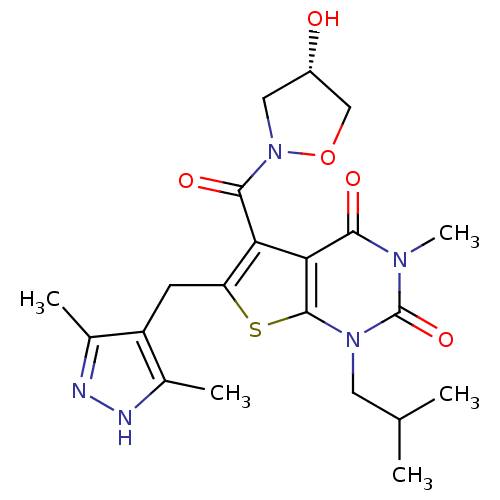
(6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-{[(4S)-...)Show SMILES CC(C)Cn1c2sc(Cc3c(C)n[nH]c3C)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H27N5O5S/c1-10(2)7-25-20-17(18(28)24(5)21(25)30)16(19(29)26-8-13(27)9-31-26)15(32-20)6-14-11(3)22-23-12(14)4/h10,13,27H,6-9H2,1-5H3,(H,22,23)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613510
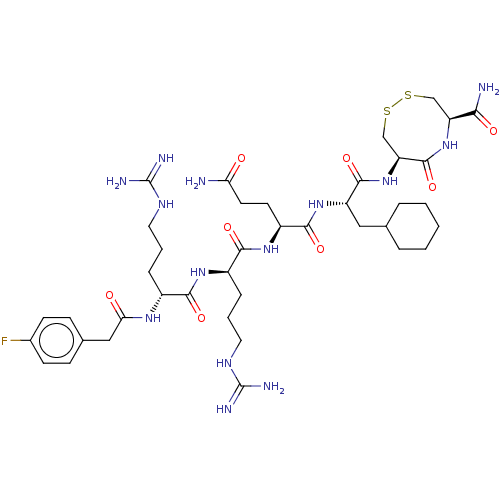
(CHEMBL5282859)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)Cc1ccc(F)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613524
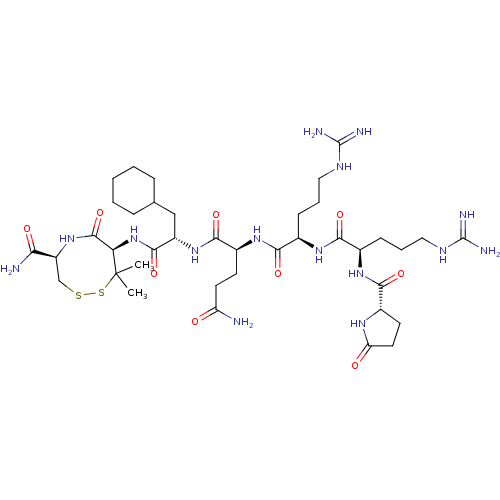
(CHEMBL5289642)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H]1NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCC(=O)N1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613494
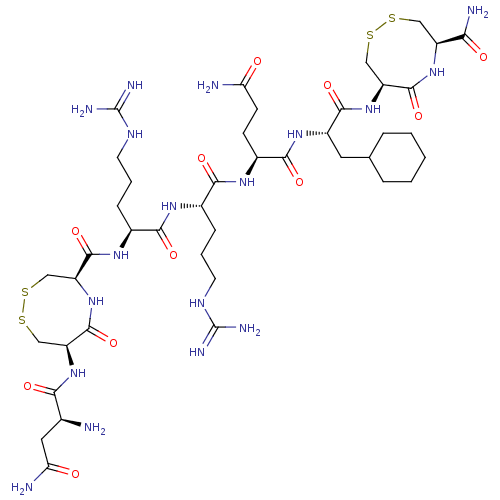
(CHEMBL5280066)Show SMILES N[C@@H](CC(N)=O)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613549
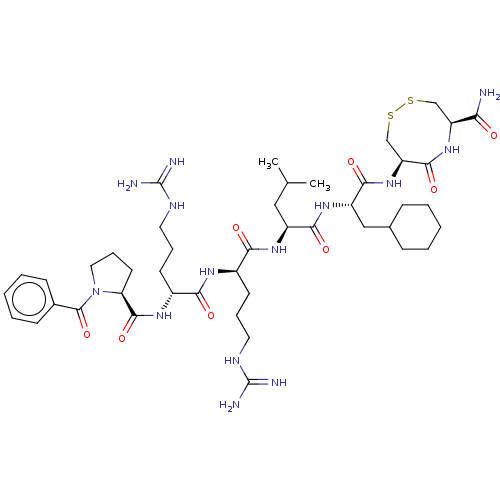
(CHEMBL5283084)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50332723
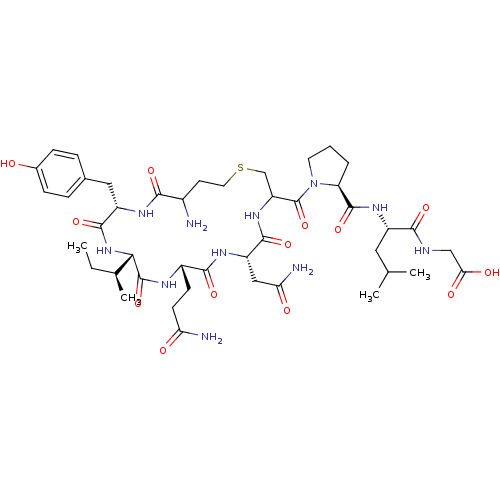
(CHEMBL1630531 | Cystathionine Oxytocin)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)C(N)CCSCC(NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O |r| Show InChI InChI=1S/C44H67N11O13S/c1-5-23(4)36-43(67)49-27(12-13-33(46)57)39(63)51-30(19-34(47)58)40(64)53-31(21-69-16-14-26(45)37(61)50-29(41(65)54-36)18-24-8-10-25(56)11-9-24)44(68)55-15-6-7-32(55)42(66)52-28(17-22(2)3)38(62)48-20-35(59)60/h8-11,22-23,26-32,36,56H,5-7,12-21,45H2,1-4H3,(H2,46,57)(H2,47,58)(H,48,62)(H,49,67)(H,50,61)(H,51,63)(H,52,66)(H,53,64)(H,54,65)(H,59,60)/t23-,26?,27-,28-,29-,30-,31?,32-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from oxytocin receptor expressed in COS1 cells |
J Med Chem 53: 8585-96 (2010)
Article DOI: 10.1021/jm100989w
BindingDB Entry DOI: 10.7270/Q2CC10X9 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21997
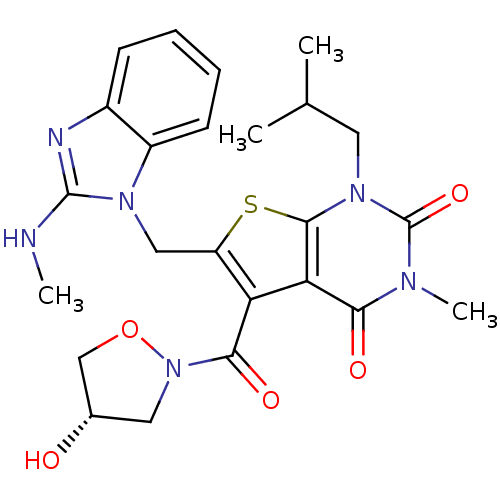
(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CNc1nc2ccccc2n1Cc1sc2n(CC(C)C)c(=O)n(C)c(=O)c2c1C(=O)N1C[C@H](O)CO1 |r| Show InChI InChI=1S/C24H28N6O5S/c1-13(2)9-29-22-19(20(32)27(4)24(29)34)18(21(33)30-10-14(31)12-35-30)17(36-22)11-28-16-8-6-5-7-15(16)26-23(28)25-3/h5-8,13-14,31H,9-12H2,1-4H3,(H,25,26)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613512
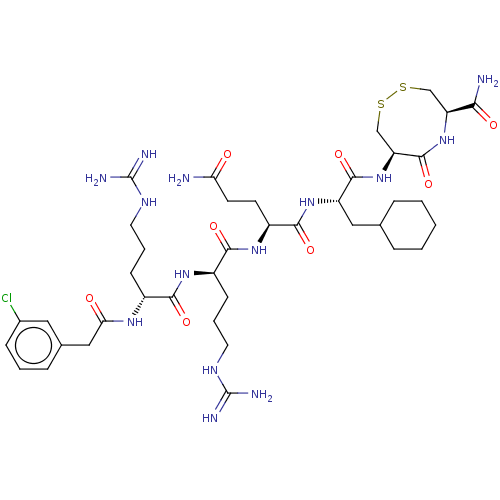
(CHEMBL5276129)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)Cc1cccc(Cl)c1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613523
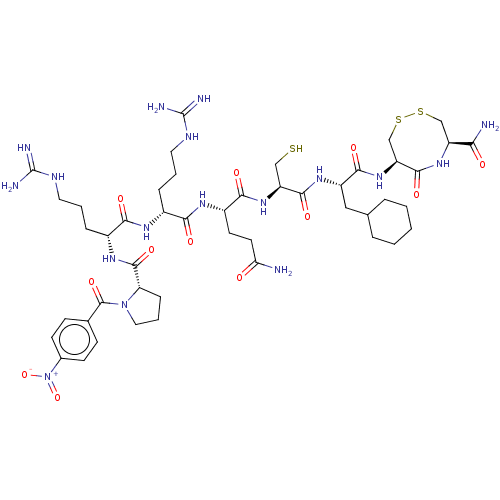
(CHEMBL5279518)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)c1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613532
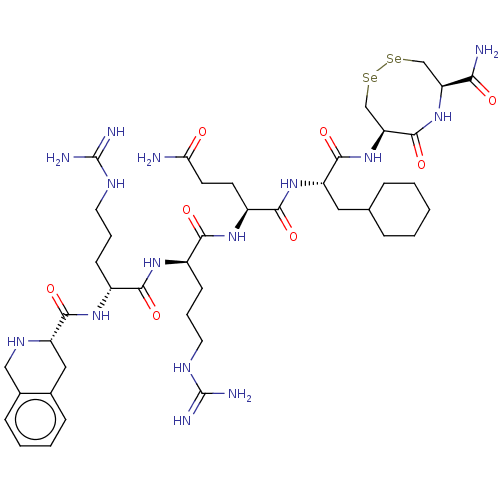
(CHEMBL5288155)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-c2ccccc2-[#6]-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@H]-1-[#6][Se;v2][Se;v2][#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](-[#7])=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613496
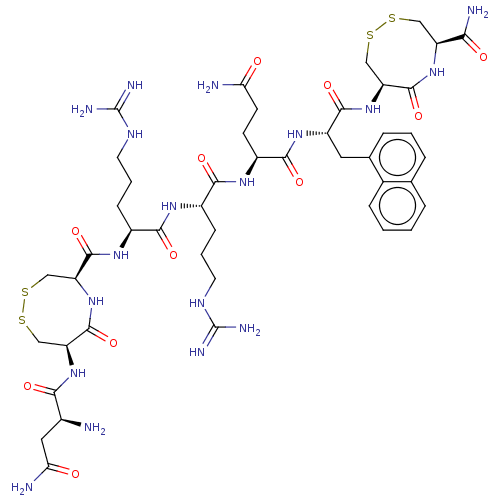
(CHEMBL5266718)Show SMILES N[C@@H](CC(N)=O)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50031347

(2-Amino-5-(2-amino-propyl)-benzene-1,4-diol | CHEM...)Show InChI InChI=1S/C9H14N2O2/c1-5(10)2-6-3-9(13)7(11)4-8(6)12/h3-5,12-13H,2,10-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oklahoma
Curated by ChEMBL
| Assay Description
Evaluated for the Competitive inhibition of uptake of dopamine transporter |
J Med Chem 38: 4087-97 (1995)
BindingDB Entry DOI: 10.7270/Q2930S73 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21993

(6-[(4,5-dichloro-2-methyl-1H-imidazol-1-yl)methyl]...)Show SMILES CON(C)C(=O)c1c(Cn2c(C)nc(Cl)c2Cl)sc2n(CC(C)C)c(=O)n(C)c(=O)c12 Show InChI InChI=1S/C19H23Cl2N5O4S/c1-9(2)7-26-18-13(16(27)23(4)19(26)29)12(17(28)24(5)30-6)11(31-18)8-25-10(3)22-14(20)15(25)21/h9H,7-8H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613506
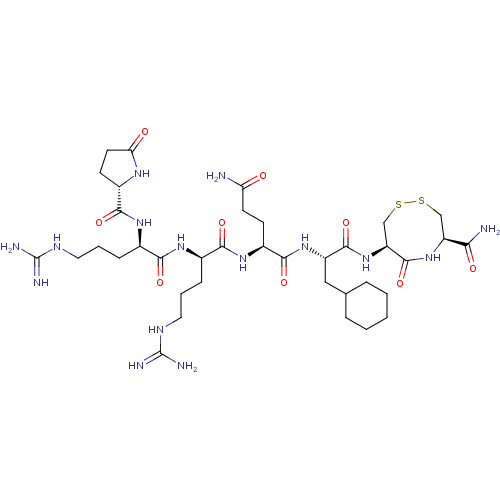
(CHEMBL5268886)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21985
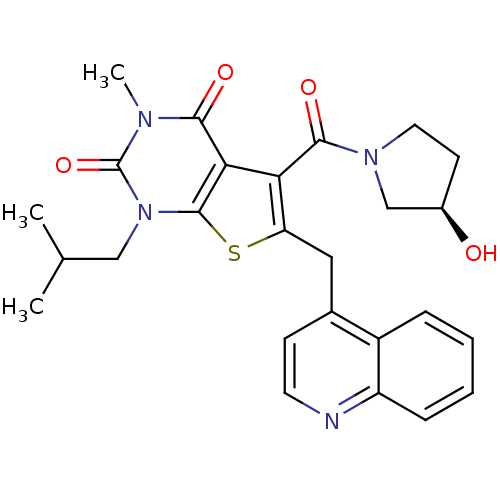
(5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccccc34)c(C(=O)N3CC[C@@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H28N4O4S/c1-15(2)13-30-25-22(23(32)28(3)26(30)34)21(24(33)29-11-9-17(31)14-29)20(35-25)12-16-8-10-27-19-7-5-4-6-18(16)19/h4-8,10,15,17,31H,9,11-14H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus) | BDBM50031343

(2-Amino-5-(2-amino-ethyl)-benzene-1,4-diol | CHEMB...)Show InChI InChI=1S/C8H12N2O2/c9-2-1-5-3-8(12)6(10)4-7(5)11/h3-4,11-12H,1-2,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oklahoma
Curated by ChEMBL
| Assay Description
Evaluated for the Competitive inhibition of uptake of norepinephrine |
J Med Chem 38: 4087-97 (1995)
BindingDB Entry DOI: 10.7270/Q2930S73 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613508
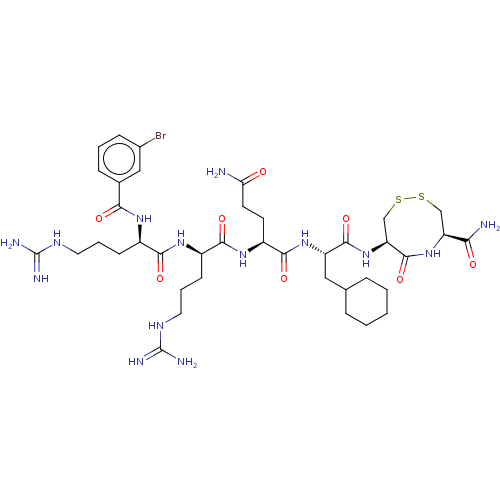
(CHEMBL5288478)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)c1cccc(Br)c1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613507
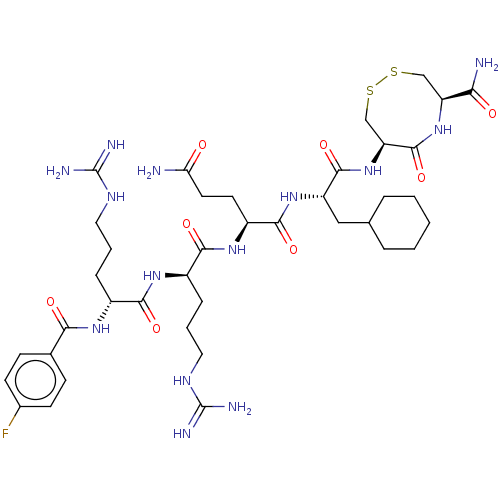
(CHEMBL5280357)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613503

(CHEMBL5267287)Show SMILES NC(=O)CC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50613509
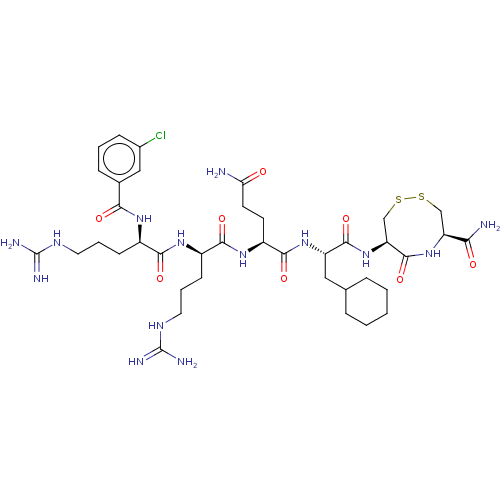
(CHEMBL5271178)Show SMILES NC(=O)CC[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)c1cccc(Cl)c1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H]1CSSC[C@H](NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data