 Found 650 hits with Last Name = 'roy' and Initial = 'rs'
Found 650 hits with Last Name = 'roy' and Initial = 'rs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50371255

(CHEMBL1203953)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)[C@H]1Cc1ccc(F)cc1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H20F7N5O/c24-14-3-1-12(2-4-14)7-19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)20(36)10-15(31)8-13-9-17(26)18(27)11-16(13)25/h1-4,9,11,15,19H,5-8,10,31H2/t15-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232520

((2R)-4-oxo-4-[3-(trifluoromethyl)-8-[2-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1C(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C24H20F9N5O/c25-16-11-18(27)17(26)8-13(16)7-14(34)10-20(39)37-5-6-38-21(35-36-22(38)24(31,32)33)19(37)9-12-3-1-2-4-15(12)23(28,29)30/h1-4,8,11,14,19H,5-7,9-10,34H2/t14-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232520

((2R)-4-oxo-4-[3-(trifluoromethyl)-8-[2-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1C(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C24H20F9N5O/c25-16-11-18(27)17(26)8-13(16)7-14(34)10-20(39)37-5-6-38-21(35-36-22(38)24(31,32)33)19(37)9-12-3-1-2-4-15(12)23(28,29)30/h1-4,8,11,14,19H,5-7,9-10,34H2/t14-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232506
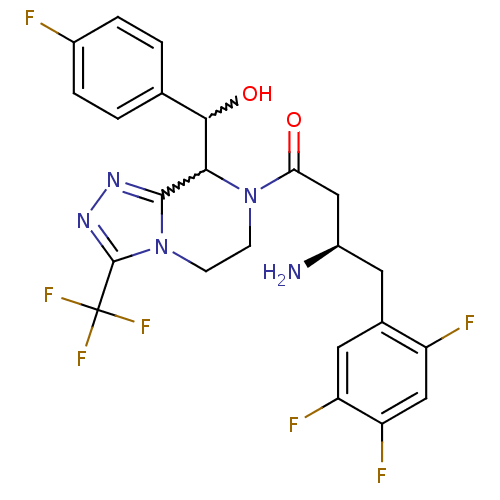
(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1C(O)c1ccc(F)cc1)Cc1cc(F)c(F)cc1F |w:17.17,18.20| Show InChI InChI=1S/C23H20F7N5O2/c24-13-3-1-11(2-4-13)20(37)19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)18(36)9-14(31)7-12-8-16(26)17(27)10-15(12)25/h1-4,8,10,14,19-20,37H,5-7,9,31H2/t14-,19?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232518
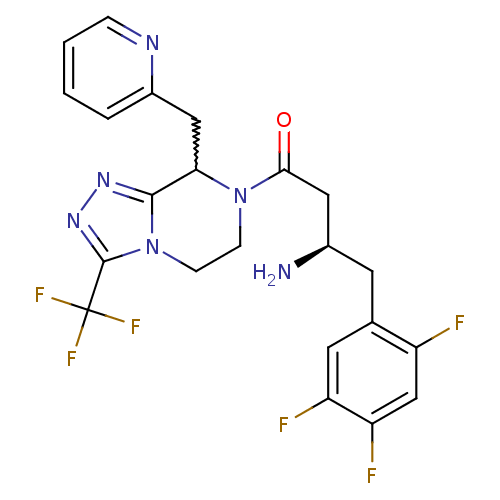
((2R)-4-oxo-4-[8-(pyridin-2-ylmethyl)-3-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccn1)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C22H20F6N6O/c23-15-11-17(25)16(24)8-12(15)7-13(29)9-19(35)33-5-6-34-20(31-32-21(34)22(26,27)28)18(33)10-14-3-1-2-4-30-14/h1-4,8,11,13,18H,5-7,9-10,29H2/t13-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232518

((2R)-4-oxo-4-[8-(pyridin-2-ylmethyl)-3-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccn1)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C22H20F6N6O/c23-15-11-17(25)16(24)8-12(15)7-13(29)9-19(35)33-5-6-34-20(31-32-21(34)22(26,27)28)18(33)10-14-3-1-2-4-30-14/h1-4,8,11,13,18H,5-7,9-10,29H2/t13-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232503
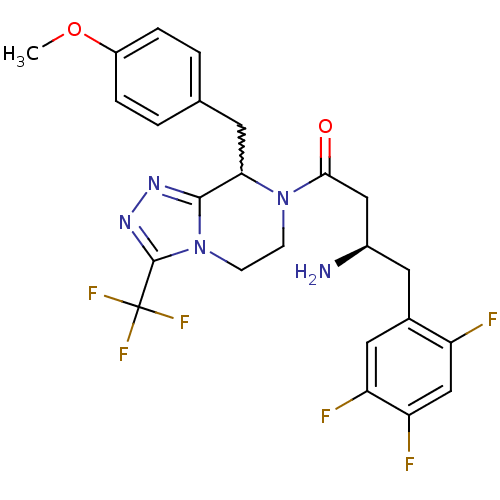
((2R)-4-[8-(4-methoxybenzyl)-3-(trifluoromethyl)-5,...)Show SMILES COc1ccc(CC2N(CCn3c2nnc3C(F)(F)F)C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1 |w:7.6| Show InChI InChI=1S/C24H23F6N5O2/c1-37-16-4-2-13(3-5-16)8-20-22-32-33-23(24(28,29)30)35(22)7-6-34(20)21(36)11-15(31)9-14-10-18(26)19(27)12-17(14)25/h2-5,10,12,15,20H,6-9,11,31H2,1H3/t15-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232503

((2R)-4-[8-(4-methoxybenzyl)-3-(trifluoromethyl)-5,...)Show SMILES COc1ccc(CC2N(CCn3c2nnc3C(F)(F)F)C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1 |w:7.6| Show InChI InChI=1S/C24H23F6N5O2/c1-37-16-4-2-13(3-5-16)8-20-22-32-33-23(24(28,29)30)35(22)7-6-34(20)21(36)11-15(31)9-14-10-18(26)19(27)12-17(14)25/h2-5,10,12,15,20H,6-9,11,31H2,1H3/t15-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232501

((2R)-4-[8-(2-fluorobenzyl)-3-(trifluoromethyl)-5,6...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C23H20F7N5O/c24-15-4-2-1-3-12(15)9-19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)20(36)10-14(31)7-13-8-17(26)18(27)11-16(13)25/h1-4,8,11,14,19H,5-7,9-10,31H2/t14-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232501

((2R)-4-[8-(2-fluorobenzyl)-3-(trifluoromethyl)-5,6...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C23H20F7N5O/c24-15-4-2-1-3-12(15)9-19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)20(36)10-14(31)7-13-8-17(26)18(27)11-16(13)25/h1-4,8,11,14,19H,5-7,9-10,31H2/t14-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232506

(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1C(O)c1ccc(F)cc1)Cc1cc(F)c(F)cc1F |w:17.17,18.20| Show InChI InChI=1S/C23H20F7N5O2/c24-13-3-1-11(2-4-13)20(37)19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)18(36)9-14(31)7-12-8-16(26)17(27)10-15(12)25/h1-4,8,10,14,19-20,37H,5-7,9,31H2/t14-,19?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232506

(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1C(O)c1ccc(F)cc1)Cc1cc(F)c(F)cc1F |w:17.17,18.20| Show InChI InChI=1S/C23H20F7N5O2/c24-13-3-1-11(2-4-13)20(37)19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)18(36)9-14(31)7-12-8-16(26)17(27)10-15(12)25/h1-4,8,10,14,19-20,37H,5-7,9,31H2/t14-,19?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232510

((2R)-4-[8-benzyl-3-(trifluoromethyl)-5,6-dihydro[1...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C23H21F6N5O/c24-16-12-18(26)17(25)10-14(16)9-15(30)11-20(35)33-6-7-34-21(31-32-22(34)23(27,28)29)19(33)8-13-4-2-1-3-5-13/h1-5,10,12,15,19H,6-9,11,30H2/t15-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232510

((2R)-4-[8-benzyl-3-(trifluoromethyl)-5,6-dihydro[1...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C23H21F6N5O/c24-16-12-18(26)17(25)10-14(16)9-15(30)11-20(35)33-6-7-34-21(31-32-22(34)23(27,28)29)19(33)8-13-4-2-1-3-5-13/h1-5,10,12,15,19H,6-9,11,30H2/t15-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328523
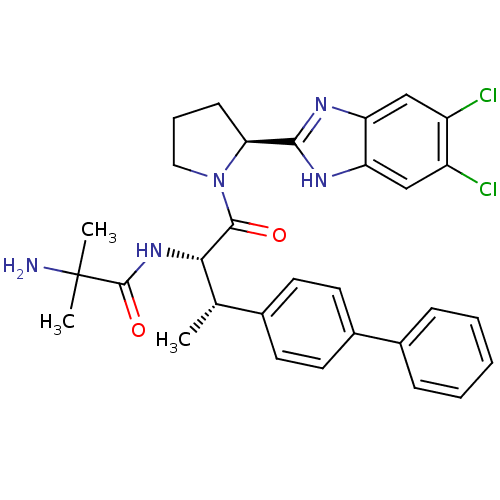
(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328523

(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50134192
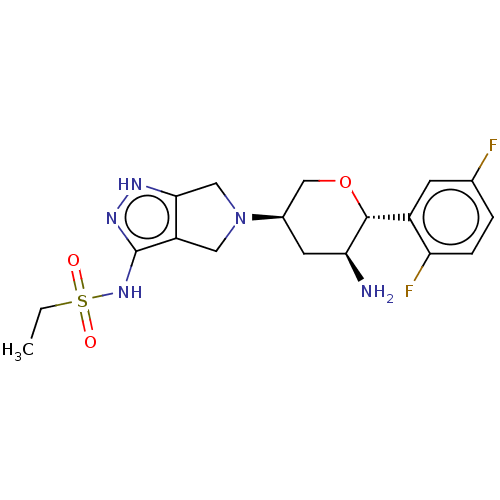
(CHEMBL3734828)Show SMILES CCS(=O)(=O)Nc1n[nH]c2CN(Cc12)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C18H23F2N5O3S/c1-2-29(26,27)24-18-13-7-25(8-16(13)22-23-18)11-6-15(21)17(28-9-11)12-5-10(19)3-4-14(12)20/h3-5,11,15,17H,2,6-9,21H2,1H3,(H2,22,23,24)/t11-,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis |
Bioorg Med Chem Lett 25: 5767-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.070
BindingDB Entry DOI: 10.7270/Q208675S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50134193
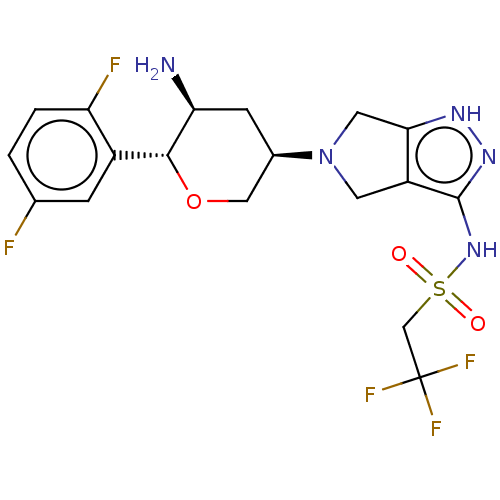
(CHEMBL3735461)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2[nH]nc(NS(=O)(=O)CC(F)(F)F)c2C1 |r| Show InChI InChI=1S/C18H20F5N5O3S/c19-9-1-2-13(20)11(3-9)16-14(24)4-10(7-31-16)28-5-12-15(6-28)25-26-17(12)27-32(29,30)8-18(21,22)23/h1-3,10,14,16H,4-8,24H2,(H2,25,26,27)/t10-,14+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis |
Bioorg Med Chem Lett 25: 5767-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.070
BindingDB Entry DOI: 10.7270/Q208675S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50134186
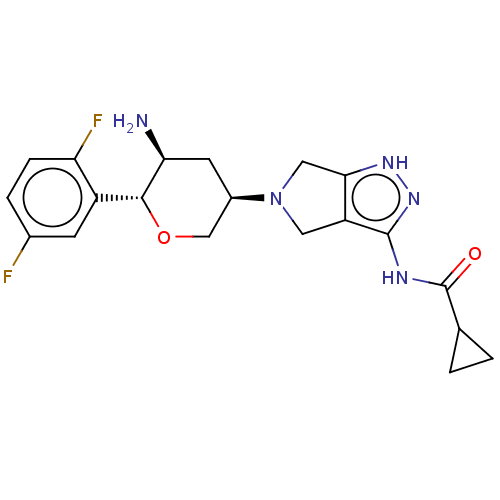
(CHEMBL3734964)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2[nH]nc(NC(=O)C3CC3)c2C1 |r| Show InChI InChI=1S/C20H23F2N5O2/c21-11-3-4-15(22)13(5-11)18-16(23)6-12(9-29-18)27-7-14-17(8-27)25-26-19(14)24-20(28)10-1-2-10/h3-5,10,12,16,18H,1-2,6-9,23H2,(H2,24,25,26,28)/t12-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis |
Bioorg Med Chem Lett 25: 5767-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.070
BindingDB Entry DOI: 10.7270/Q208675S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50134195
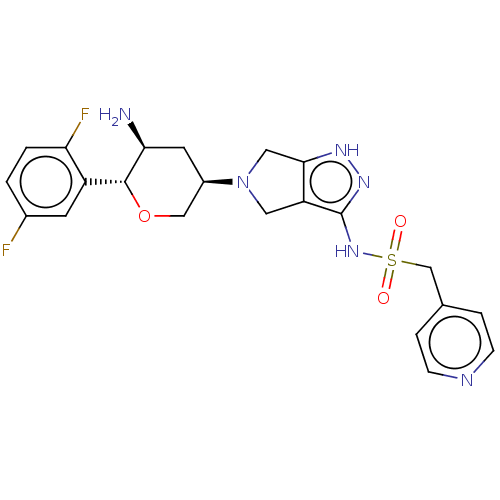
(CHEMBL3735433)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2[nH]nc(NS(=O)(=O)Cc3ccncc3)c2C1 |r| Show InChI InChI=1S/C22H24F2N6O3S/c23-14-1-2-18(24)16(7-14)21-19(25)8-15(11-33-21)30-9-17-20(10-30)27-28-22(17)29-34(31,32)12-13-3-5-26-6-4-13/h1-7,15,19,21H,8-12,25H2,(H2,27,28,29)/t15-,19+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis |
Bioorg Med Chem Lett 25: 5767-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.070
BindingDB Entry DOI: 10.7270/Q208675S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50134194
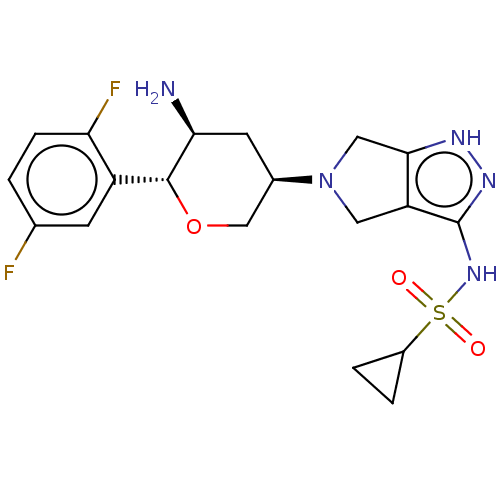
(CHEMBL3734999)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2[nH]nc(NS(=O)(=O)C3CC3)c2C1 |r| Show InChI InChI=1S/C19H23F2N5O3S/c20-10-1-4-15(21)13(5-10)18-16(22)6-11(9-29-18)26-7-14-17(8-26)23-24-19(14)25-30(27,28)12-2-3-12/h1,4-5,11-12,16,18H,2-3,6-9,22H2,(H2,23,24,25)/t11-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis |
Bioorg Med Chem Lett 25: 5767-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.070
BindingDB Entry DOI: 10.7270/Q208675S |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328527
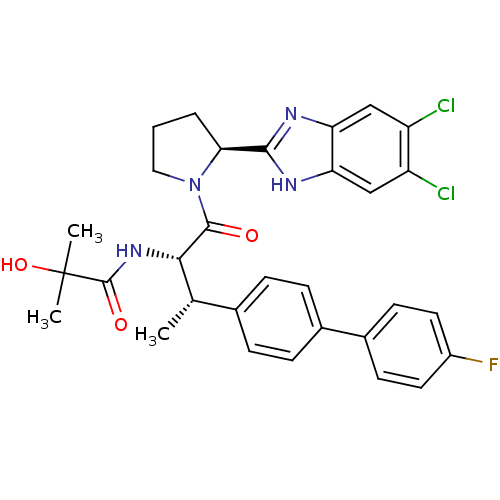
(CHEMBL1259208 | N-[(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H31Cl2FN4O3/c1-17(18-6-8-19(9-7-18)20-10-12-21(34)13-11-20)27(37-30(40)31(2,3)41)29(39)38-14-4-5-26(38)28-35-24-15-22(32)23(33)16-25(24)36-28/h6-13,15-17,26-27,41H,4-5,14H2,1-3H3,(H,35,36)(H,37,40)/t17-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328527

(CHEMBL1259208 | N-[(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H31Cl2FN4O3/c1-17(18-6-8-19(9-7-18)20-10-12-21(34)13-11-20)27(37-30(40)31(2,3)41)29(39)38-14-4-5-26(38)28-35-24-15-22(32)23(33)16-25(24)36-28/h6-13,15-17,26-27,41H,4-5,14H2,1-3H3,(H,35,36)(H,37,40)/t17-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328539

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5-ter...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)N |r| Show InChI InChI=1S/C30H39N5O2/c1-29(2,3)25-19-32-26(34-25)24-12-9-17-35(24)27(36)23(33-28(37)30(4,5)31)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24H,9,12,17-18,31H2,1-5H3,(H,32,34)(H,33,37)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328523

(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11151
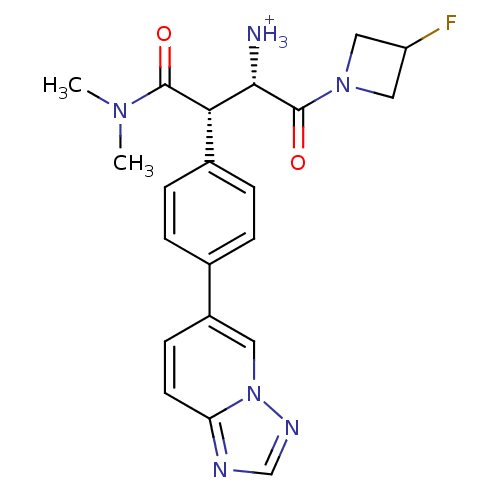
((1S,2S)-1-(dimethylcarbamoyl)-3-(3-fluoroazetidin-...)Show SMILES CN(C)C(=O)[C@H]([C@H]([NH3+])C(=O)N1CC(F)C1)c1ccc(cc1)-c1ccc2ncnn2c1 |r| Show InChI InChI=1S/C21H23FN6O2/c1-26(2)20(29)18(19(23)21(30)27-10-16(22)11-27)14-5-3-13(4-6-14)15-7-8-17-24-12-25-28(17)9-15/h3-9,12,16,18-19H,10-11,23H2,1-2H3/p+1/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 49: 3614-27 (2006)
Article DOI: 10.1021/jm060015t
BindingDB Entry DOI: 10.7270/Q2N87807 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232507
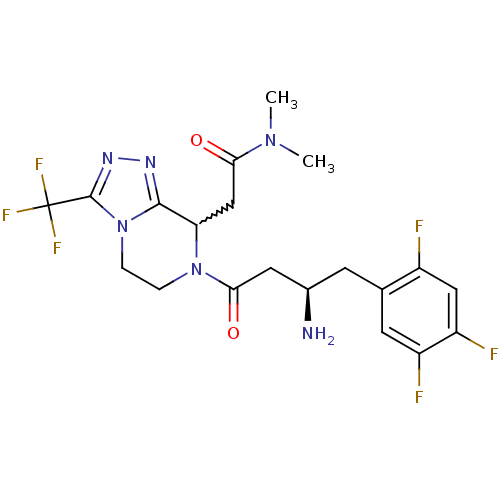
(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CN(C)C(=O)CC1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |w:6.5| Show InChI InChI=1S/C20H22F6N6O2/c1-30(2)16(33)9-15-18-28-29-19(20(24,25)26)32(18)4-3-31(15)17(34)7-11(27)5-10-6-13(22)14(23)8-12(10)21/h6,8,11,15H,3-5,7,9,27H2,1-2H3/t11-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232507

(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CN(C)C(=O)CC1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |w:6.5| Show InChI InChI=1S/C20H22F6N6O2/c1-30(2)16(33)9-15-18-28-29-19(20(24,25)26)32(18)4-3-31(15)17(34)7-11(27)5-10-6-13(22)14(23)8-12(10)21/h6,8,11,15H,3-5,7,9,27H2,1-2H3/t11-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328537

(3-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(CN)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-31(2,18-34)30(40)37-26(15-19-10-12-21(13-11-19)20-7-4-3-5-8-20)29(39)38-14-6-9-27(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h3-5,7-8,10-13,16-17,26-27H,6,9,14-15,18,34H2,1-2H3,(H,35,36)(H,37,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50134191
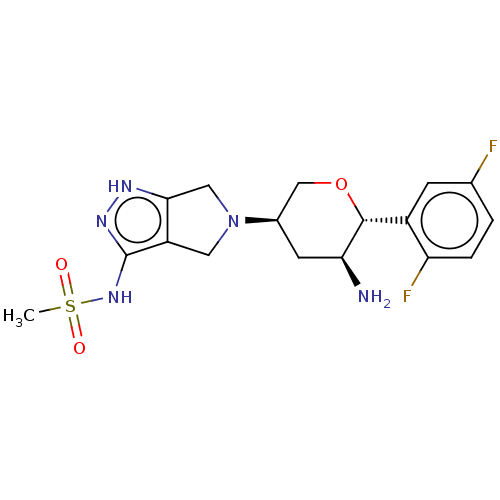
(CHEMBL3734769)Show SMILES CS(=O)(=O)Nc1n[nH]c2CN(Cc12)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C17H21F2N5O3S/c1-28(25,26)23-17-12-6-24(7-15(12)21-22-17)10-5-14(20)16(27-8-10)11-4-9(18)2-3-13(11)19/h2-4,10,14,16H,5-8,20H2,1H3,(H2,21,22,23)/t10-,14+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis |
Bioorg Med Chem Lett 25: 5767-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.070
BindingDB Entry DOI: 10.7270/Q208675S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50134190
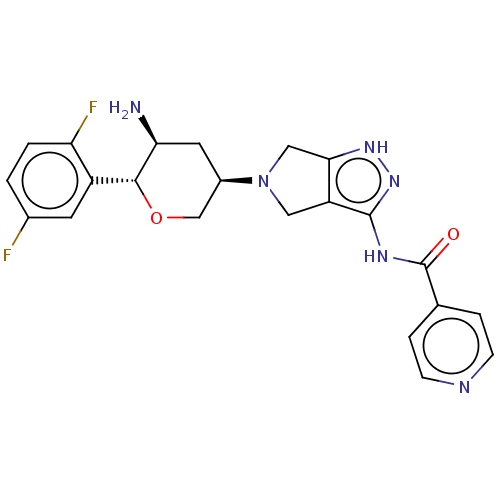
(CHEMBL3735925)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2[nH]nc(NC(=O)c3ccncc3)c2C1 |r| Show InChI InChI=1S/C22H22F2N6O2/c23-13-1-2-17(24)15(7-13)20-18(25)8-14(11-32-20)30-9-16-19(10-30)28-29-21(16)27-22(31)12-3-5-26-6-4-12/h1-7,14,18,20H,8-11,25H2,(H2,27,28,29,31)/t14-,18+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis |
Bioorg Med Chem Lett 25: 5767-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.070
BindingDB Entry DOI: 10.7270/Q208675S |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328534
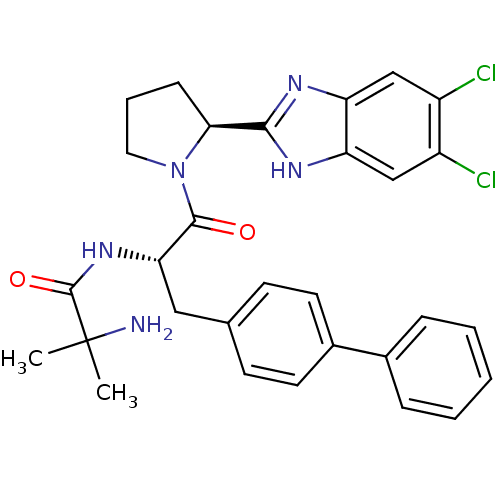
(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C30H31Cl2N5O2/c1-30(2,33)29(39)36-25(15-18-10-12-20(13-11-18)19-7-4-3-5-8-19)28(38)37-14-6-9-26(37)27-34-23-16-21(31)22(32)17-24(23)35-27/h3-5,7-8,10-13,16-17,25-26H,6,9,14-15,33H2,1-2H3,(H,34,35)(H,36,39)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328522

(CHEMBL1259179 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)O |r| Show InChI InChI=1S/C30H38N4O3/c1-29(2,3)25-19-31-26(33-25)24-12-9-17-34(24)27(35)23(32-28(36)30(4,5)37)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24,37H,9,12,17-18H2,1-5H3,(H,31,33)(H,32,36)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328522

(CHEMBL1259179 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)O |r| Show InChI InChI=1S/C30H38N4O3/c1-29(2,3)25-19-31-26(33-25)24-12-9-17-34(24)27(35)23(32-28(36)30(4,5)37)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24,37H,9,12,17-18H2,1-5H3,(H,31,33)(H,32,36)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11143

((1S,2S)-1-(dimethylcarbamoyl)-3-[(3S)-3-fluoropyrr...)Show SMILES CN(C)C(=O)[C@H]([C@H]([NH3+])C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc2nncn2c1 |r| Show InChI InChI=1S/C22H25FN6O2/c1-27(2)21(30)19(20(24)22(31)28-10-9-17(23)12-28)15-5-3-14(4-6-15)16-7-8-18-26-25-13-29(18)11-16/h3-8,11,13,17,19-20H,9-10,12,24H2,1-2H3/p+1/t17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 49: 3614-27 (2006)
Article DOI: 10.1021/jm060015t
BindingDB Entry DOI: 10.7270/Q2N87807 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50296488

(CHEMBL559940 | Trifluoro-acetate(2R,3R)-7-methanes...)Show SMILES CS(=O)(=O)c1ccc2n3C[C@H]([NH3+])[C@H](Cc3nc2c1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C18H16F3N3O2S/c1-27(25,26)9-2-3-17-16(4-9)23-18-6-11(15(22)8-24(17)18)10-5-13(20)14(21)7-12(10)19/h2-5,7,11,15H,6,8,22H2,1H3/p+1/t11-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 19: 4097-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.011
BindingDB Entry DOI: 10.7270/Q2513Z7C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11144
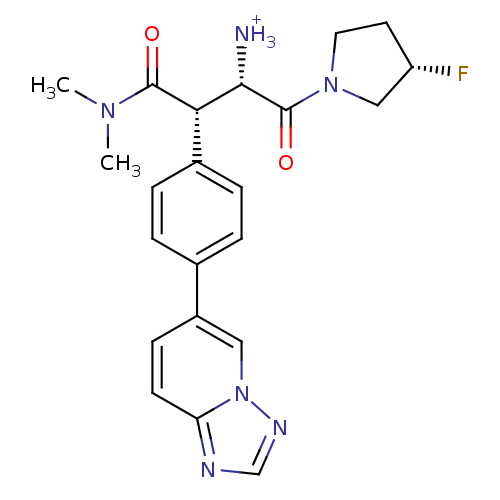
((1S,2S)-1-(dimethylcarbamoyl)-3-[(3S)-3-fluoropyrr...)Show SMILES CN(C)C(=O)[C@H]([C@H]([NH3+])C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc2ncnn2c1 |r| Show InChI InChI=1S/C22H25FN6O2/c1-27(2)21(30)19(20(24)22(31)28-10-9-17(23)12-28)15-5-3-14(4-6-15)16-7-8-18-25-13-26-29(18)11-16/h3-8,11,13,17,19-20H,9-10,12,24H2,1-2H3/p+1/t17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 49: 3614-27 (2006)
Article DOI: 10.1021/jm060015t
BindingDB Entry DOI: 10.7270/Q2N87807 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232502
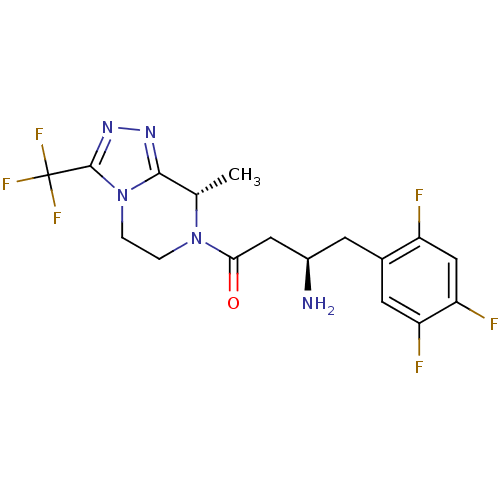
((2R)-4-[(8S)-8-methyl-3-(trifluoromethyl)-5,6-dihy...)Show SMILES C[C@@H]1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C17H17F6N5O/c1-8-15-25-26-16(17(21,22)23)28(15)3-2-27(8)14(29)6-10(24)4-9-5-12(19)13(20)7-11(9)18/h5,7-8,10H,2-4,6,24H2,1H3/t8-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50134189
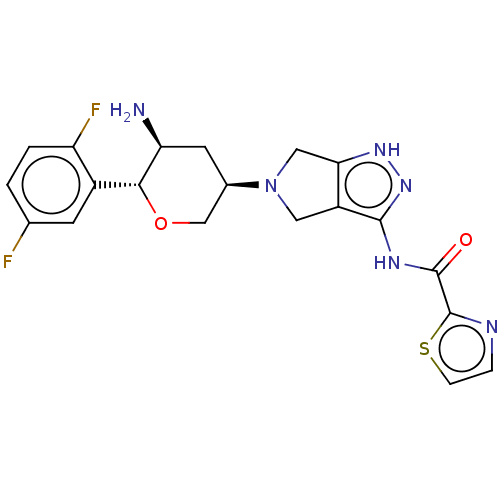
(CHEMBL3735129)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2[nH]nc(NC(=O)c3nccs3)c2C1 |r| Show InChI InChI=1S/C20H20F2N6O2S/c21-10-1-2-14(22)12(5-10)17-15(23)6-11(9-30-17)28-7-13-16(8-28)26-27-18(13)25-19(29)20-24-3-4-31-20/h1-5,11,15,17H,6-9,23H2,(H2,25,26,27,29)/t11-,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis |
Bioorg Med Chem Lett 25: 5767-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.070
BindingDB Entry DOI: 10.7270/Q208675S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232511

((2R)-4-[8-ethyl-3-(trifluoromethyl)-5,6-dihydro[1,...)Show SMILES CCC1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |w:2.1| Show InChI InChI=1S/C18H19F6N5O/c1-2-14-16-26-27-17(18(22,23)24)29(16)4-3-28(14)15(30)7-10(25)5-9-6-12(20)13(21)8-11(9)19/h6,8,10,14H,2-5,7,25H2,1H3/t10-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232511

((2R)-4-[8-ethyl-3-(trifluoromethyl)-5,6-dihydro[1,...)Show SMILES CCC1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |w:2.1| Show InChI InChI=1S/C18H19F6N5O/c1-2-14-16-26-27-17(18(22,23)24)29(16)4-3-28(14)15(30)7-10(25)5-9-6-12(20)13(21)8-11(9)19/h6,8,10,14H,2-5,7,25H2,1H3/t10-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328538

(3-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)CC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-31(2,34)18-28(39)35-26(15-19-10-12-21(13-11-19)20-7-4-3-5-8-20)30(40)38-14-6-9-27(38)29-36-24-16-22(32)23(33)17-25(24)37-29/h3-5,7-8,10-13,16-17,26-27H,6,9,14-15,18,34H2,1-2H3,(H,35,39)(H,36,37)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328528
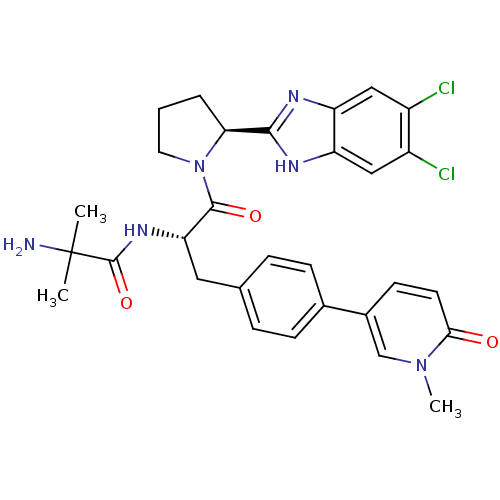
(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-4-(1-methyl...)Show SMILES Cn1cc(ccc1=O)-c1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C30H32Cl2N6O3/c1-30(2,33)29(41)36-24(13-17-6-8-18(9-7-17)19-10-11-26(39)37(3)16-19)28(40)38-12-4-5-25(38)27-34-22-14-20(31)21(32)15-23(22)35-27/h6-11,14-16,24-25H,4-5,12-13,33H2,1-3H3,(H,34,35)(H,36,41)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328524

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-(betaS)-bet...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H29Cl2N5O2/c1-14(15-8-5-4-6-9-15)21(31-24(34)25(2,3)28)23(33)32-11-7-10-20(32)22-29-18-12-16(26)17(27)13-19(18)30-22/h4-6,8-9,12-14,20-21H,7,10-11,28H2,1-3H3,(H,29,30)(H,31,34)/t14-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11152
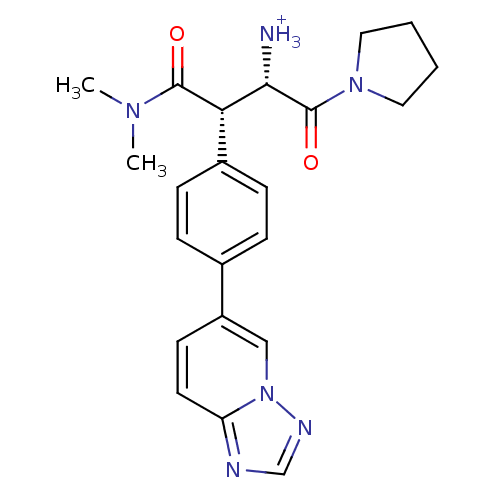
((1S,2S)-1-(dimethylcarbamoyl)-3-oxo-3-(pyrrolidin-...)Show SMILES CN(C)C(=O)[C@H]([C@H]([NH3+])C(=O)N1CCCC1)c1ccc(cc1)-c1ccc2ncnn2c1 |r| Show InChI InChI=1S/C22H26N6O2/c1-26(2)21(29)19(20(23)22(30)27-11-3-4-12-27)16-7-5-15(6-8-16)17-9-10-18-24-14-25-28(18)13-17/h5-10,13-14,19-20H,3-4,11-12,23H2,1-2H3/p+1/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 49: 3614-27 (2006)
Article DOI: 10.1021/jm060015t
BindingDB Entry DOI: 10.7270/Q2N87807 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11145

((1S,2S)-1-(dimethylcarbamoyl)-3-[(3S)-3-fluoropyrr...)Show SMILES CN(C)C(=O)[C@H]([C@H]([NH3+])C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccn2ncnc2c1 |r| Show InChI InChI=1S/C22H25FN6O2/c1-27(2)21(30)19(20(24)22(31)28-9-8-17(23)12-28)15-5-3-14(4-6-15)16-7-10-29-18(11-16)25-13-26-29/h3-7,10-11,13,17,19-20H,8-9,12,24H2,1-2H3/p+1/t17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 49: 3614-27 (2006)
Article DOI: 10.1021/jm060015t
BindingDB Entry DOI: 10.7270/Q2N87807 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232508
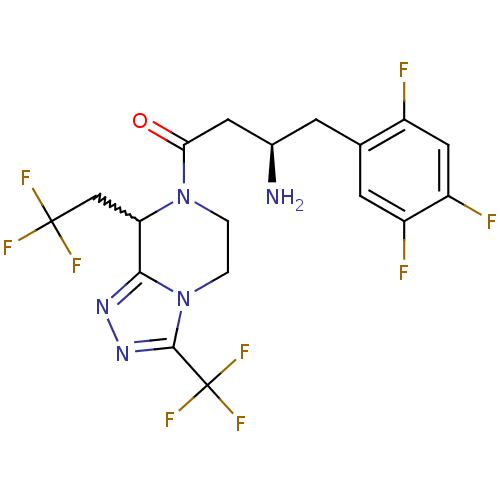
((2R)-4-[8-(2,2,2-trifluoroethyl)-3-(trifluoromethy...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1CC(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C18H16F9N5O/c19-10-6-12(21)11(20)4-8(10)3-9(28)5-14(33)31-1-2-32-15(13(31)7-17(22,23)24)29-30-16(32)18(25,26)27/h4,6,9,13H,1-3,5,7,28H2/t9-,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232508

((2R)-4-[8-(2,2,2-trifluoroethyl)-3-(trifluoromethy...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1CC(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C18H16F9N5O/c19-10-6-12(21)11(20)4-8(10)3-9(28)5-14(33)31-1-2-32-15(13(31)7-17(22,23)24)29-30-16(32)18(25,26)27/h4,6,9,13H,1-3,5,7,28H2/t9-,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11154

((2S,3S)-1-(3,3-difluoropyrrolidin-1-yl)-3-(dimethy...)Show SMILES CN(C)C(=O)[C@H]([C@H]([NH3+])C(=O)N1CCC(F)(F)C1)c1ccc(cc1)-c1ccn2ncnc2c1 |r| Show InChI InChI=1S/C22H24F2N6O2/c1-28(2)20(31)18(19(25)21(32)29-10-8-22(23,24)12-29)15-5-3-14(4-6-15)16-7-9-30-17(11-16)26-13-27-30/h3-7,9,11,13,18-19H,8,10,12,25H2,1-2H3/p+1/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 49: 3614-27 (2006)
Article DOI: 10.1021/jm060015t
BindingDB Entry DOI: 10.7270/Q2N87807 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11153
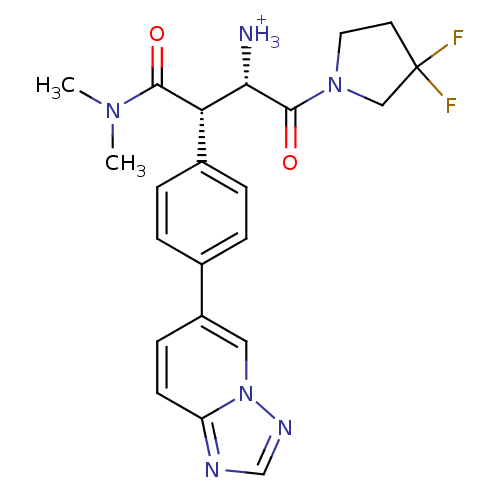
((2S,3S)-1-(3,3-difluoropyrrolidin-1-yl)-3-(dimethy...)Show SMILES CN(C)C(=O)[C@H]([C@H]([NH3+])C(=O)N1CCC(F)(F)C1)c1ccc(cc1)-c1ccc2ncnn2c1 |r| Show InChI InChI=1S/C22H24F2N6O2/c1-28(2)20(31)18(19(25)21(32)29-10-9-22(23,24)12-29)15-5-3-14(4-6-15)16-7-8-17-26-13-27-30(17)11-16/h3-8,11,13,18-19H,9-10,12,25H2,1-2H3/p+1/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 49: 3614-27 (2006)
Article DOI: 10.1021/jm060015t
BindingDB Entry DOI: 10.7270/Q2N87807 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data