 Found 329 hits with Last Name = 'arnold' and Initial = 's'
Found 329 hits with Last Name = 'arnold' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50601872

(CHEMBL5174733)Show SMILES [11CH3]Oc1ccc2c(NC(=O)Nc3cccc(c3)C(F)(F)F)ccnc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072352
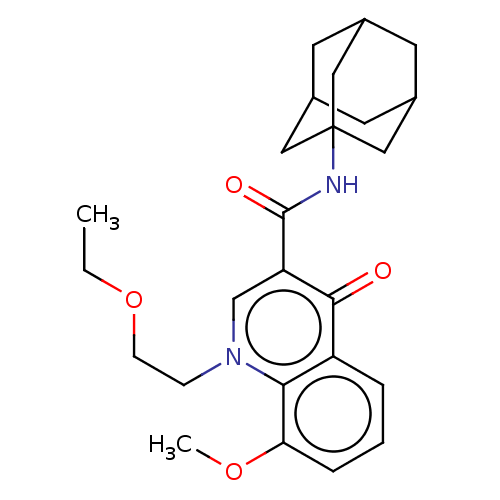
(CHEMBL3409318)Show SMILES CCOCCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c(=O)c2cccc(OC)c12 |TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C25H32N2O4/c1-3-31-8-7-27-15-20(23(28)19-5-4-6-21(30-2)22(19)27)24(29)26-25-12-16-9-17(13-25)11-18(10-16)14-25/h4-6,15-18H,3,7-14H2,1-2H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50601864

(CHEMBL5171821) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50601855
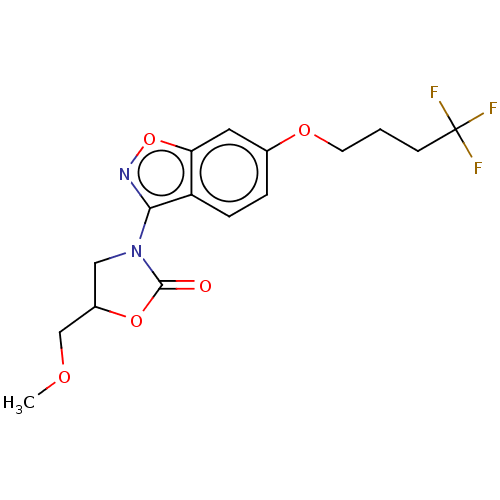
(CHEMBL5189391)Show SMILES COCC1CN(c2noc3cc(OCCCC(F)(F)F)ccc23)[11C](=O)O1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50601854

(CHEMBL4117628)Show SMILES COC(=O)c1c(NC(=O)C23CC4CC(CC(O)(C4)C2)C3)sc2CCCCc12 |TLB:16:15:12:19.9.10,7:9:12:17.14.15,THB:14:13:10:17.15.18,14:15:12.13.19:10,18:15:12:19.9.10,18:9:12:17.14.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50506752
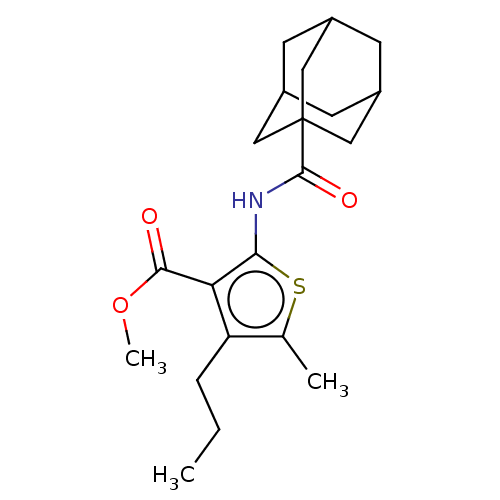
(CHEMBL4441693)Show SMILES CCCc1c(C)sc(NC(=O)C23CC4CC(CC(C4)C2)C3)c1C(=O)OC |TLB:9:11:14.13.18:16,THB:9:11:14:18.17.16,12:13:16:20.11.19,12:11:14.13.18:16,19:11:14:18.17.16,19:17:14:20.12.11| Show InChI InChI=1S/C21H29NO3S/c1-4-5-16-12(2)26-18(17(16)19(23)25-3)22-20(24)21-9-13-6-14(10-21)8-15(7-13)11-21/h13-15H,4-11H2,1-3H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50397745
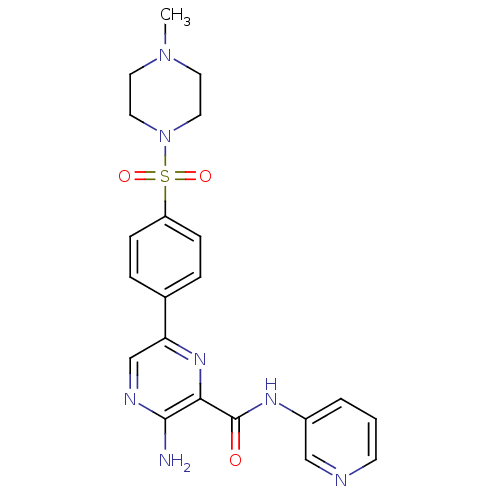
(CHEMBL2177161)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(cc1)-c1cnc(N)c(n1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C21H23N7O3S/c1-27-9-11-28(12-10-27)32(30,31)17-6-4-15(5-7-17)18-14-24-20(22)19(26-18)21(29)25-16-3-2-8-23-13-16/h2-8,13-14H,9-12H2,1H3,(H2,22,24)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50601865

(CHEMBL5180946)Show SMILES CCOc1cc2ccc(C(N)=O)c(Nc3ccc(F)cc3F)c2cc1N1CCN([11CH3])CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM8296
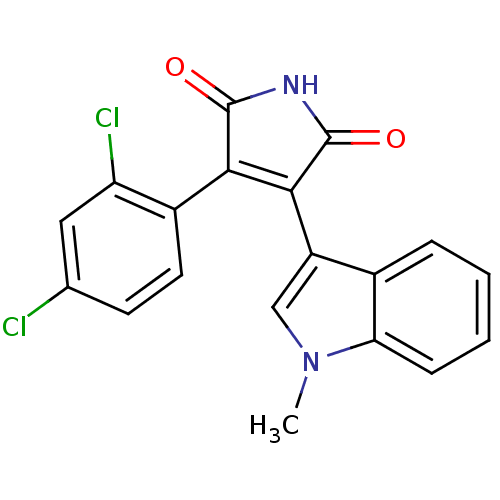
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50312837
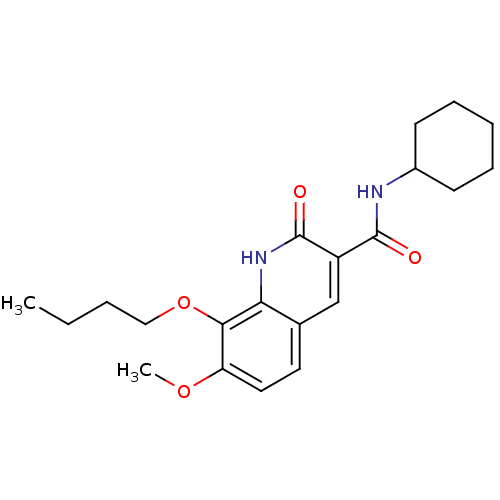
(CHEMBL1081610 | [11C]8-butoxy-N-cyclohexyl-7-metho...)Show SMILES CCCCOc1c(OC)ccc2cc(C(=O)NC3CCCCC3)c(=O)[nH]c12 Show InChI InChI=1S/C21H28N2O4/c1-3-4-12-27-19-17(26-2)11-10-14-13-16(21(25)23-18(14)19)20(24)22-15-8-6-5-7-9-15/h10-11,13,15H,3-9,12H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50249570
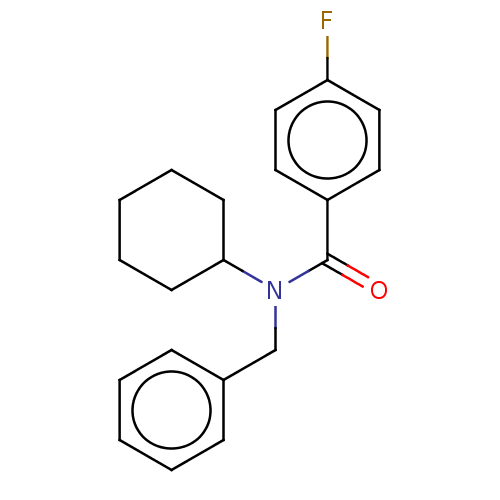
(CHEMBL4066660)Show InChI InChI=1S/C20H22FNO/c21-18-13-11-17(12-14-18)20(23)22(19-9-5-2-6-10-19)15-16-7-3-1-4-8-16/h1,3-4,7-8,11-14,19H,2,5-6,9-10,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50285227
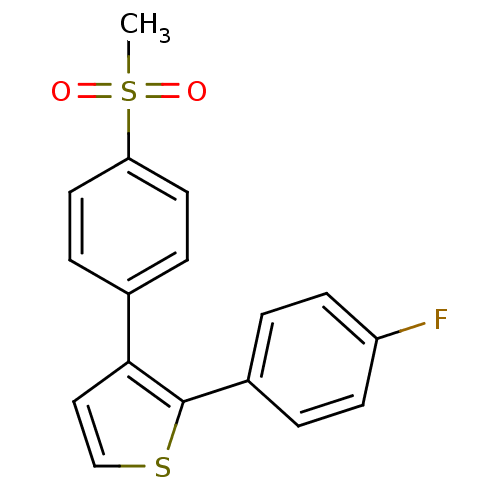
(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-t...)Show InChI InChI=1S/C17H13FO2S2/c1-22(19,20)15-8-4-12(5-9-15)16-10-11-21-17(16)13-2-6-14(18)7-3-13/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Rattus norvegicus (rat)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50601854

(CHEMBL4117628)Show SMILES COC(=O)c1c(NC(=O)C23CC4CC(CC(O)(C4)C2)C3)sc2CCCCc12 |TLB:16:15:12:19.9.10,7:9:12:17.14.15,THB:14:13:10:17.15.18,14:15:12.13.19:10,18:15:12:19.9.10,18:9:12:17.14.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50506752

(CHEMBL4441693)Show SMILES CCCc1c(C)sc(NC(=O)C23CC4CC(CC(C4)C2)C3)c1C(=O)OC |TLB:9:11:14.13.18:16,THB:9:11:14:18.17.16,12:13:16:20.11.19,12:11:14.13.18:16,19:11:14:18.17.16,19:17:14:20.12.11| Show InChI InChI=1S/C21H29NO3S/c1-4-5-16-12(2)26-18(17(16)19(23)25-3)22-20(24)21-9-13-6-14(10-21)8-15(7-13)11-21/h13-15H,4-11H2,1-3H3,(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272095
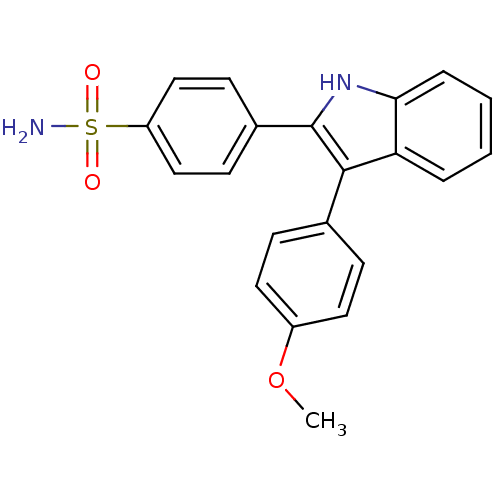
(4-(3-(4-methoxyphenyl)-1H-indol-2-yl)benzenesulfon...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O3S/c1-26-16-10-6-14(7-11-16)20-18-4-2-3-5-19(18)23-21(20)15-8-12-17(13-9-15)27(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50601869
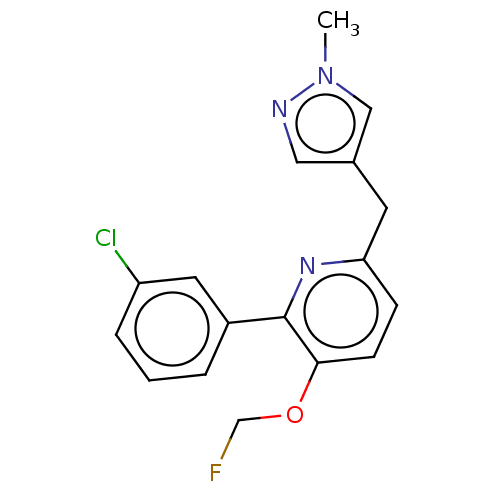
(CHEMBL5184286)Show SMILES [11CH3]n1cc(Cc2ccc(OCF)c(n2)-c2cccc(Cl)c2)cn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50233715
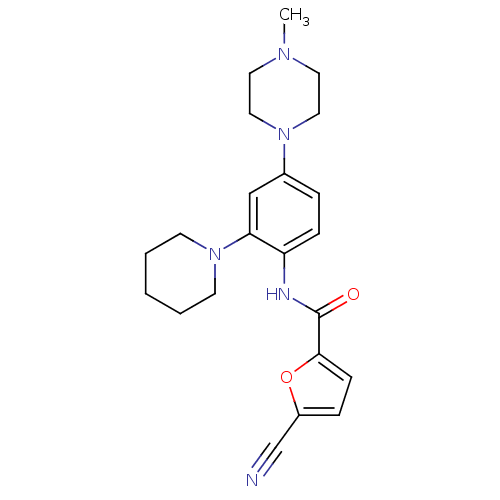
(5-cyano-N-(4-(4-methylpiperazin-1-yl)-2-(piperidin...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2ccc(o2)C#N)c(c1)N1CCCCC1 Show InChI InChI=1S/C22H27N5O2/c1-25-11-13-26(14-12-25)17-5-7-19(20(15-17)27-9-3-2-4-10-27)24-22(28)21-8-6-18(16-23)29-21/h5-8,15H,2-4,9-14H2,1H3,(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50601852
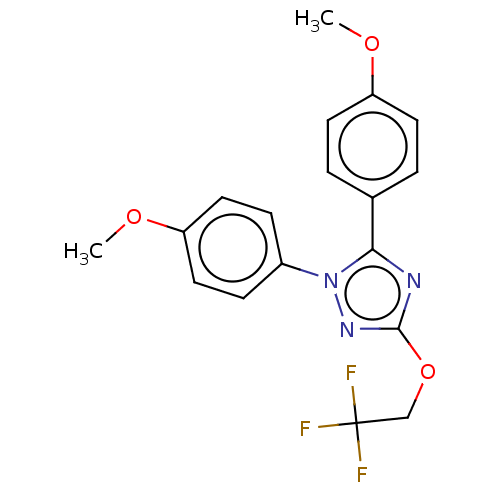
(CHEMBL5206077)Show SMILES COc1ccc(cc1)-n1nc(OCC(F)(F)F)nc1-c1ccc(O[11CH3])cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50601860

(CHEMBL5186767)Show SMILES C[C@H]1Cc2c(CN1C(=O)c1ccnc(c1[18F])C(F)(F)F)nnn2-c1ncccn1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50601851
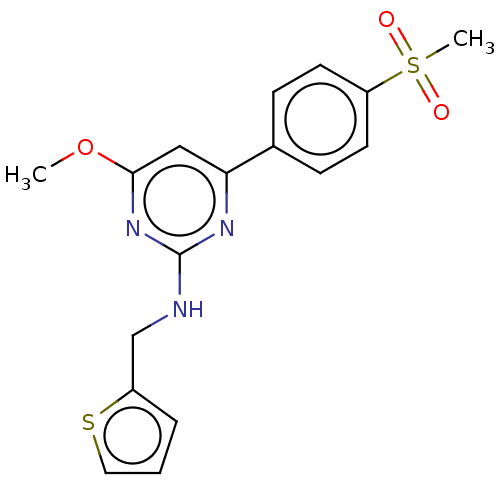
(CHEMBL5200454)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(O[11CH3])nc(NCc2cccs2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057581
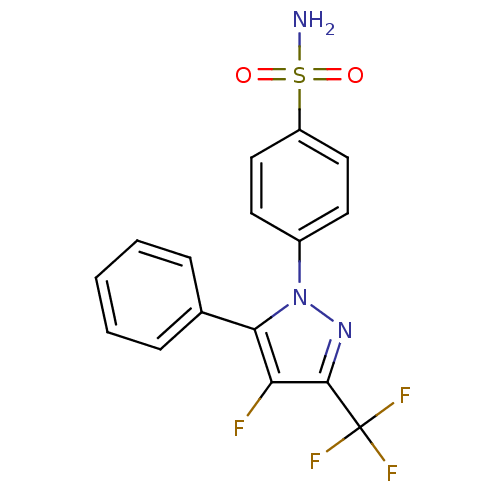
(4-(4-Fluoro-5-phenyl-3-trifluoromethyl-pyrazol-1-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(c(F)c1-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C16H11F4N3O2S/c17-13-14(10-4-2-1-3-5-10)23(22-15(13)16(18,19)20)11-6-8-12(9-7-11)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50601873
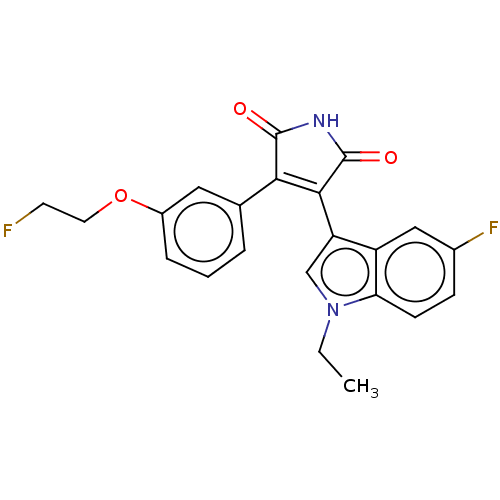
(CHEMBL5190086)Show SMILES CCn1cc(C2=C(C(=O)NC2=O)c2cccc(OCC[18F])c2)c2cc(F)ccc12 |t:5| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM450758
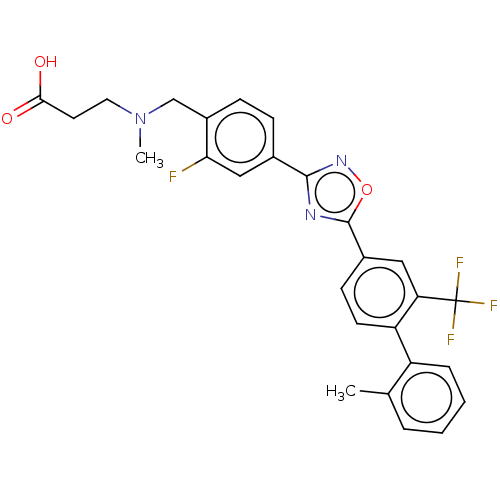
(US10676467, Compound TZ 33 21)Show SMILES CN(CCC(O)=O)Cc1ccc(cc1F)-c1noc(n1)-c1ccc(-c2ccccc2C)c(c1)C(F)(F)F Show InChI InChI=1S/C27H23F4N3O3/c1-16-5-3-4-6-20(16)21-10-9-18(13-22(21)27(29,30)31)26-32-25(33-37-26)17-7-8-19(23(28)14-17)15-34(2)12-11-24(35)36/h3-10,13-14H,11-12,15H2,1-2H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50601414
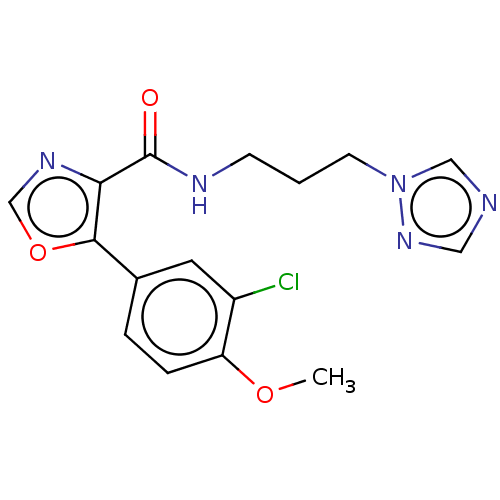
(CHEMBL5173945) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Toll-like receptor 4
(Homo sapiens (Human)) | BDBM50275658

((S)-2-((R)-3-(hexanoyloxy)tetradecanamido)-3-((2R,...)Show SMILES CCCCCCCCCCC[C@H](CC(=O)N[C@@H](CO[C@@H]1O[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCC)[C@H]1NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCC)C(O)=O)OC(=O)CCCCC |r| Show InChI InChI=1S/C69H127N2O19P/c1-7-13-19-22-25-28-31-34-40-43-54(85-61(75)46-37-16-10-4)49-59(73)70-57(68(79)80)53-84-69-65(71-60(74)50-55(86-62(76)47-38-17-11-5)44-41-35-32-29-26-23-20-14-8-2)67(66(58(52-72)88-69)90-91(81,82)83)89-64(78)51-56(87-63(77)48-39-18-12-6)45-42-36-33-30-27-24-21-15-9-3/h54-58,65-67,69,72H,7-53H2,1-6H3,(H,70,73)(H,71,74)(H,79,80)(H2,81,82,83)/t54-,55-,56-,57+,58-,65-,66-,67-,69-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Biologicals
Curated by ChEMBL
| Assay Description
Antagonist activity at TLR4 in human PBMC assessed as inhibition of LPS-stimulated TNFalpha production preincubated for 30 mins before LPS challenge ... |
Bioorg Med Chem Lett 18: 5350-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.060
BindingDB Entry DOI: 10.7270/Q2DB81P6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50601866

(CHEMBL5197130)Show SMILES CCCCNc1ncc(c(NC2CCC(O)CC2)n1)-c1cccc([18F])n1 |(-6.67,-3.85,;-5.33,-3.08,;-4,-3.85,;-2.66,-3.08,;-1.33,-3.85,;0,-3.08,;1.34,-3.84,;2.66,-3.08,;2.66,-1.54,;1.34,-.77,;1.34,.77,;0,1.54,;-1.33,.77,;-2.66,1.54,;-2.66,3.08,;-4,3.85,;-1.33,3.85,;0,3.08,;0,-1.54,;4,-.77,;5.34,-1.54,;6.67,-.76,;6.66,.77,;5.33,1.54,;5.33,3.08,;4,.77,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50601871

(CHEMBL5207508)Show SMILES [11CH3]Oc1ccc(Cc2ccncc2)nc1-c1cccc(c1)[N+]([O-])=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416603
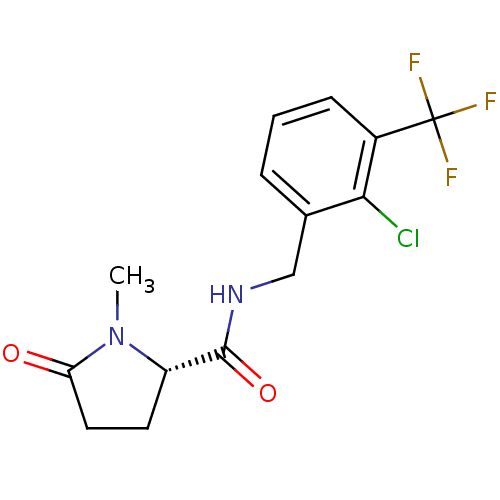
(CHEMBL1222883)Show SMILES CN1[C@@H](CCC1=O)C(=O)NCc1cccc(c1Cl)C(F)(F)F |r| Show InChI InChI=1S/C14H14ClF3N2O2/c1-20-10(5-6-11(20)21)13(22)19-7-8-3-2-4-9(12(8)15)14(16,17)18/h2-4,10H,5-7H2,1H3,(H,19,22)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50601870

(CHEMBL5181462)Show SMILES [11CH3]Oc1ccc(Cc2cn[nH]c2)nc1-c1cccc(c1)[N+]([O-])=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50029825
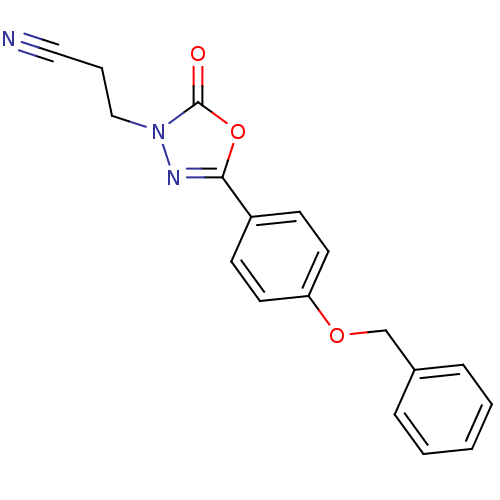
(3-[5-(4-Benzyloxy-phenyl)-2-oxo-[1,3,4]oxadiazol-3...)Show InChI InChI=1S/C18H15N3O3/c19-11-4-12-21-18(22)24-17(20-21)15-7-9-16(10-8-15)23-13-14-5-2-1-3-6-14/h1-3,5-10H,4,12-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50601850

(CHEMBL5178995)Show SMILES [11CH3]Oc1ccc(cc1F)-c1cnc(Cl)n1-c1ccc(cc1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50601853
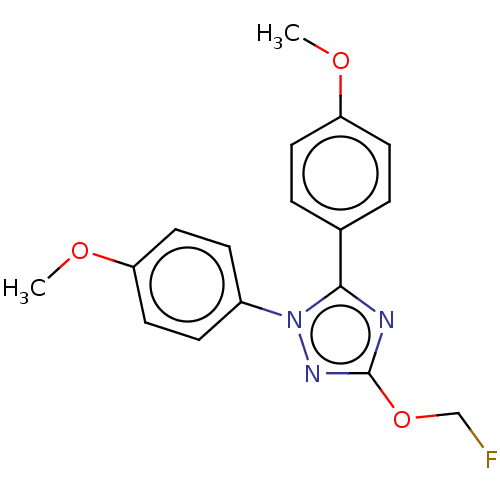
(CHEMBL5184826)Show SMILES [2H]C([2H])([18F])Oc1nc(-c2ccc(OC)cc2)n(n1)-c1ccc(OC)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50601859

(CHEMBL5200183)Show SMILES C[C@@H]1Cn2c(CN1C(=O)c1cccc(Cl)c1Cl)nnc2-c1cc(O[11CH3])ccn1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084631

(CHEMBL3427203)Show InChI InChI=1S/C17H17N3O3/c1-21-14-8-4-12(5-9-14)16-18-17(23-3)19-20(16)13-6-10-15(22-2)11-7-13/h4-11H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50601848

(CHEMBL5169729)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1c(Cl)ncc1-c1ccc(O[11CH3])c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50601868

(CHEMBL5172644) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Toll-like receptor 4
(Homo sapiens (Human)) | BDBM50275659
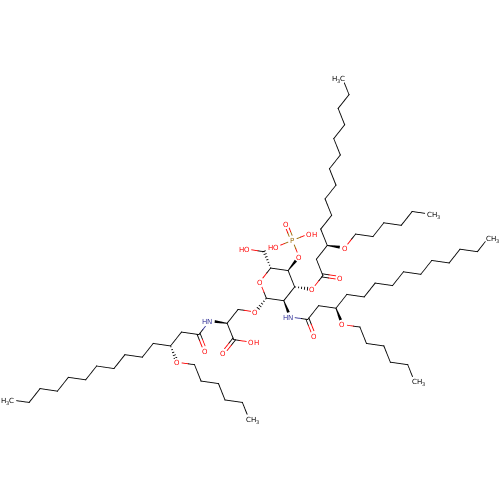
((S)-2-((R)-3-(hexyloxy)tetradecanamido)-3-((2R,3R,...)Show SMILES CCCCCCCCCCC[C@H](CC(=O)N[C@@H](CO[C@@H]1O[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OCCCCCC)[C@H]1NC(=O)C[C@@H](CCCCCCCCCCC)OCCCCCC)C(O)=O)OCCCCCC |r| Show InChI InChI=1S/C69H133N2O16P/c1-7-13-19-25-28-31-34-37-40-46-57(81-49-43-22-16-10-4)52-62(73)70-60(68(76)77)56-84-69-65(71-63(74)53-58(82-50-44-23-17-11-5)47-41-38-35-32-29-26-20-14-8-2)67(66(61(55-72)85-69)87-88(78,79)80)86-64(75)54-59(83-51-45-24-18-12-6)48-42-39-36-33-30-27-21-15-9-3/h57-61,65-67,69,72H,7-56H2,1-6H3,(H,70,73)(H,71,74)(H,76,77)(H2,78,79,80)/t57-,58-,59-,60+,61-,65-,66-,67-,69-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Biologicals
Curated by ChEMBL
| Assay Description
Antagonist activity at TLR4 in human PBMC assessed as inhibition of LPS-stimulated TNFalpha production preincubated for 30 mins before LPS challenge ... |
Bioorg Med Chem Lett 18: 5350-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.060
BindingDB Entry DOI: 10.7270/Q2DB81P6 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436963
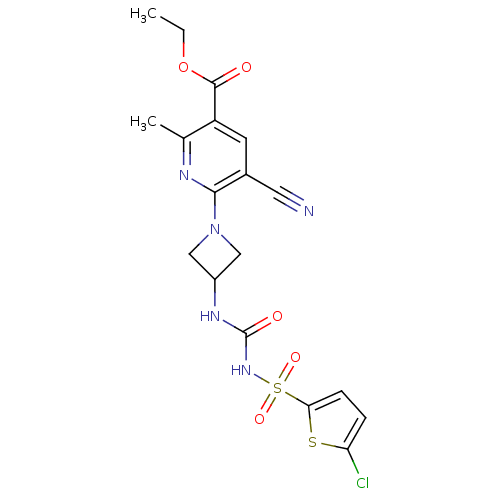
(CHEMBL2402255)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C18H18ClN5O5S2/c1-3-29-17(25)13-6-11(7-20)16(21-10(13)2)24-8-12(9-24)22-18(26)23-31(27,28)15-5-4-14(19)30-15/h4-6,12H,3,8-9H2,1-2H3,(H2,22,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50601865

(CHEMBL5180946)Show SMILES CCOc1cc2ccc(C(N)=O)c(Nc3ccc(F)cc3F)c2cc1N1CCN([11CH3])CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM450730
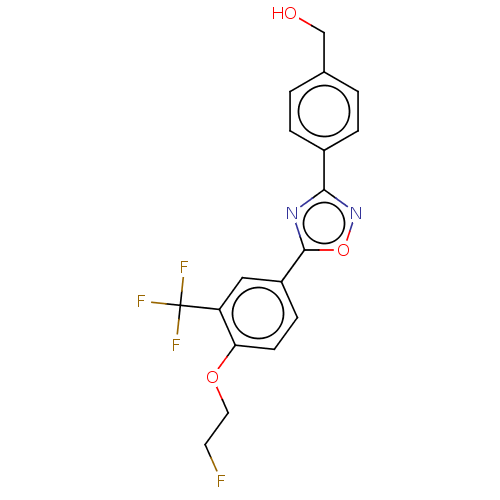
(US10676467, Compound 8c | US10676467, Compound TZ ...)Show SMILES OCc1ccc(cc1)-c1noc(n1)-c1ccc(OCCF)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O3/c19-7-8-26-15-6-5-13(9-14(15)18(20,21)22)17-23-16(24-27-17)12-3-1-11(10-25)2-4-12/h1-6,9,25H,7-8,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50601862

(CHEMBL5194016)Show SMILES FC(F)(F)c1cccc(CNC(=O)[C@@H]2CCC(=O)N2CC[18F])c1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057589
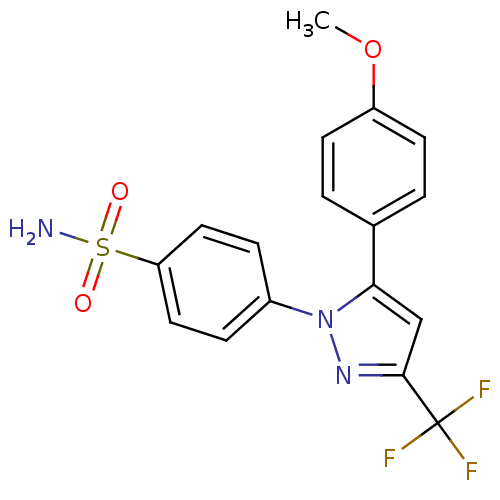
(4-[5-(4-Methoxy-phenyl)-3-trifluoromethyl-pyrazol-...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O3S/c1-26-13-6-2-11(3-7-13)15-10-16(17(18,19)20)22-23(15)12-4-8-14(9-5-12)27(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM31501
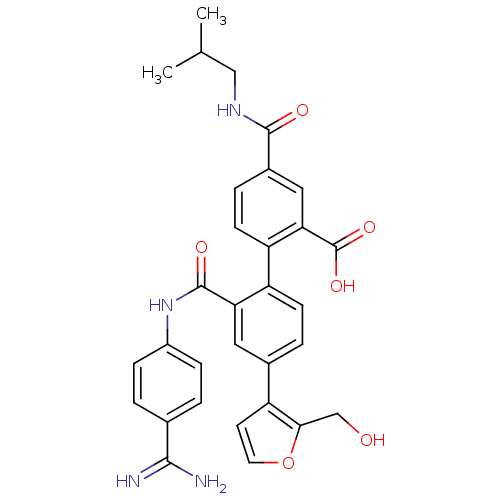
(substituted biphenyl derivative, 36ao)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(cc1C(=O)Nc1ccc(cc1)C(N)=N)-c1ccoc1CO Show InChI InChI=1S/C31H30N4O6/c1-17(2)15-34-29(37)20-6-10-24(26(14-20)31(39)40)23-9-5-19(22-11-12-41-27(22)16-36)13-25(23)30(38)35-21-7-3-18(4-8-21)28(32)33/h3-14,17,36H,15-16H2,1-2H3,(H3,32,33)(H,34,37)(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals
| Assay Description
TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50601867

(CHEMBL5191424) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM31497

(substituted biphenyl derivative, 36ak)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(cc1C(=O)Nc1ccc(cc1)C(N)=N)-c1cscc1CO Show InChI InChI=1S/C31H30N4O5S/c1-17(2)13-34-29(37)20-6-10-24(26(12-20)31(39)40)23-9-5-19(27-16-41-15-21(27)14-36)11-25(23)30(38)35-22-7-3-18(4-8-22)28(32)33/h3-12,15-17,36H,13-14H2,1-2H3,(H3,32,33)(H,34,37)(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals
| Assay Description
TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM31462

(substituted biphenyl derivative, 36b)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(C=C)cc1C(=O)Nc1ccc(cc1)C(N)=N Show InChI InChI=1S/C28H28N4O4/c1-4-17-5-11-21(23(13-17)27(34)32-20-9-6-18(7-10-20)25(29)30)22-12-8-19(14-24(22)28(35)36)26(33)31-15-16(2)3/h4-14,16H,1,15H2,2-3H3,(H3,29,30)(H,31,33)(H,32,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals
| Assay Description
TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM31499
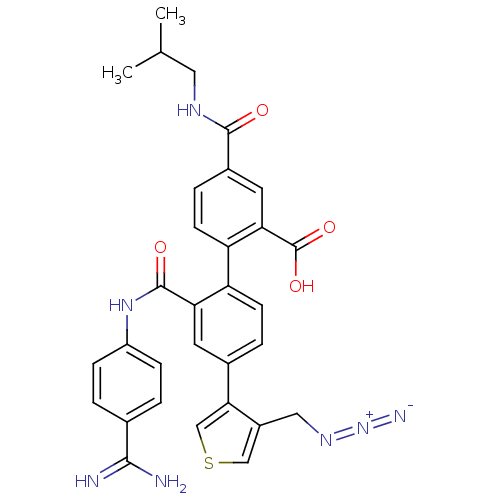
(substituted biphenyl derivative, 36am)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(cc1C(=O)Nc1ccc(cc1)C(N)=N)-c1cscc1CN=[N+]=[N-] Show InChI InChI=1S/C31H29N7O4S/c1-17(2)13-35-29(39)20-6-10-24(26(12-20)31(41)42)23-9-5-19(27-16-43-15-21(27)14-36-38-34)11-25(23)30(40)37-22-7-3-18(4-8-22)28(32)33/h3-12,15-17H,13-14H2,1-2H3,(H3,32,33)(H,35,39)(H,37,40)(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals
| Assay Description
TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM31488

(substituted biphenyl derivative, 36ab)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(cc1C(=O)Nc1ccc(cc1)C(N)=N)-c1ccco1 Show InChI InChI=1S/C30H28N4O5/c1-17(2)16-33-28(35)20-8-12-23(25(15-20)30(37)38)22-11-7-19(26-4-3-13-39-26)14-24(22)29(36)34-21-9-5-18(6-10-21)27(31)32/h3-15,17H,16H2,1-2H3,(H3,31,32)(H,33,35)(H,34,36)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals
| Assay Description
TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM31468

(substituted biphenyl derivative, 36h)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(C=C=C)cc1C(=O)Nc1ccc(cc1)C(N)=N Show InChI InChI=1S/C29H28N4O4/c1-4-5-18-6-12-22(24(14-18)28(35)33-21-10-7-19(8-11-21)26(30)31)23-13-9-20(15-25(23)29(36)37)27(34)32-16-17(2)3/h5-15,17H,1,16H2,2-3H3,(H3,30,31)(H,32,34)(H,33,35)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals
| Assay Description
TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data