

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma kallikrein (Homo sapiens (Human)) | BDBM218837 (TBMB-PK15 (10)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow | Assay Description The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... | Chembiochem 18: 387-395 (2017) Article DOI: 10.1002/cbic.201600612 BindingDB Entry DOI: 10.7270/Q2H9942B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM218839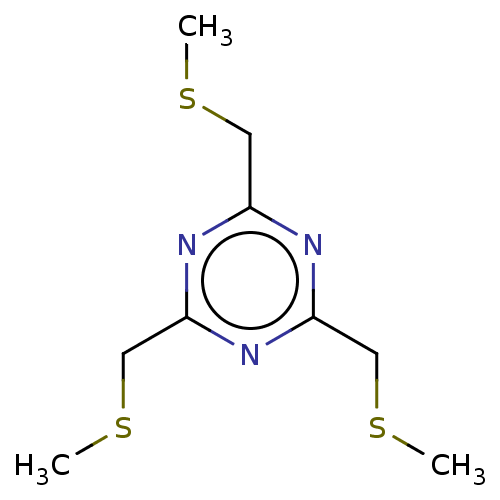 (TBMT-FX618 (16) | TBMT-PK15 (12)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow | Assay Description The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... | Chembiochem 18: 387-395 (2017) Article DOI: 10.1002/cbic.201600612 BindingDB Entry DOI: 10.7270/Q2H9942B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM218840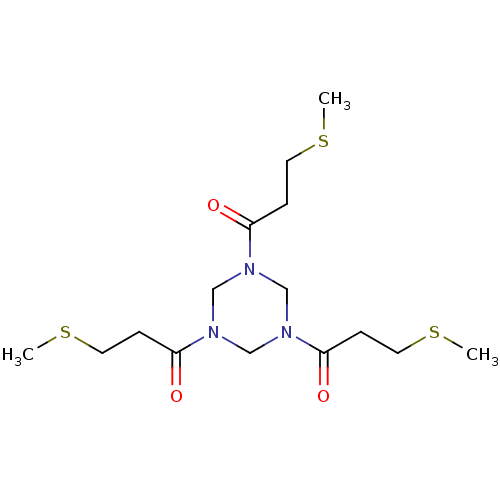 (TATA-FXII618 (14)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow | Assay Description The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... | Chembiochem 18: 387-395 (2017) Article DOI: 10.1002/cbic.201600612 BindingDB Entry DOI: 10.7270/Q2H9942B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM218838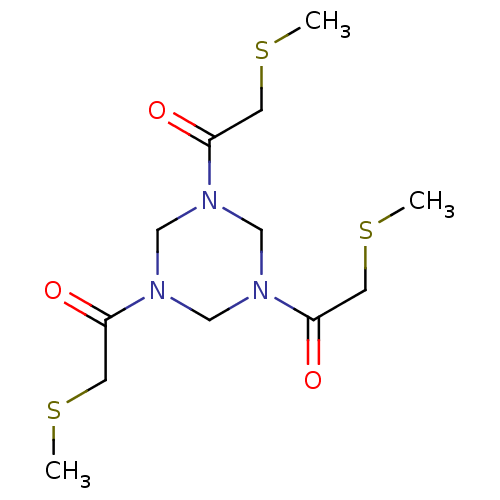 (TATB-FXII618 (15) | TATB-PK15 (11)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow | Assay Description The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... | Chembiochem 18: 387-395 (2017) Article DOI: 10.1002/cbic.201600612 BindingDB Entry DOI: 10.7270/Q2H9942B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM218838 (TATB-FXII618 (15) | TATB-PK15 (11)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow | Assay Description The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... | Chembiochem 18: 387-395 (2017) Article DOI: 10.1002/cbic.201600612 BindingDB Entry DOI: 10.7270/Q2H9942B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM218839 (TBMT-FX618 (16) | TBMT-PK15 (12)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow | Assay Description The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... | Chembiochem 18: 387-395 (2017) Article DOI: 10.1002/cbic.201600612 BindingDB Entry DOI: 10.7270/Q2H9942B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (RAT) | BDBM50318385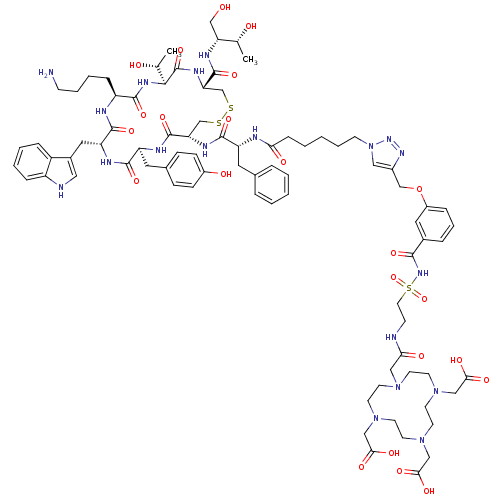 (2-(4-{[(2-{[(3-{[1-(5-{[(1R)-1-{[(4R,7S,10S,13R,16...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [111In-DOTA0,Tyr3]octreotide from SSTR2 receptor in rat AR42J cells | J Med Chem 53: 3944-53 (2010) Article DOI: 10.1021/jm100246m BindingDB Entry DOI: 10.7270/Q2PG1RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (RAT) | BDBM50318388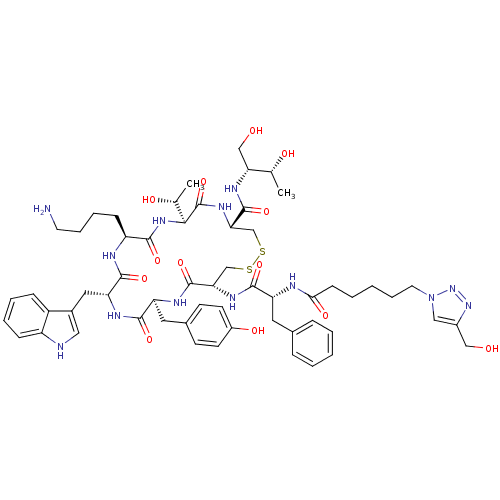 ((4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-N-[(2R,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [111In-DOTA0,Tyr3]octreotide from SSTR2 receptor in rat AR42J cells | J Med Chem 53: 3944-53 (2010) Article DOI: 10.1021/jm100246m BindingDB Entry DOI: 10.7270/Q2PG1RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (RAT) | BDBM50318386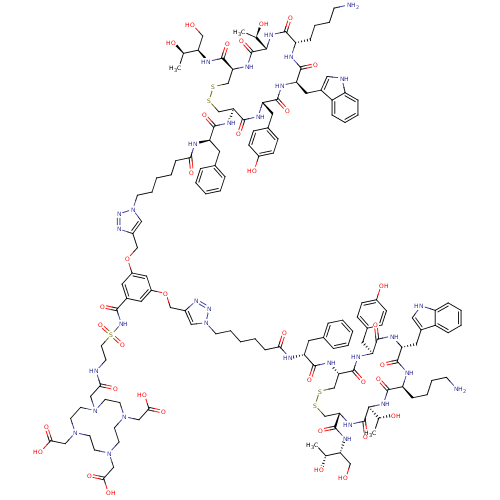 (2-[7-({[2-({[3,5-bis({[1-(5-{[(1R)-1-{[(4R,7S,10S,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [111In-DOTA0,Tyr3]octreotide from SSTR2 receptor in rat AR42J cells | J Med Chem 53: 3944-53 (2010) Article DOI: 10.1021/jm100246m BindingDB Entry DOI: 10.7270/Q2PG1RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50410236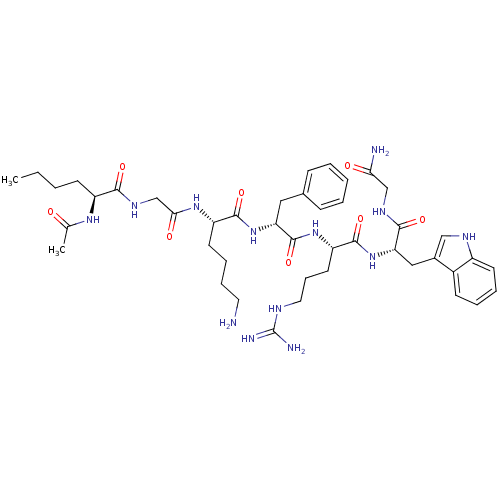 (CHEMBL365913) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human melanocortin receptor-4 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50174609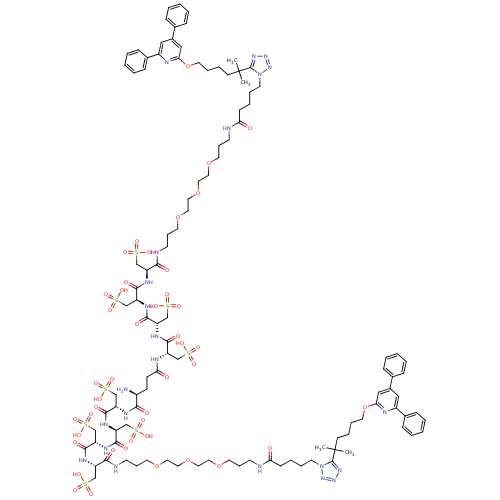 ((LTB4-(Csa)4)2-Glu-H Conjugate | CHEMBL412510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Medical Imaging Curated by ChEMBL | Assay Description Inhibitory concentration against human leukotriene B4 receptor using competing agent [3H]-LTB4 as radioligand in pH 7.2 buffer, for 1 h at 37 degree ... | J Med Chem 48: 6442-53 (2005) Article DOI: 10.1021/jm050383h BindingDB Entry DOI: 10.7270/Q2959H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50426994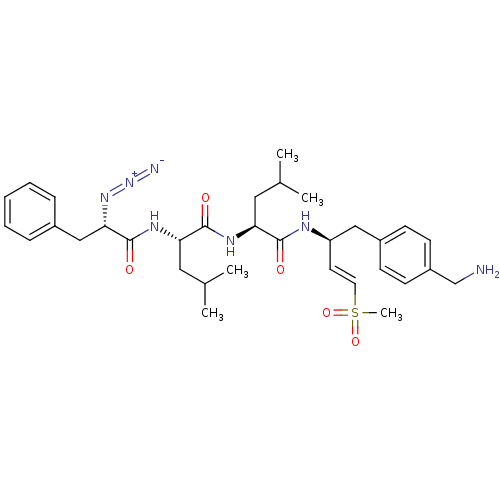 (CHEMBL2326533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of human 20S constitutive proteasome beta-2 using Bz-VGR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 61: 5395-5411 (2018) Article DOI: 10.1021/acs.jmedchem.8b00685 BindingDB Entry DOI: 10.7270/Q29K4DRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (RAT) | BDBM50318387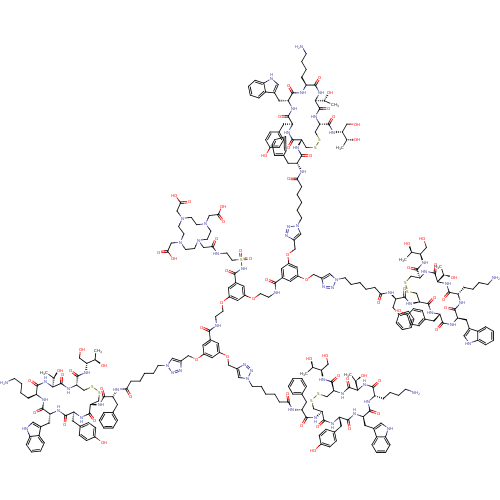 (CHEMBL1099306) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [111In-DOTA0,Tyr3]octreotide from SSTR2 receptor in rat AR42J cells | J Med Chem 53: 3944-53 (2010) Article DOI: 10.1021/jm100246m BindingDB Entry DOI: 10.7270/Q2PG1RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50410248 (CHEMBL186970) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human melanocortin receptor-4 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50174608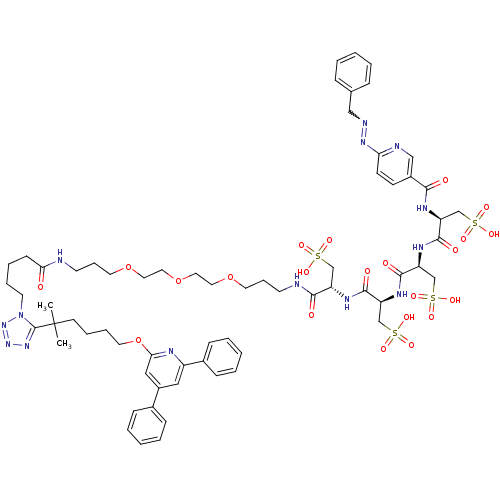 (CHEMBL406432 | HYNIC Conjugate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Medical Imaging Curated by ChEMBL | Assay Description Inhibitory concentration against human leukotriene B4 receptor using competing agent [111In]-(17)] as radioligand in pH 7.2 buffer, for 1 h at 37 deg... | J Med Chem 48: 6442-53 (2005) Article DOI: 10.1021/jm050383h BindingDB Entry DOI: 10.7270/Q2959H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50308672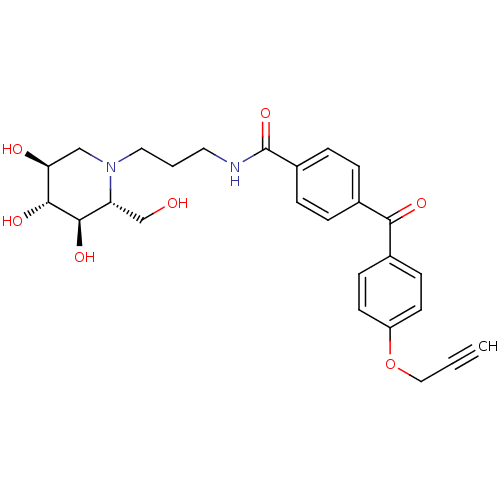 (CHEMBL590070 | N-(Propylamide-benzophenone)-1-deox...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferyl from non-lysosomal GBA2 by fluorometry | Bioorg Med Chem 18: 267-73 (2010) Article DOI: 10.1016/j.bmc.2009.10.060 BindingDB Entry DOI: 10.7270/Q2ZC82ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50174610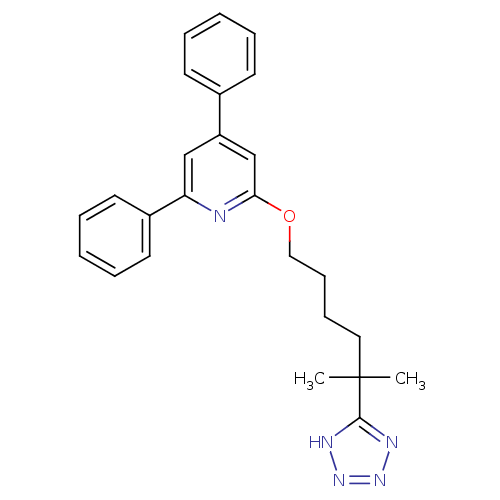 (2-[5-Methyl-5-(1H-tetrazol-5-yl)-hexyloxy]-4,6-dip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Medical Imaging Curated by ChEMBL | Assay Description Inhibitory concentration against human leukotriene B4 receptor using competing agent [3H]-LTB4 as radioligand in pH 7.2 buffer, for 1 h at 37 degree ... | J Med Chem 48: 6442-53 (2005) Article DOI: 10.1021/jm050383h BindingDB Entry DOI: 10.7270/Q2959H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50410249 (CHEMBL373344) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human melanocortin receptor-4 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-10 (Homo sapiens (Human)) | BDBM50426994 (CHEMBL2326533) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of human 20S immunoproteasome beta-2 using Bz-VGR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 61: 5395-5411 (2018) Article DOI: 10.1021/acs.jmedchem.8b00685 BindingDB Entry DOI: 10.7270/Q29K4DRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50174607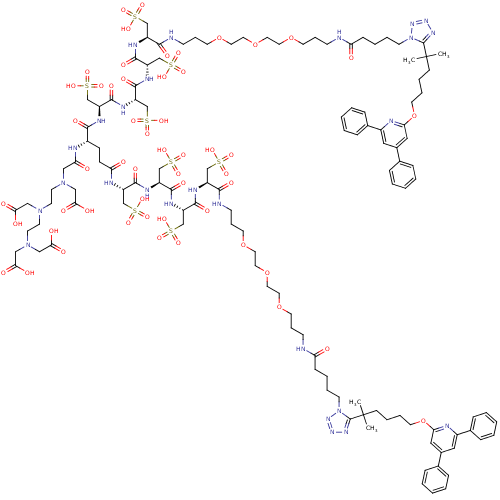 (CHEMBL415548 | DTPA Conjugate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Medical Imaging Curated by ChEMBL | Assay Description Inhibitory concentration against human leukotriene B4 receptor using competing agent [111In]-(17)] as radioligand in pH 7.2 buffer, for 1 h at 37 deg... | J Med Chem 48: 6442-53 (2005) Article DOI: 10.1021/jm050383h BindingDB Entry DOI: 10.7270/Q2959H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50174606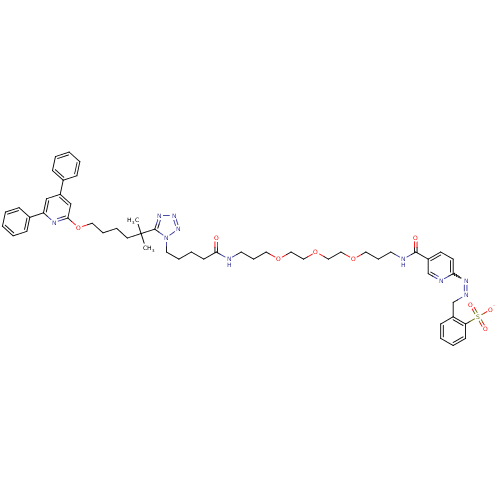 (CHEMBL384950 | HYNIC Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Medical Imaging Curated by ChEMBL | Assay Description Inhibitory concentration against human leukotriene B4 receptor using competing agent [111In]-(17)] as radioligand in pH 7.2 buffer, for 1 h at 37 deg... | J Med Chem 48: 6442-53 (2005) Article DOI: 10.1021/jm050383h BindingDB Entry DOI: 10.7270/Q2959H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50174606 (CHEMBL384950 | HYNIC Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Medical Imaging Curated by ChEMBL | Assay Description Inhibitory concentration against human leukotriene B4 receptor using competing agent [3H]-LTB4 as radioligand in pH 7.2 buffer upon incubation for 1 ... | J Med Chem 48: 6442-53 (2005) Article DOI: 10.1021/jm050383h BindingDB Entry DOI: 10.7270/Q2959H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50174607 (CHEMBL415548 | DTPA Conjugate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Medical Imaging Curated by ChEMBL | Assay Description Inhibitory concentration against human leukotriene B4 receptor using competing agent [3H]-LTB4 as radioligand in pH 7.2 buffer, for 1 h at 37 degree ... | J Med Chem 48: 6442-53 (2005) Article DOI: 10.1021/jm050383h BindingDB Entry DOI: 10.7270/Q2959H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50410241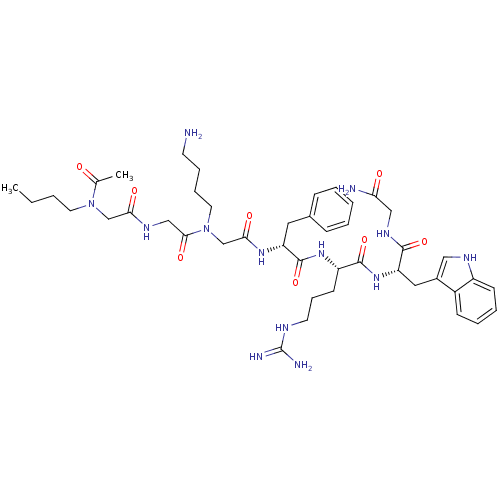 (CHEMBL188459) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human melanocortin receptor-4 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50308673 (CHEMBL589341 | N-(Propylamide-acetophenone)-1-deox...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferyl from non-lysosomal GBA2 by fluorometry | Bioorg Med Chem 18: 267-73 (2010) Article DOI: 10.1016/j.bmc.2009.10.060 BindingDB Entry DOI: 10.7270/Q2ZC82ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50410252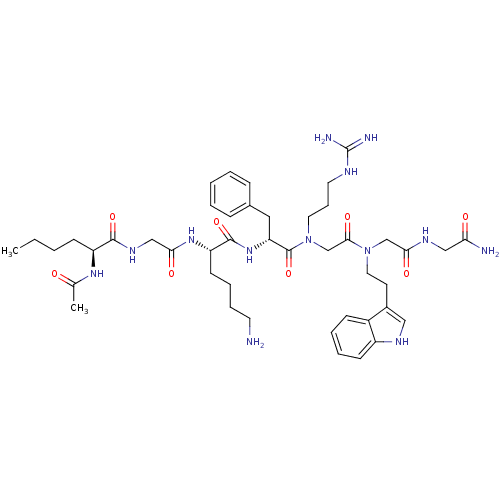 (CHEMBL190551) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human melanocortin receptor-4 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50229129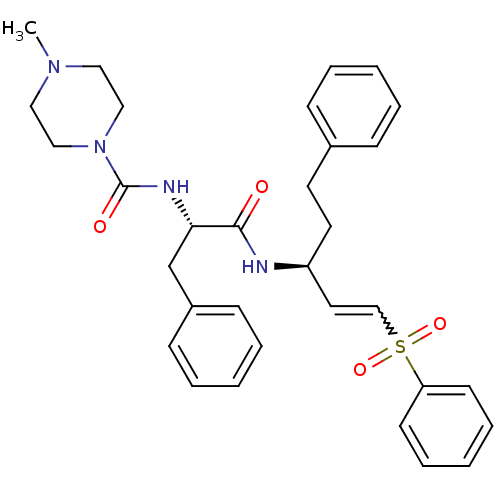 (4-Methyl-piperazine-1-carboxylic acid [(S)-1-((E)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50366880 (CHEMBL1793842) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Binding affinity for Syk tandem SH2 domain in surface plasmon resonance assay (SPR) | Bioorg Med Chem Lett 13: 1241-4 (2003) BindingDB Entry DOI: 10.7270/Q2XD127G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546078 (CHEMBL4750628) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50274010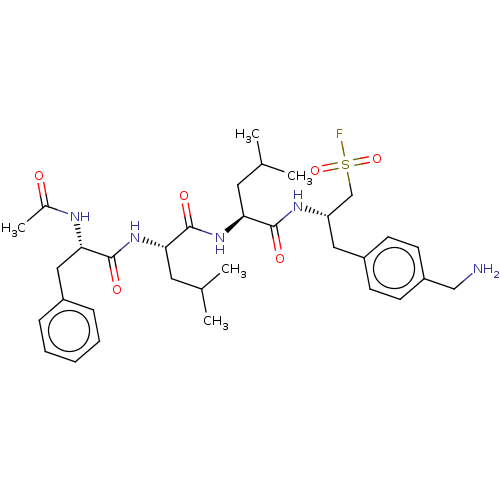 (CHEMBL4126411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of human 20S constitutive proteasome beta-2 using Bz-VGR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 61: 5395-5411 (2018) Article DOI: 10.1021/acs.jmedchem.8b00685 BindingDB Entry DOI: 10.7270/Q29K4DRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546076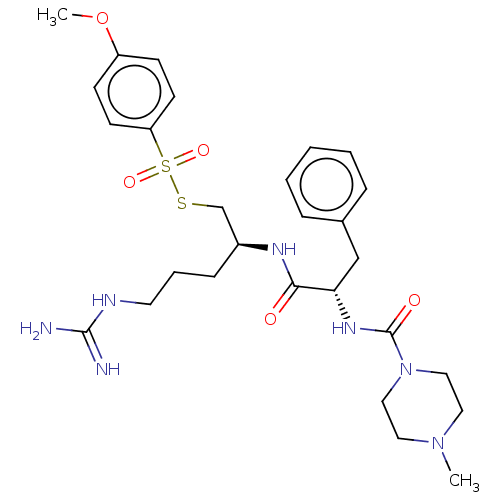 (CHEMBL4744708) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50410249 (CHEMBL373344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Effective concentration against human melanocortin receptor-3 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546077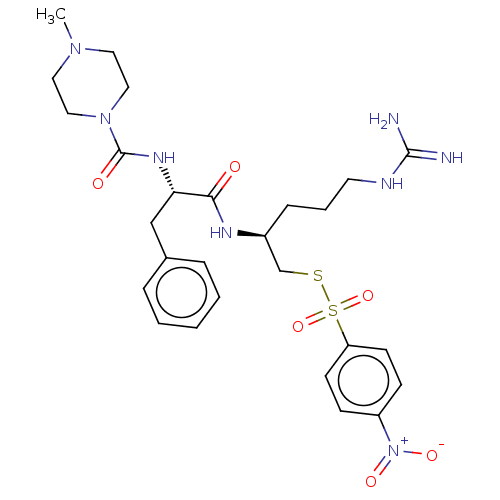 (CHEMBL4742162) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50274036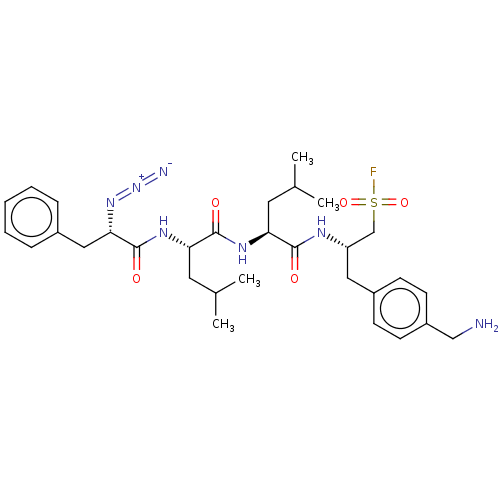 (CHEMBL4128478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of human 20S constitutive proteasome beta-2 using Bz-VGR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 61: 5395-5411 (2018) Article DOI: 10.1021/acs.jmedchem.8b00685 BindingDB Entry DOI: 10.7270/Q29K4DRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50366877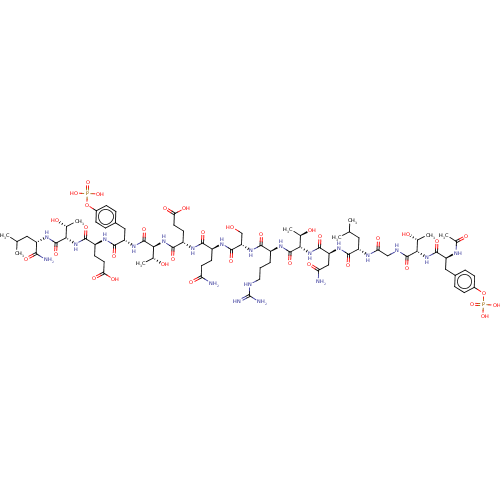 (CHEMBL2369726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Binding affinity for Syk tandem SH2 domain in surface plasmon resonance assay (SPR) | Bioorg Med Chem Lett 13: 1241-4 (2003) BindingDB Entry DOI: 10.7270/Q2XD127G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-10 (Homo sapiens (Human)) | BDBM50274036 (CHEMBL4128478) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of human 20S immunoproteasome beta-2 using Bz-VGR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 61: 5395-5411 (2018) Article DOI: 10.1021/acs.jmedchem.8b00685 BindingDB Entry DOI: 10.7270/Q29K4DRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50273999 (CHEMBL4129702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of human 20S constitutive proteasome beta-2 using Bz-VGR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 61: 5395-5411 (2018) Article DOI: 10.1021/acs.jmedchem.8b00685 BindingDB Entry DOI: 10.7270/Q29K4DRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546071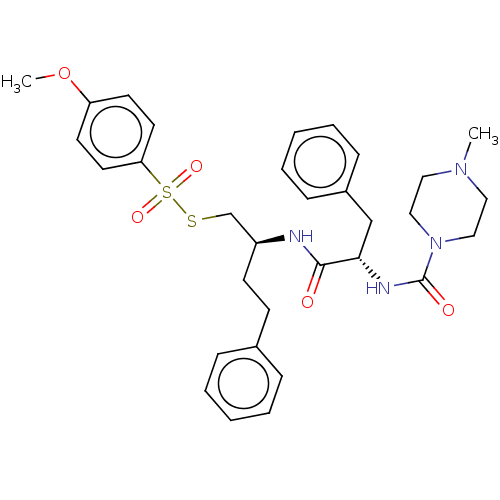 (CHEMBL4762654) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50274011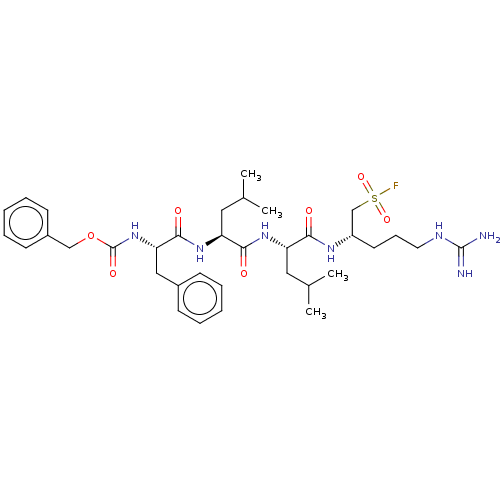 (CHEMBL4125911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of human 20S constitutive proteasome beta-2 using Bz-VGR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 61: 5395-5411 (2018) Article DOI: 10.1021/acs.jmedchem.8b00685 BindingDB Entry DOI: 10.7270/Q29K4DRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50410239 (CHEMBL190953) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human melanocortin receptor-4 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50410247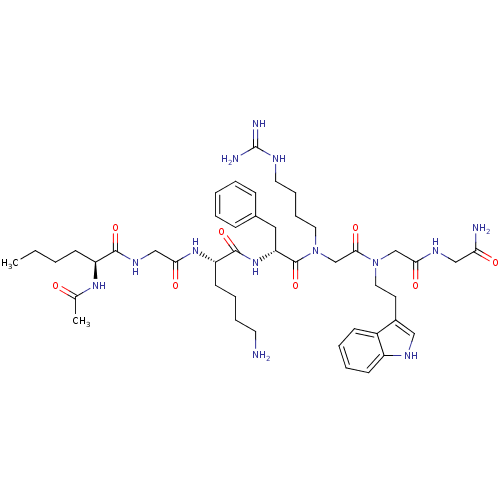 (CHEMBL190080) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human melanocortin receptor-4 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50410248 (CHEMBL186970) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Effective concentration against human melanocortin receptor-3 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50410251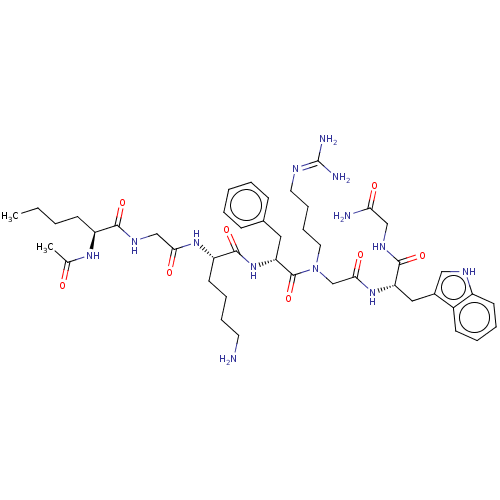 (CHEMBL2371835) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [125I]NDP-MSH from human melanocortin receptor-4 expressed in 293 HEK cells | J Med Chem 48: 4224-30 (2005) Article DOI: 10.1021/jm0490033 BindingDB Entry DOI: 10.7270/Q2QV3NQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546075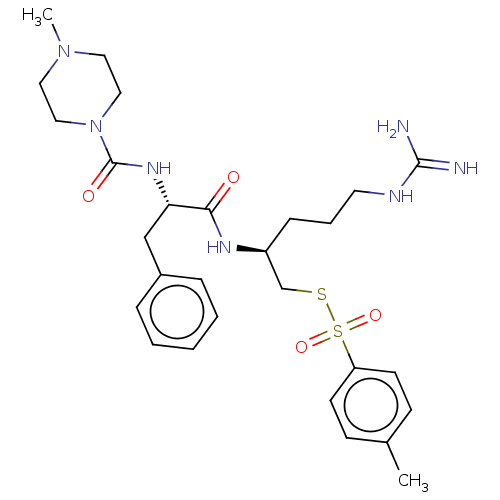 (CHEMBL4755488) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546074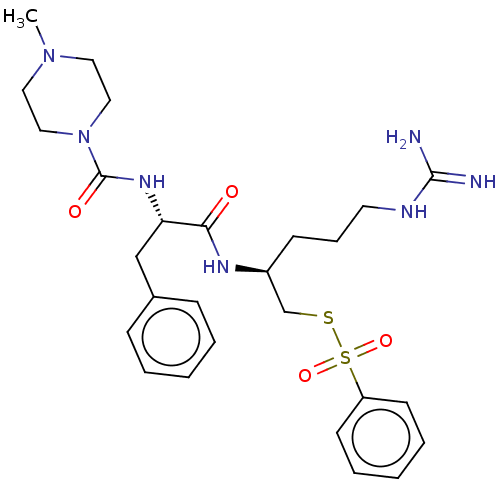 (CHEMBL4799021) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546073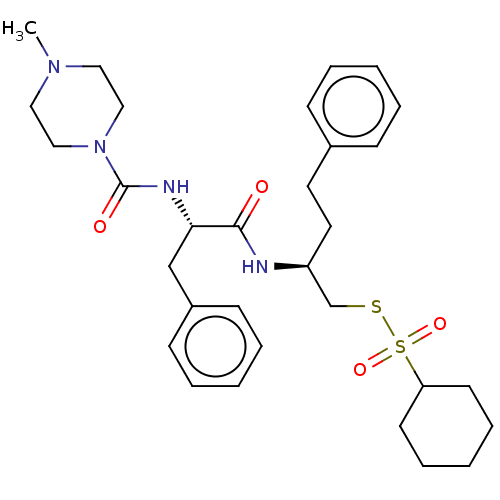 (CHEMBL4740177) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546070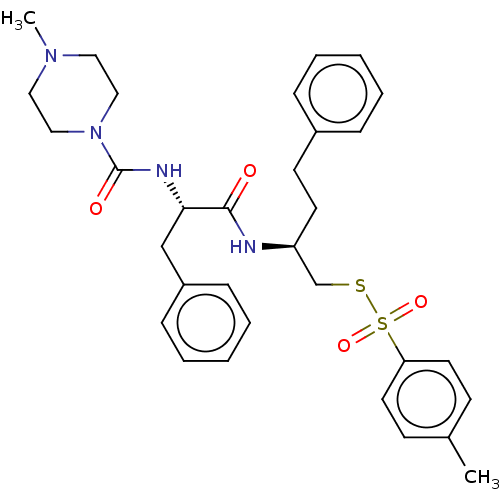 (CHEMBL4764681) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546072 (CHEMBL4794953) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50546069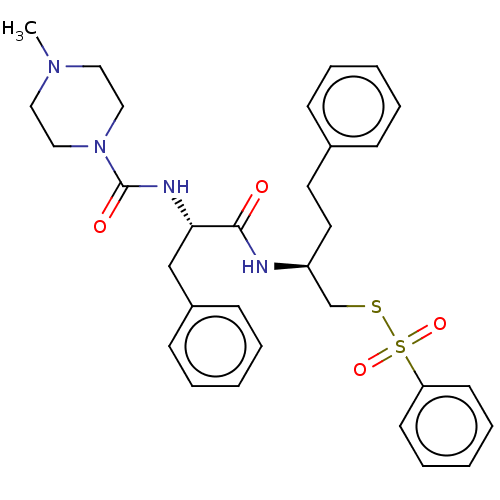 (CHEMBL4758732) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain assessed as reduction in p-nitoaniline release using Nalpha-benzoyl-L-arginine 4-nitroanilide hydrochloride as substrate ... | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.014 BindingDB Entry DOI: 10.7270/Q2DV1PGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-10 (Homo sapiens (Human)) | BDBM50274033 (CHEMBL4128898) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of human 20S immunoproteasome beta-2 using Bz-VGR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 61: 5395-5411 (2018) Article DOI: 10.1021/acs.jmedchem.8b00685 BindingDB Entry DOI: 10.7270/Q29K4DRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 225 total ) | Next | Last >> |