

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM18796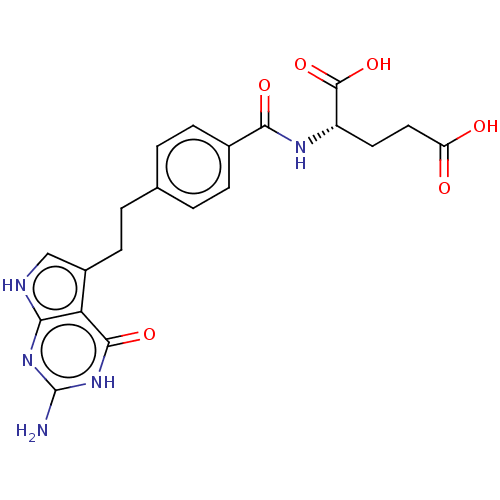 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 5.5 by Dixon plot | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50306576 ((S)-2-({5-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 5.5 by Dixon plot | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50393640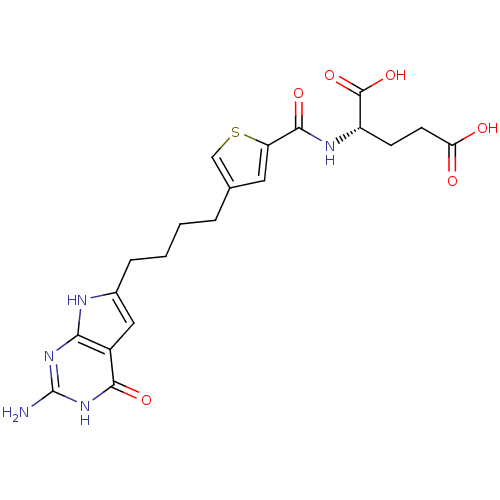 (CHEMBL2158681) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 5.5 by Dixon plot | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50393639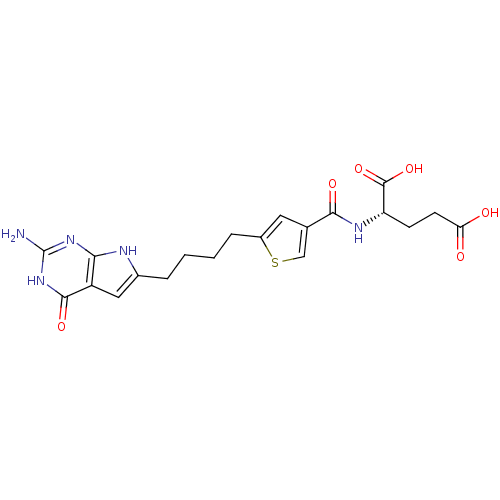 (CHEMBL2158682) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 5.5 by Dixon plot | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM18796 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 6.8 by Dixon plot | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50306576 ((S)-2-({5-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 6.8 by Dixon plot | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50393640 (CHEMBL2158681) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 6.8 by Dixon plot | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50393639 (CHEMBL2158682) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 6.8 by Dixon plot | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50393640 (CHEMBL2158681) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of GARFTase in human IGROV1 cells assessed as reduction in [14C]glycine incorporation into [14C]formyl GAR incubated for 15 hrs in complet... | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50393639 (CHEMBL2158682) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of GARFTase in human IGROV1 cells assessed as reduction in [14C]glycine incorporation into [14C]formyl GAR incubated for 15 hrs in complet... | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50306576 ((S)-2-({5-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of GARFTase in human IGROV1 cells assessed as reduction in [14C]glycine incorporation into [14C]formyl GAR incubated for 15 hrs in complet... | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005518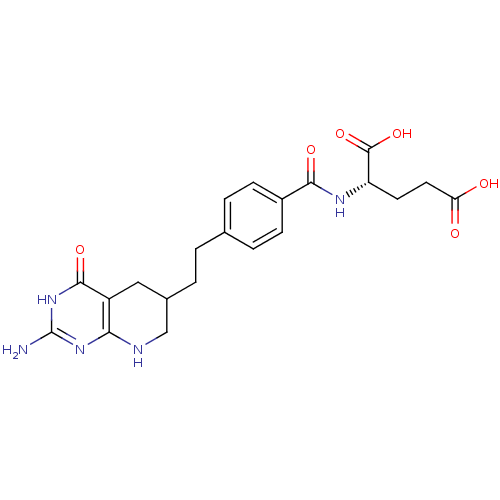 ((S)-2-(4-(2-((R)-2-amino-4-oxo-1,4,5,6,7,8-hexahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of GARFTase in human IGROV1 cells assessed as reduction in [14C]glycine incorporation into [14C]formyl GAR incubated for 15 hrs in complet... | J Med Chem 55: 1758-70 (2012) Article DOI: 10.1021/jm201688n BindingDB Entry DOI: 10.7270/Q26Q1ZB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50514760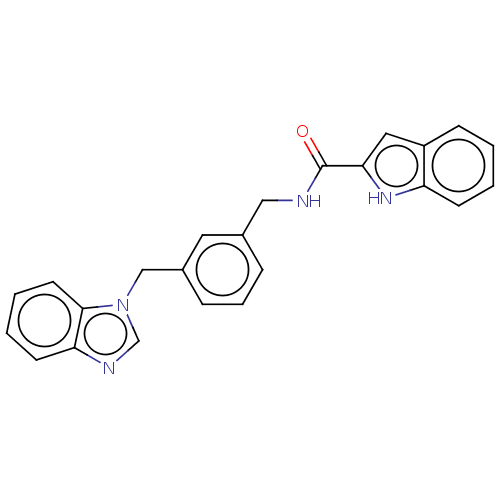 (CHEMBL4448402) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50514753 (CHEMBL4557994) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50249877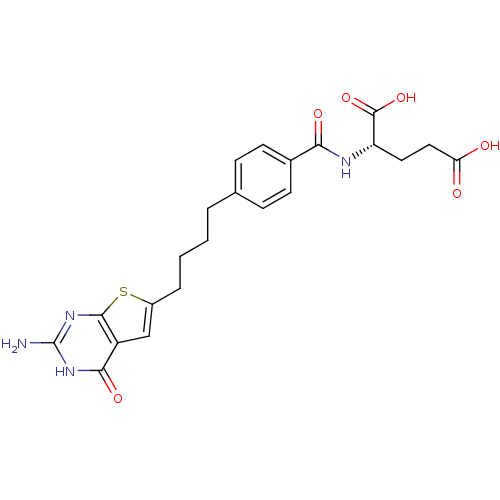 ((S)-2-(4-(4-(2-amino-4-oxo-3,4-dihydrothieno[2,3-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of GARFTase in human KB cells assessed as inhibition of incorporation of [14C]glycine into [14C]formyl GAR after 30 mins in presence of az... | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50249876 (CHEMBL490934 | N-{4-[3-(2-Amino-4-oxo-3,4-dihydrot...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of GARFTase in human KB cells assessed as inhibition of incorporation of [14C]glycine into [14C]formyl GAR after 30 mins in presence of az... | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human LXF-289 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22590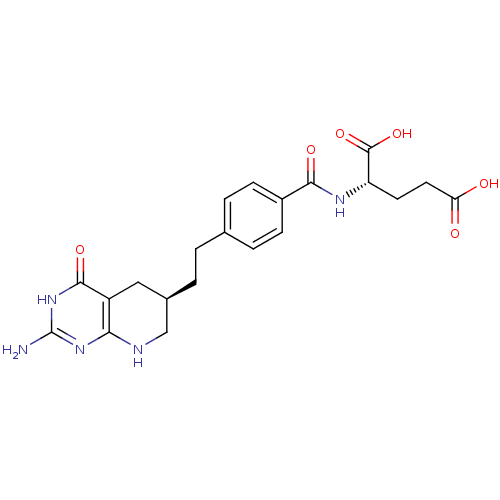 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of GARFTase in human KB cells assessed as inhibition of incorporation of [14C]glycine into [14C]formyl GAR after 30 mins in presence of az... | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514759 (CHEMBL4571131) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50249953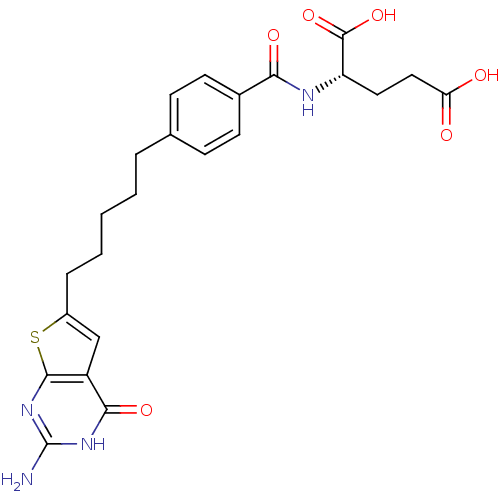 (CHEMBL491129 | N-{4-[5-(2-Amino-4-oxo-3,4-dihydrot...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of GARFTase in human KB cells assessed as inhibition of incorporation of [14C]glycine into [14C]formyl GAR after 30 mins in presence of az... | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50249954 (CHEMBL491298 | N-{4-[6-(2-Amino-4-oxo-3,4-dihydrot...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of GARFTase in human KB cells assessed as inhibition of incorporation of [14C]glycine into [14C]formyl GAR after 30 mins in presence of az... | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM18796 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of GARFTase in human KB cells assessed as inhibition of incorporation of [14C]glycine into [14C]formyl GAR after 30 mins in presence of az... | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50249875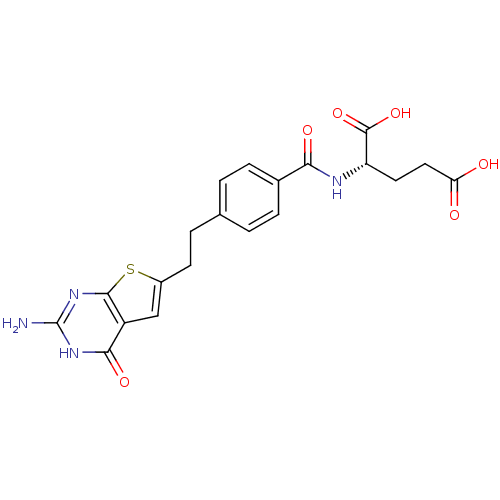 (CHEMBL522455 | N-{4-[2-(2-Amino-4-oxo-3,4-dihydrot...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of GARFTase in human KB cells assessed as inhibition of incorporation of [14C]glycine into [14C]formyl GAR after 30 mins in presence of az... | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HepG2 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514760 (CHEMBL4448402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514760 (CHEMBL4448402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514760 (CHEMBL4448402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514752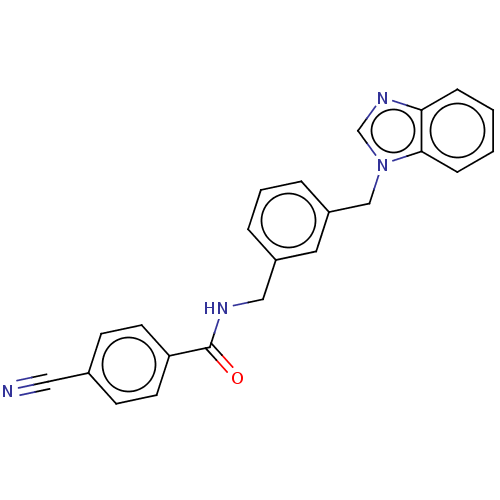 (CHEMBL4458549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514752 (CHEMBL4458549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514752 (CHEMBL4458549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50514752 (CHEMBL4458549) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514757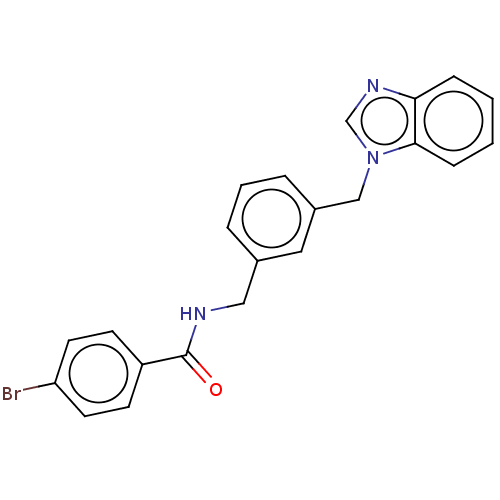 (CHEMBL4466117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514760 (CHEMBL4448402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human DAN-G cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514762 (CHEMBL4531545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 327 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514761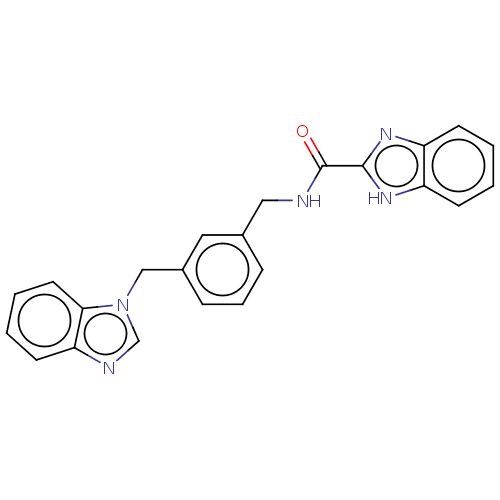 (CHEMBL4472707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514754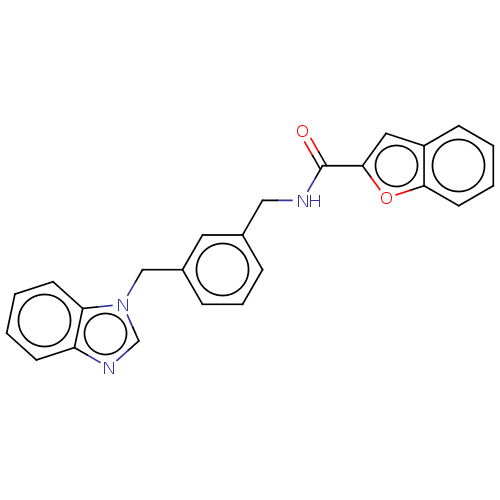 (CHEMBL4588288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 413 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514758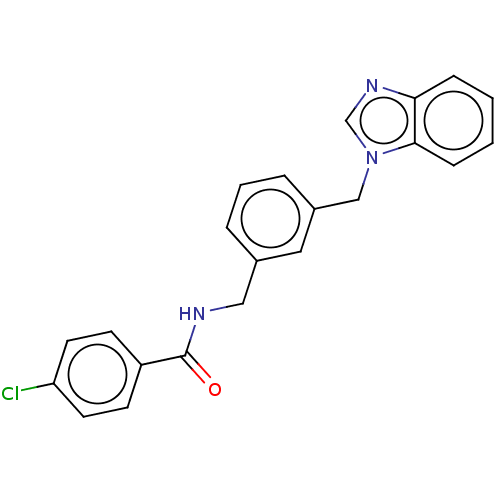 (CHEMBL4451614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 477 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 605 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human DAN-G cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514755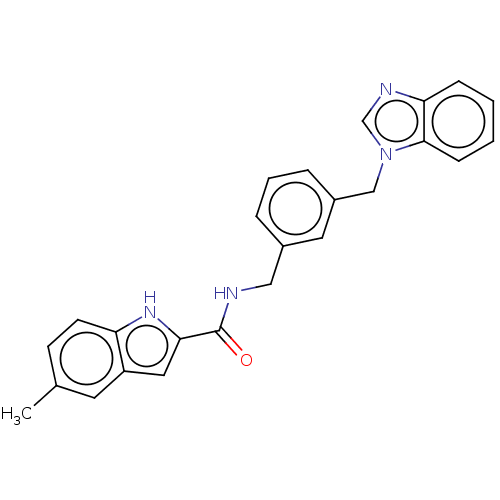 (CHEMBL4443904) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 636 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant GARFTase | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514756 (CHEMBL4439258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 781 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514763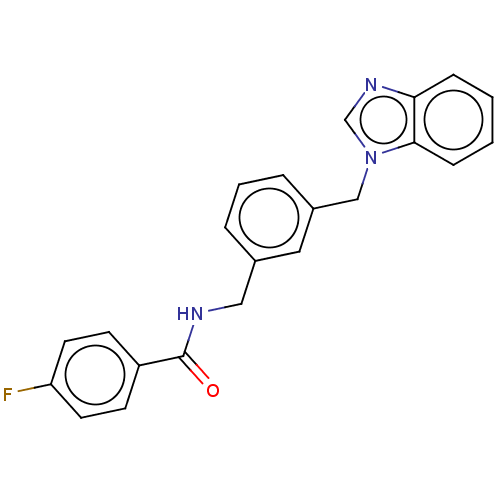 (CHEMBL4457663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 961 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514752 (CHEMBL4458549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human DAN-G cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM50514753 (CHEMBL4557994) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of mouse TDO transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50249877 ((S)-2-(4-(4-(2-amino-4-oxo-3,4-dihydrothieno[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant GARFTase | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50249876 (CHEMBL490934 | N-{4-[3-(2-Amino-4-oxo-3,4-dihydrot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant GARFTase | J Med Chem 52: 2940-51 (2009) Article DOI: 10.1021/jm8011323 BindingDB Entry DOI: 10.7270/Q2NG4QH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |