 Found 27879 hits with Last Name = 'sen' and Initial = 's'
Found 27879 hits with Last Name = 'sen' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(RAT) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85020
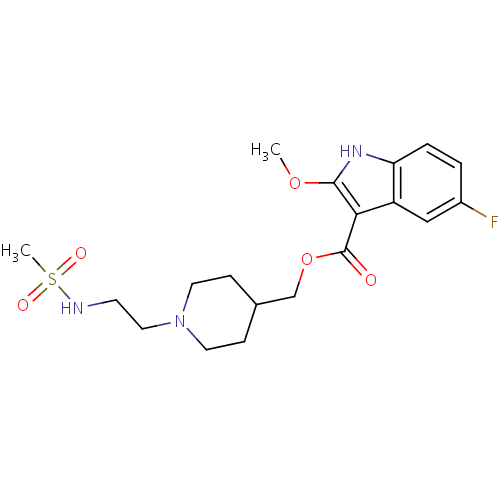
(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50166288
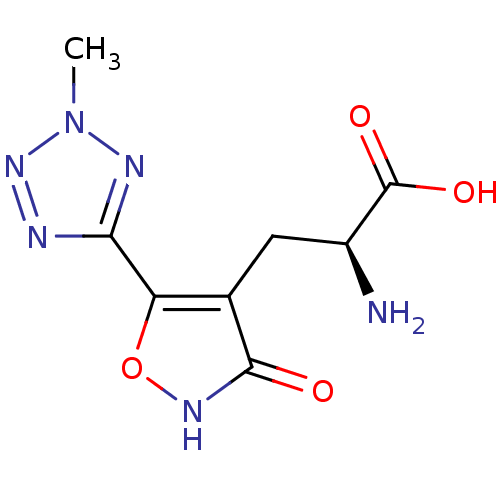
((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...)Show InChI InChI=1S/C8H10N6O4/c1-14-11-6(10-13-14)5-3(7(15)12-18-5)2-4(9)8(16)17/h4H,2,9H2,1H3,(H,12,15)(H,16,17)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR3 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 4
(Rattus norvegicus) | BDBM50166288

((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...)Show InChI InChI=1S/C8H10N6O4/c1-14-11-6(10-13-14)5-3(7(15)12-18-5)2-4(9)8(16)17/h4H,2,9H2,1H3,(H,12,15)(H,16,17)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR4 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Rattus norvegicus) | BDBM50166288

((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...)Show InChI InChI=1S/C8H10N6O4/c1-14-11-6(10-13-14)5-3(7(15)12-18-5)2-4(9)8(16)17/h4H,2,9H2,1H3,(H,12,15)(H,16,17)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR2 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50166288

((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...)Show InChI InChI=1S/C8H10N6O4/c1-14-11-6(10-13-14)5-3(7(15)12-18-5)2-4(9)8(16)17/h4H,2,9H2,1H3,(H,12,15)(H,16,17)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR1 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85026
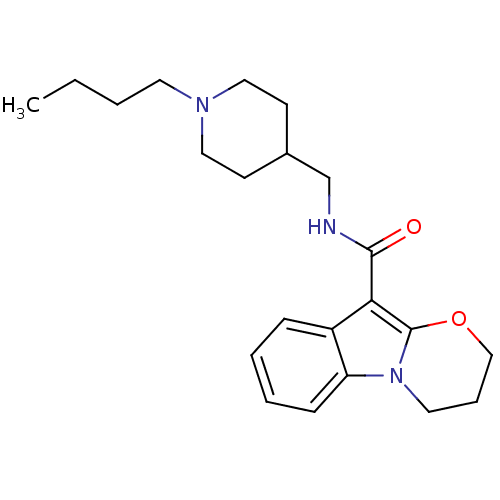
(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.00890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101499
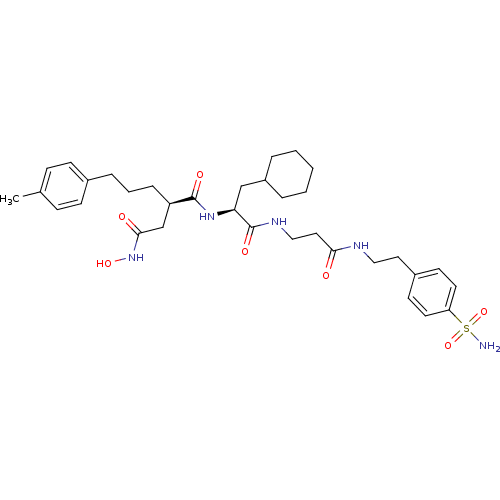
(CHEMBL74040 | N*1*-(2-Cyclohexyl-1-{2-[2-(4-sulfam...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C34H49N5O7S/c1-24-10-12-25(13-11-24)8-5-9-28(23-32(41)39-44)33(42)38-30(22-27-6-3-2-4-7-27)34(43)37-21-19-31(40)36-20-18-26-14-16-29(17-15-26)47(35,45)46/h10-17,27-28,30,44H,2-9,18-23H2,1H3,(H,36,40)(H,37,43)(H,38,42)(H,39,41)(H2,35,45,46)/t28-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101512
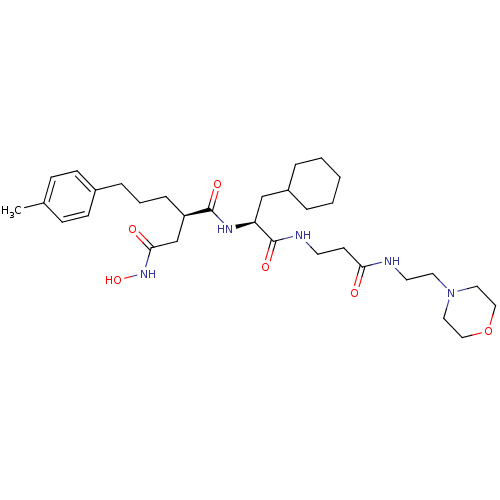
(CHEMBL306033 | N*1*-{2-Cyclohexyl-1-[2-(2-morpholi...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCN2CCOCC2)cc1 Show InChI InChI=1S/C32H51N5O6/c1-24-10-12-25(13-11-24)8-5-9-27(23-30(39)36-42)31(40)35-28(22-26-6-3-2-4-7-26)32(41)34-15-14-29(38)33-16-17-37-18-20-43-21-19-37/h10-13,26-28,42H,2-9,14-23H2,1H3,(H,33,38)(H,34,41)(H,35,40)(H,36,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101509
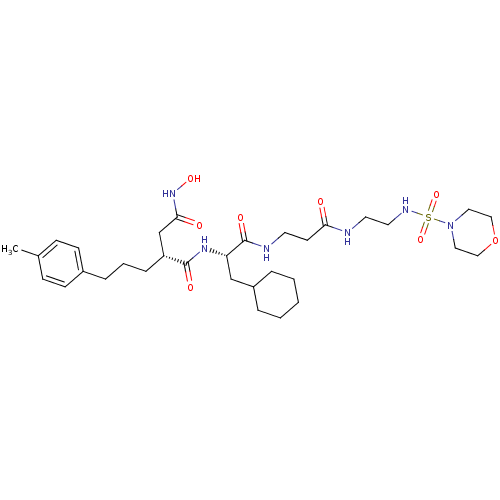
(CHEMBL306947 | N*1*-(2-Cyclohexyl-1-{2-[2-(morphol...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCNS(=O)(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C32H52N6O8S/c1-24-10-12-25(13-11-24)8-5-9-27(23-30(40)37-43)31(41)36-28(22-26-6-3-2-4-7-26)32(42)34-15-14-29(39)33-16-17-35-47(44,45)38-18-20-46-21-19-38/h10-13,26-28,35,43H,2-9,14-23H2,1H3,(H,33,39)(H,34,42)(H,36,41)(H,37,40)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101508
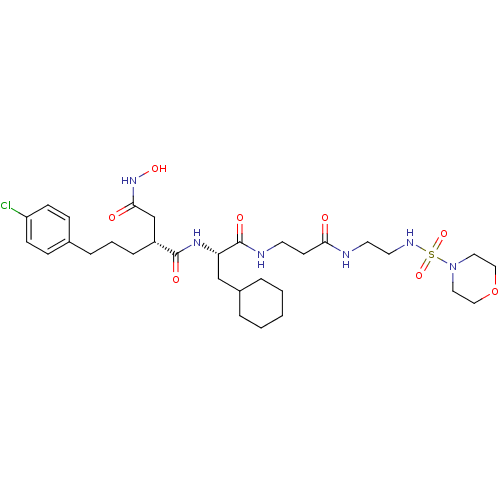
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-(2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCNS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C31H49ClN6O8S/c32-26-11-9-23(10-12-26)7-4-8-25(22-29(40)37-43)30(41)36-27(21-24-5-2-1-3-6-24)31(42)34-14-13-28(39)33-15-16-35-47(44,45)38-17-19-46-20-18-38/h9-12,24-25,27,35,43H,1-8,13-22H2,(H,33,39)(H,34,42)(H,36,41)(H,37,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030752
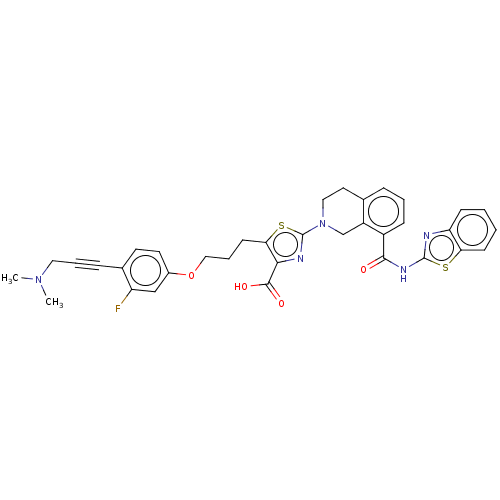
(CHEMBL3342333)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-9-23-14-15-24(20-27(23)36)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-22-8-5-10-25(26(22)21-41)32(42)39-34-37-28-11-3-4-12-29(28)46-34/h3-5,8,10-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030754
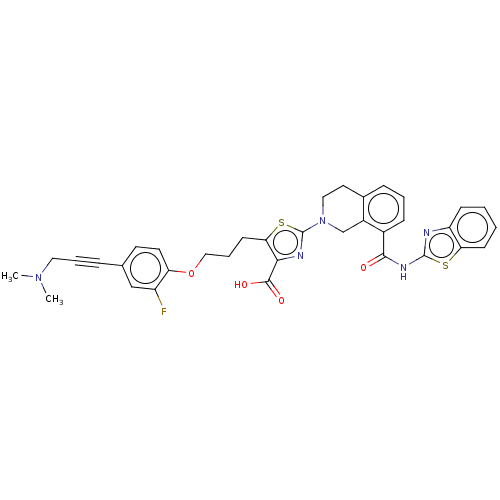
(CHEMBL3342332)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(F)c1 Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757
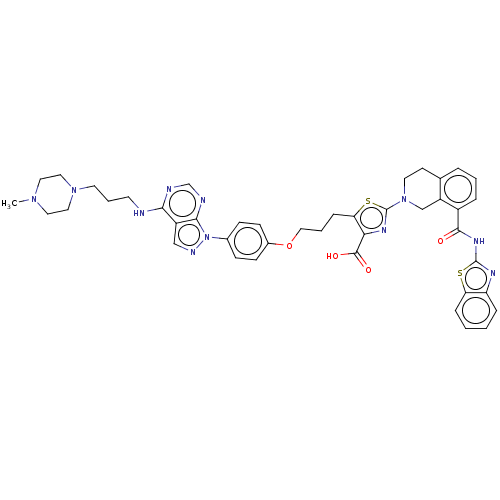
(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030758
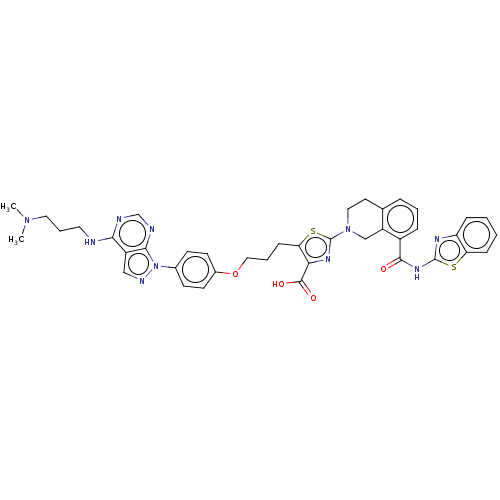
(CHEMBL3342195)Show SMILES CN(C)CCCNc1ncnc2n(ncc12)-c1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C40H40N10O4S2/c1-48(2)19-7-18-41-35-29-22-44-50(36(29)43-24-42-35)26-13-15-27(16-14-26)54-21-6-12-33-34(38(52)53)46-40(56-33)49-20-17-25-8-5-9-28(30(25)23-49)37(51)47-39-45-31-10-3-4-11-32(31)55-39/h3-5,8-11,13-16,22,24H,6-7,12,17-21,23H2,1-2H3,(H,52,53)(H,41,42,43)(H,45,47,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030759
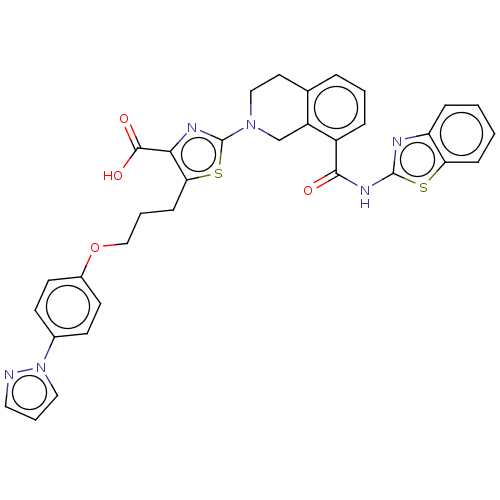
(CHEMBL3342194)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1cccn1)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C33H28N6O4S2/c40-30(37-32-35-26-8-1-2-9-27(26)44-32)24-7-3-6-21-15-18-38(20-25(21)24)33-36-29(31(41)42)28(45-33)10-4-19-43-23-13-11-22(12-14-23)39-17-5-16-34-39/h1-3,5-9,11-14,16-17H,4,10,15,18-20H2,(H,41,42)(H,35,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101505
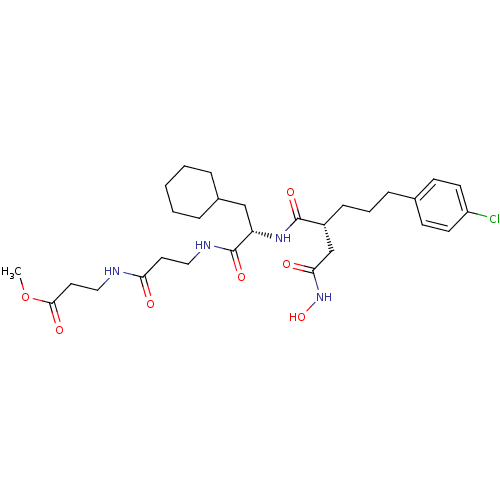
(3-(3-{2-[5-(4-Chloro-phenyl)-2-hydroxycarbamoylmet...)Show SMILES COC(=O)CCNC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C29H43ClN4O7/c1-41-27(37)15-17-31-25(35)14-16-32-29(39)24(18-21-6-3-2-4-7-21)33-28(38)22(19-26(36)34-40)9-5-8-20-10-12-23(30)13-11-20/h10-13,21-22,24,40H,2-9,14-19H2,1H3,(H,31,35)(H,32,39)(H,33,38)(H,34,36)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101516
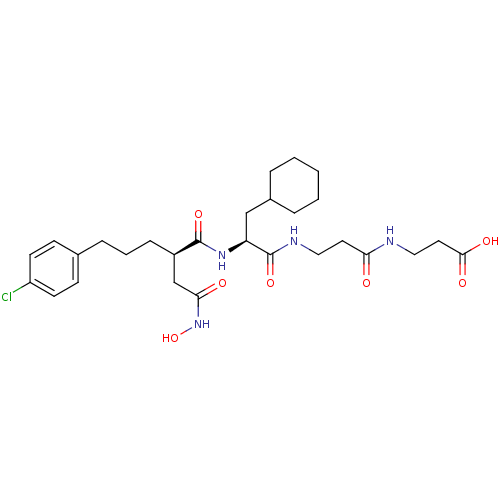
(3-(3-{2-[5-(4-Chloro-phenyl)-2-hydroxycarbamoylmet...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCC(O)=O Show InChI InChI=1S/C28H41ClN4O7/c29-22-11-9-19(10-12-22)7-4-8-21(18-25(35)33-40)27(38)32-23(17-20-5-2-1-3-6-20)28(39)31-15-13-24(34)30-16-14-26(36)37/h9-12,20-21,23,40H,1-8,13-18H2,(H,30,34)(H,31,39)(H,32,38)(H,33,35)(H,36,37)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Rattus norvegicus) | BDBM50002370
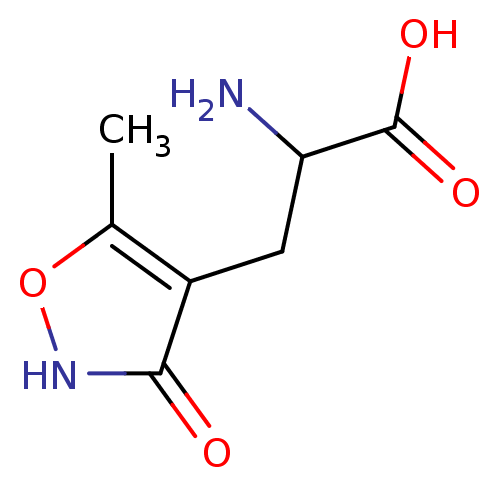
((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR2 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101520
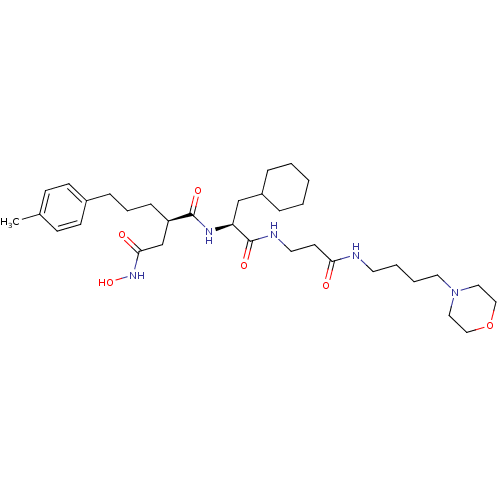
(CHEMBL77663 | N*1*-{2-Cyclohexyl-1-[2-(4-morpholin...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCC(=O)NCCCCN2CCOCC2)cc1 Show InChI InChI=1S/C34H55N5O6/c1-26-12-14-27(15-13-26)10-7-11-29(25-32(41)38-44)33(42)37-30(24-28-8-3-2-4-9-28)34(43)36-18-16-31(40)35-17-5-6-19-39-20-22-45-23-21-39/h12-15,28-30,44H,2-11,16-25H2,1H3,(H,35,40)(H,36,43)(H,37,42)(H,38,41)/t29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50078280

((R)-2-Cyano-N-[(R)-2-(2,5-difluoro-phenoxy)-1-meth...)Show SMILES C[C@H](COc1cc(F)ccc1F)NC(=O)[C@@H](C#N)C(C)(C)C Show InChI InChI=1S/C16H20F2N2O2/c1-10(20-15(21)12(8-19)16(2,3)4)9-22-14-7-11(17)5-6-13(14)18/h5-7,10,12H,9H2,1-4H3,(H,20,21)/t10-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine-Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against scytalone dehydratase enzyme obtained from Magnaporthe grisea |
Bioorg Med Chem Lett 9: 1607-12 (1999)
BindingDB Entry DOI: 10.7270/Q2JH3KBD |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101518
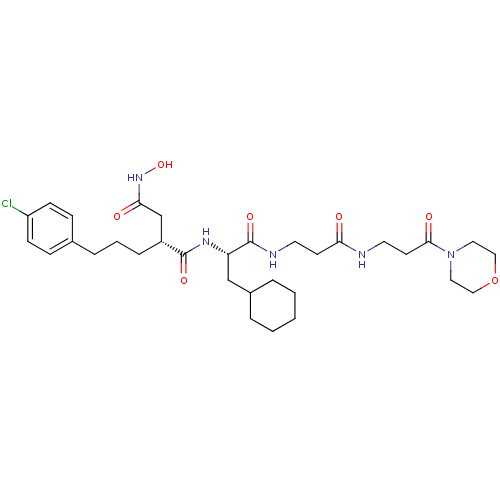
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C32H48ClN5O7/c33-26-11-9-23(10-12-26)7-4-8-25(22-29(40)37-44)31(42)36-27(21-24-5-2-1-3-6-24)32(43)35-15-13-28(39)34-16-14-30(41)38-17-19-45-20-18-38/h9-12,24-25,27,44H,1-8,13-22H2,(H,34,39)(H,35,43)(H,36,42)(H,37,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101504
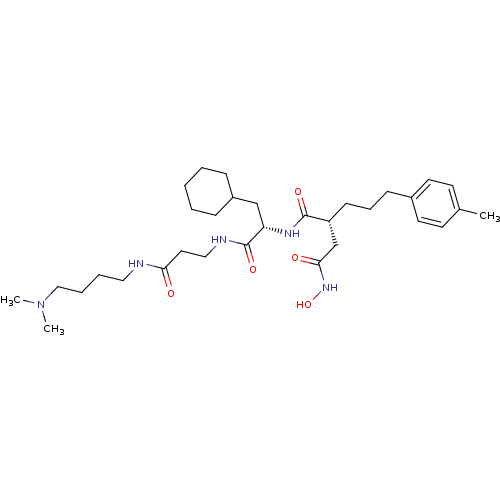
(CHEMBL77057 | N*1*-{2-Cyclohexyl-1-[2-(4-dimethyla...)Show SMILES CN(C)CCCCNC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(C)cc1)CC(=O)NO Show InChI InChI=1S/C32H53N5O5/c1-24-14-16-25(17-15-24)12-9-13-27(23-30(39)36-42)31(40)35-28(22-26-10-5-4-6-11-26)32(41)34-20-18-29(38)33-19-7-8-21-37(2)3/h14-17,26-28,42H,4-13,18-23H2,1-3H3,(H,33,38)(H,34,41)(H,35,40)(H,36,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030756
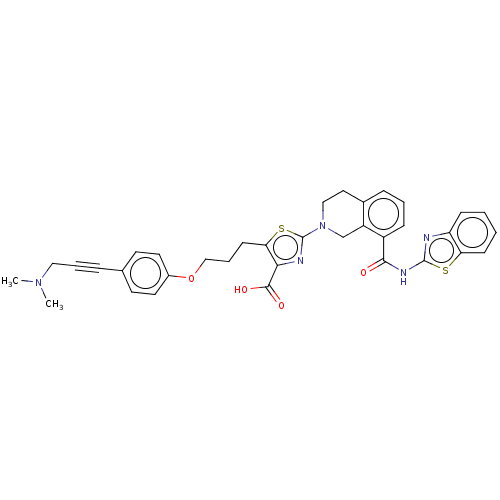
(CHEMBL3342197)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C35H33N5O4S2/c1-39(2)19-6-8-23-14-16-25(17-15-23)44-21-7-13-30-31(33(42)43)37-35(46-30)40-20-18-24-9-5-10-26(27(24)22-40)32(41)38-34-36-28-11-3-4-12-29(28)45-34/h3-5,9-12,14-17H,7,13,18-22H2,1-2H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50002370

((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR3 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50002370

((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR1 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602306
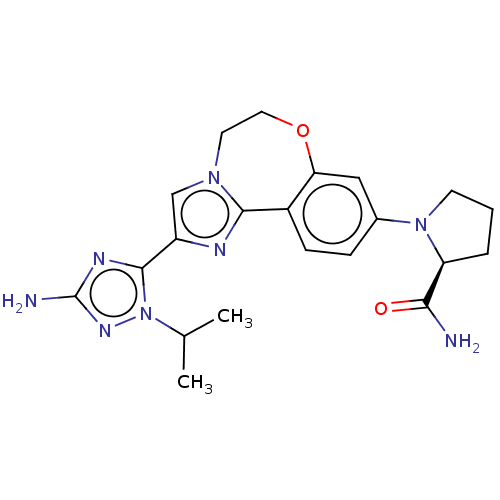
(CHEMBL5208487)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101511
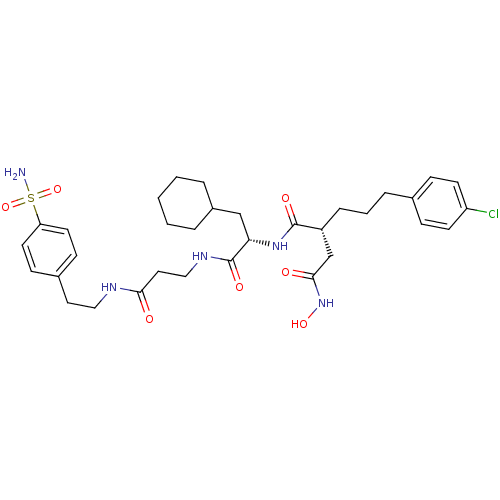
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-(2-cyclohexyl-...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CCNC(=O)[C@H](CC2CCCCC2)NC(=O)[C@H](CCCc2ccc(Cl)cc2)CC(=O)NO)cc1 Show InChI InChI=1S/C33H46ClN5O7S/c34-27-13-9-23(10-14-27)7-4-8-26(22-31(41)39-44)32(42)38-29(21-25-5-2-1-3-6-25)33(43)37-20-18-30(40)36-19-17-24-11-15-28(16-12-24)47(35,45)46/h9-16,25-26,29,44H,1-8,17-22H2,(H,36,40)(H,37,43)(H,38,42)(H,39,41)(H2,35,45,46)/t26-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101495
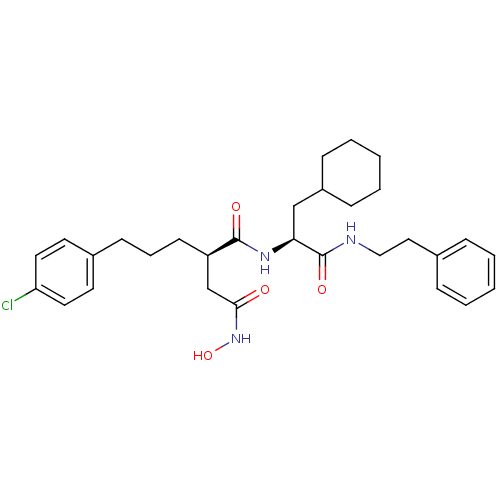
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-((S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40ClN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757

(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030751
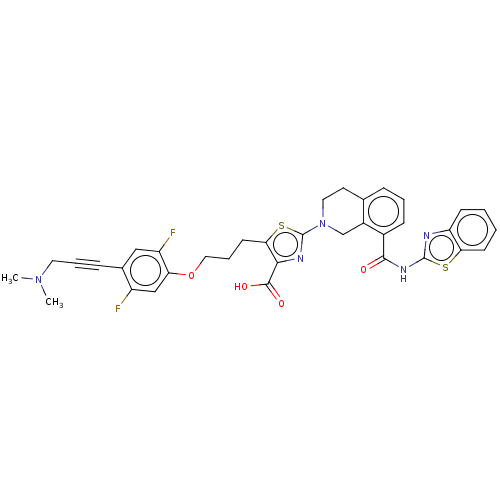
(CHEMBL3342334)Show SMILES CN(C)CC#Cc1cc(F)c(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H31F2N5O4S2/c1-41(2)15-6-9-22-18-26(37)28(19-25(22)36)46-17-7-13-30-31(33(44)45)39-35(48-30)42-16-14-21-8-5-10-23(24(21)20-42)32(43)40-34-38-27-11-3-4-12-29(27)47-34/h3-5,8,10-12,18-19H,7,13-17,20H2,1-2H3,(H,44,45)(H,38,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101503
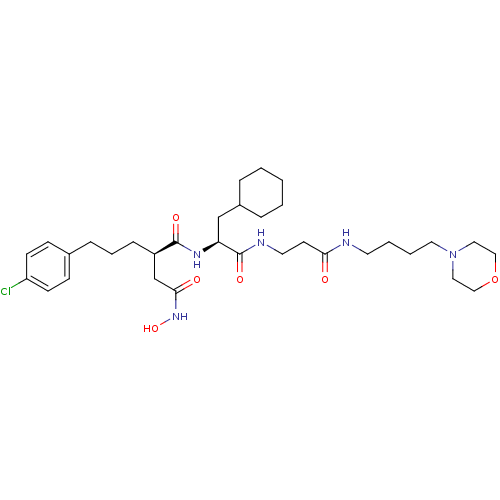
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCCCN1CCOCC1 Show InChI InChI=1S/C33H52ClN5O6/c34-28-13-11-25(12-14-28)9-6-10-27(24-31(41)38-44)32(42)37-29(23-26-7-2-1-3-8-26)33(43)36-17-15-30(40)35-16-4-5-18-39-19-21-45-22-20-39/h11-14,26-27,29,44H,1-10,15-24H2,(H,35,40)(H,36,43)(H,37,42)(H,38,41)/t27-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-2 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295665
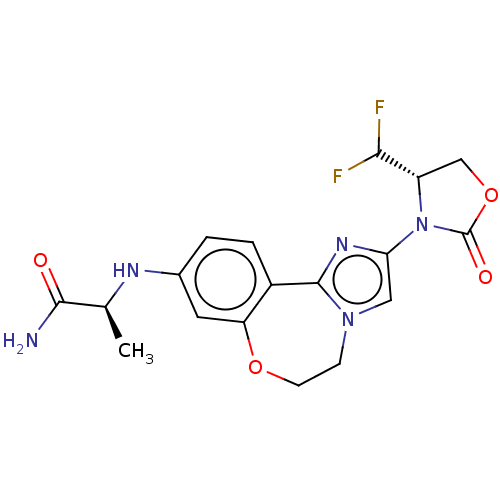
((S)-2-((2-((S)-4-(difluoromethyl)- 2-oxooxazolidin...)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C(N)=O |r| Show InChI InChI=1S/C18H19F2N5O4/c1-9(16(21)26)22-10-2-3-11-13(6-10)28-5-4-24-7-14(23-17(11)24)25-12(15(19)20)8-29-18(25)27/h2-3,6-7,9,12,15,22H,4-5,8H2,1H3,(H2,21,26)/t9-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor 4
(Rattus norvegicus) | BDBM50002370

((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR4 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50508839

(CHEMBL4579554)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)C(\Cc2ccc(O)c(Br)c2)=N\O)cc1 Show InChI InChI=1S/C17H18BrN3O5S/c18-14-9-12(3-6-16(14)22)10-15(21-24)17(23)20-8-7-11-1-4-13(5-2-11)27(19,25)26/h1-6,9,22,24H,7-8,10H2,(H,20,23)(H2,19,25,26)/b21-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 expressed in Escherichia coli BL21 (DE3) by stopped-flow CO2 hydration assay |
J Med Chem 62: 4174-4192 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00282
BindingDB Entry DOI: 10.7270/Q20868M1 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030755
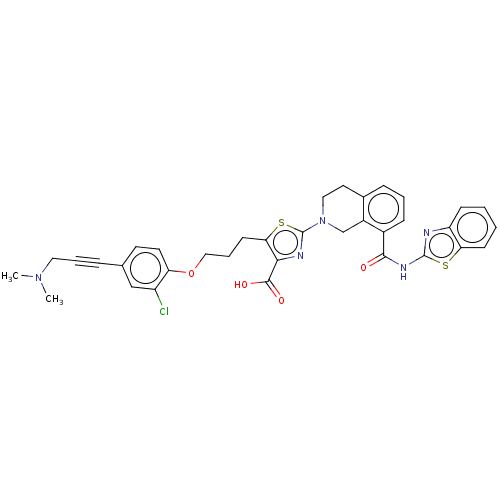
(CHEMBL3342198)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(Cl)c1 Show InChI InChI=1S/C35H32ClN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030761
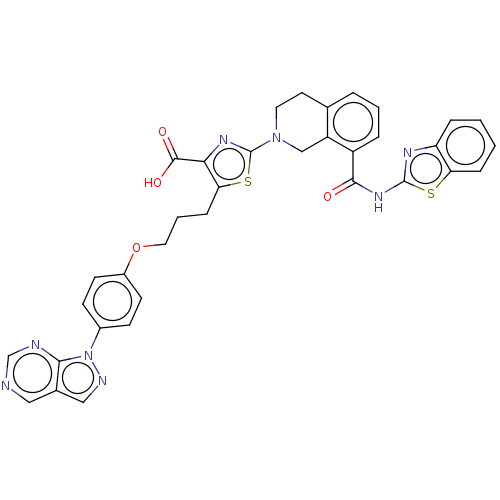
(CHEMBL3342192)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1ncc2cncnc12)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C35H28N8O4S2/c44-32(41-34-39-27-7-1-2-8-28(27)48-34)25-6-3-5-21-14-15-42(19-26(21)25)35-40-30(33(45)46)29(49-35)9-4-16-47-24-12-10-23(11-13-24)43-31-22(18-38-43)17-36-20-37-31/h1-3,5-8,10-13,17-18,20H,4,9,14-16,19H2,(H,45,46)(H,39,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602320
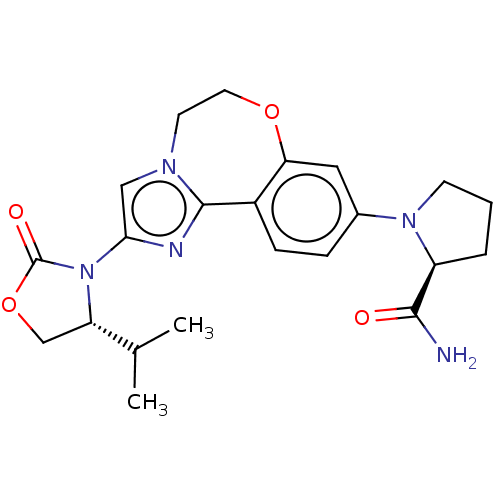
(CHEMBL5199631)Show SMILES CC(C)[C@@H]1COC(=O)N1c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data