 Found 614 hits with Last Name = 'xiang' and Initial = 's'
Found 614 hits with Last Name = 'xiang' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Threonine--tRNA ligase
(Phytophthora sojae) | BDBM50129555
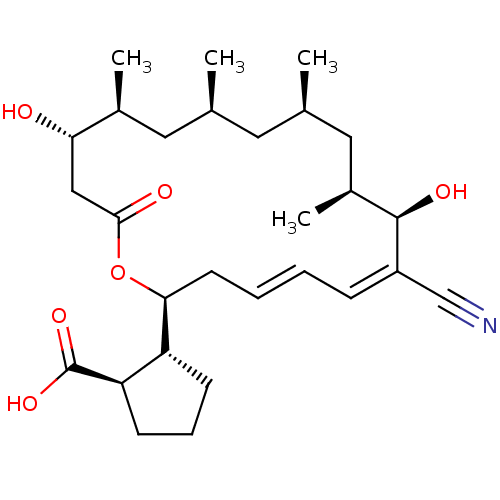
((1R,2R)-2-((4E,6Z)-(2S,8R,9S,11R,13S,15S,16S)-7-cy...)Show SMILES C[C@H]1C[C@@H](C)C[C@H](C)[C@@H](O)\C(=C/C=C/C[C@H](OC(=O)C[C@H](O)[C@@H](C)C1)[C@@H]1CCC[C@H]1C(O)=O)C#N |c:10,t:12| Show InChI InChI=1S/C28H43NO6/c1-17-12-18(2)14-20(4)27(32)21(16-29)8-5-6-11-25(22-9-7-10-23(22)28(33)34)35-26(31)15-24(30)19(3)13-17/h5-6,8,17-20,22-25,27,30,32H,7,9-15H2,1-4H3,(H,33,34)/b6-5+,21-8-/t17-,18+,19-,20-,22+,23+,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Agricultural University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged Phytophthora sojae race 1 recombinant ThrRS expressed in Escherichia coli BL21 (Codonplus DE3) assessed as reduc... |
J Agric Food Chem 60: 9874-81 (2012)
Article DOI: 10.1021/jf302857x
BindingDB Entry DOI: 10.7270/Q2B56NMN |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50604182
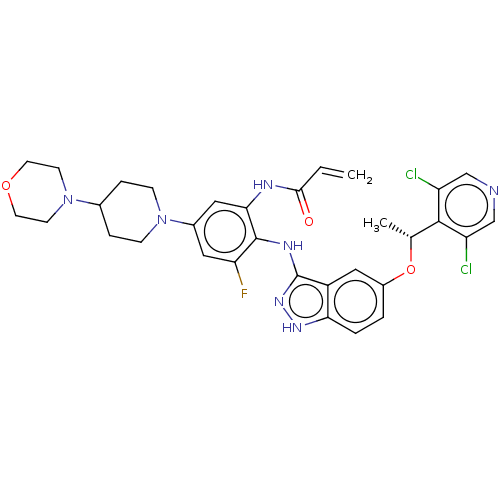
(CHEMBL5194425)Show SMILES C[C@@H](Oc1ccc2[nH]nc(Nc3c(F)cc(cc3NC(=O)C=C)N3CCC(CC3)N3CCOCC3)c2c1)c1c(Cl)cncc1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50189781
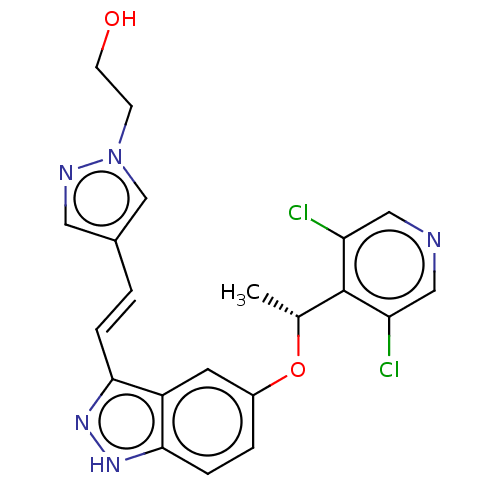
(CHEMBL3828009)Show SMILES C[C@@H](Oc1ccc2[nH]nc(\C=C\c3cnn(CCO)c3)c2c1)c1c(Cl)cncc1Cl |r| Show InChI InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50189781

(CHEMBL3828009)Show SMILES C[C@@H](Oc1ccc2[nH]nc(\C=C\c3cnn(CCO)c3)c2c1)c1c(Cl)cncc1Cl |r| Show InChI InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM435010

(US10584114, Compound 130 | US11279688, Compound 13...)Show SMILES CO[C@@]1(CC[C@@H](CC1)c1nc(C)cc(Nc2cc(C)[nH]n2)n1)C(=O)N[C@@H](C)c1ccc(nc1)-n1cc(F)cn1 |r,wU:2.1,5.8,25.28,wD:2.24,(1.11,-1.56,;1.11,-.02,;-.22,.75,;-.99,2.08,;-2.53,2.08,;-3.3,.75,;-2.53,-.59,;-.99,-.59,;-4.84,.75,;-5.61,2.08,;-7.15,2.08,;-7.92,3.41,;-7.92,.75,;-7.15,-.59,;-7.92,-1.92,;-9.46,-1.92,;-10.37,-.67,;-11.83,-1.15,;-13.08,-.24,;-11.83,-2.69,;-10.37,-3.17,;-5.61,-.59,;1.11,1.52,;1.11,3.06,;2.44,.75,;3.78,1.52,;3.78,3.06,;5.11,.75,;6.44,1.52,;7.78,.75,;7.78,-.79,;6.44,-1.56,;5.11,-.79,;9.11,-1.56,;10.52,-.94,;11.55,-2.08,;13.08,-1.92,;10.78,-3.41,;9.27,-3.09,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM435010

(US10584114, Compound 130 | US11279688, Compound 13...)Show SMILES CO[C@@]1(CC[C@@H](CC1)c1nc(C)cc(Nc2cc(C)[nH]n2)n1)C(=O)N[C@@H](C)c1ccc(nc1)-n1cc(F)cn1 |r,wU:2.1,5.8,25.28,wD:2.24,(1.11,-1.56,;1.11,-.02,;-.22,.75,;-.99,2.08,;-2.53,2.08,;-3.3,.75,;-2.53,-.59,;-.99,-.59,;-4.84,.75,;-5.61,2.08,;-7.15,2.08,;-7.92,3.41,;-7.92,.75,;-7.15,-.59,;-7.92,-1.92,;-9.46,-1.92,;-10.37,-.67,;-11.83,-1.15,;-13.08,-.24,;-11.83,-2.69,;-10.37,-3.17,;-5.61,-.59,;1.11,1.52,;1.11,3.06,;2.44,.75,;3.78,1.52,;3.78,3.06,;5.11,.75,;6.44,1.52,;7.78,.75,;7.78,-.79,;6.44,-1.56,;5.11,-.79,;9.11,-1.56,;10.52,-.94,;11.55,-2.08,;13.08,-1.92,;10.78,-3.41,;9.27,-3.09,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173235

((S)-2-{4'-[(5-Bromo-4-methoxy-benzofuran-2-carbony...)Show SMILES COc1c(Br)ccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H25BrN2O7S/c1-15(2)24(27(32)33)30-38(34,35)19-10-6-17(7-11-19)16-4-8-18(9-5-16)29-26(31)23-14-20-22(37-23)13-12-21(28)25(20)36-3/h4-15,24,30H,1-3H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM296429

(US10112942, Example 163 | US10112942, Example 166 ...)Show SMILES COc1ccc(CN2C3CC2CN(C3)c2ccc(cn2)-c2cc(OCC(C)(C)O)cn3ncc(C#N)c23)cn1 Show InChI InChI=1S/C29H31N7O3/c1-29(2,37)18-39-24-9-25(28-21(10-30)13-33-36(28)17-24)20-5-6-26(31-12-20)34-15-22-8-23(16-34)35(22)14-19-4-7-27(38-3)32-11-19/h4-7,9,11-13,17,22-23,37H,8,14-16,18H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM435010

(US10584114, Compound 130 | US11279688, Compound 13...)Show SMILES CO[C@@]1(CC[C@@H](CC1)c1nc(C)cc(Nc2cc(C)[nH]n2)n1)C(=O)N[C@@H](C)c1ccc(nc1)-n1cc(F)cn1 |r,wU:2.1,5.8,25.28,wD:2.24,(1.11,-1.56,;1.11,-.02,;-.22,.75,;-.99,2.08,;-2.53,2.08,;-3.3,.75,;-2.53,-.59,;-.99,-.59,;-4.84,.75,;-5.61,2.08,;-7.15,2.08,;-7.92,3.41,;-7.92,.75,;-7.15,-.59,;-7.92,-1.92,;-9.46,-1.92,;-10.37,-.67,;-11.83,-1.15,;-13.08,-.24,;-11.83,-2.69,;-10.37,-3.17,;-5.61,-.59,;1.11,1.52,;1.11,3.06,;2.44,.75,;3.78,1.52,;3.78,3.06,;5.11,.75,;6.44,1.52,;7.78,.75,;7.78,-.79,;6.44,-1.56,;5.11,-.79,;9.11,-1.56,;10.52,-.94,;11.55,-2.08,;13.08,-1.92,;10.78,-3.41,;9.27,-3.09,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM296429

(US10112942, Example 163 | US10112942, Example 166 ...)Show SMILES COc1ccc(CN2C3CC2CN(C3)c2ccc(cn2)-c2cc(OCC(C)(C)O)cn3ncc(C#N)c23)cn1 Show InChI InChI=1S/C29H31N7O3/c1-29(2,37)18-39-24-9-25(28-21(10-30)13-33-36(28)17-24)20-5-6-26(31-12-20)34-15-22-8-23(16-34)35(22)14-19-4-7-27(38-3)32-11-19/h4-7,9,11-13,17,22-23,37H,8,14-16,18H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50189781

(CHEMBL3828009)Show SMILES C[C@@H](Oc1ccc2[nH]nc(\C=C\c3cnn(CCO)c3)c2c1)c1c(Cl)cncc1Cl |r| Show InChI InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50604182

(CHEMBL5194425)Show SMILES C[C@@H](Oc1ccc2[nH]nc(Nc3c(F)cc(cc3NC(=O)C=C)N3CCC(CC3)N3CCOCC3)c2c1)c1c(Cl)cncc1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM394655
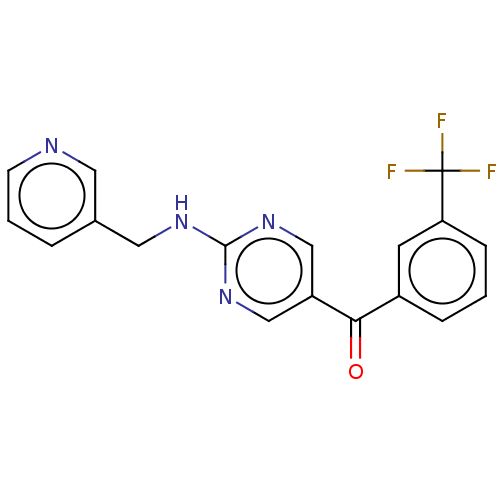
(US10308615, Example 12 | preparation of {2-[(pyrid...)Show SMILES FC(F)(F)c1cccc(c1)C(=O)c1cnc(NCc2cccnc2)nc1 Show InChI InChI=1S/C18H13F3N4O/c19-18(20,21)15-5-1-4-13(7-15)16(26)14-10-24-17(25-11-14)23-9-12-3-2-6-22-8-12/h1-8,10-11H,9H2,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50189781

(CHEMBL3828009)Show SMILES C[C@@H](Oc1ccc2[nH]nc(\C=C\c3cnn(CCO)c3)c2c1)c1c(Cl)cncc1Cl |r| Show InChI InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173236

((S)-2-{4'-[(5-Bromo-benzofuran-2-carbonyl)-amino]-...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3cc(Br)ccc3o2)cc1)C(O)=O Show InChI InChI=1S/C26H23BrN2O6S/c1-15(2)24(26(31)32)29-36(33,34)21-10-5-17(6-11-21)16-3-8-20(9-4-16)28-25(30)23-14-18-13-19(27)7-12-22(18)35-23/h3-15,24,29H,1-2H3,(H,28,30)(H,31,32)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM296429

(US10112942, Example 163 | US10112942, Example 166 ...)Show SMILES COc1ccc(CN2C3CC2CN(C3)c2ccc(cn2)-c2cc(OCC(C)(C)O)cn3ncc(C#N)c23)cn1 Show InChI InChI=1S/C29H31N7O3/c1-29(2,37)18-39-24-9-25(28-21(10-30)13-33-36(28)17-24)20-5-6-26(31-12-20)34-15-22-8-23(16-34)35(22)14-19-4-7-27(38-3)32-11-19/h4-7,9,11-13,17,22-23,37H,8,14-16,18H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pantetheinase
(Homo sapiens (Human)) | BDBM394660
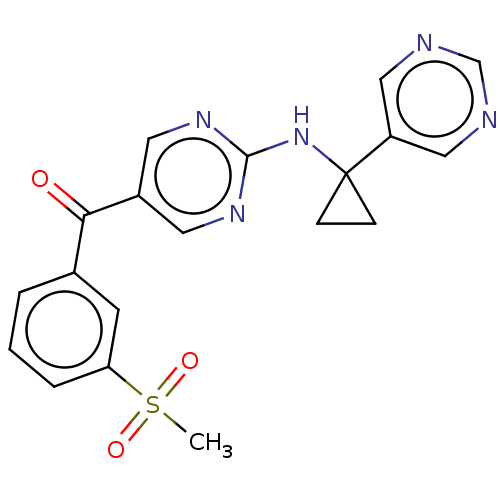
(US10308615, Example 17 | preparation of [3-(methyl...)Show SMILES CS(=O)(=O)c1cccc(c1)C(=O)c1cnc(NC2(CC2)c2cncnc2)nc1 Show InChI InChI=1S/C19H17N5O3S/c1-28(26,27)16-4-2-3-13(7-16)17(25)14-8-22-18(23-9-14)24-19(5-6-19)15-10-20-12-21-11-15/h2-4,7-12H,5-6H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173236

((S)-2-{4'-[(5-Bromo-benzofuran-2-carbonyl)-amino]-...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3cc(Br)ccc3o2)cc1)C(O)=O Show InChI InChI=1S/C26H23BrN2O6S/c1-15(2)24(26(31)32)29-36(33,34)21-10-5-17(6-11-21)16-3-8-20(9-4-16)28-25(30)23-14-18-13-19(27)7-12-22(18)35-23/h3-15,24,29H,1-2H3,(H,28,30)(H,31,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50459416

(CHEMBL4216473)Show SMILES COCCOc1cc(F)c2c(Nc3ccc(NC(=O)Cn4cc(nn4)C(C)C)cc3)ncnc2c1 Show InChI InChI=1S/C24H26FN7O3/c1-15(2)21-12-32(31-30-21)13-22(33)28-16-4-6-17(7-5-16)29-24-23-19(25)10-18(35-9-8-34-3)11-20(23)26-14-27-24/h4-7,10-12,14-15H,8-9,13H2,1-3H3,(H,28,33)(H,26,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50259007
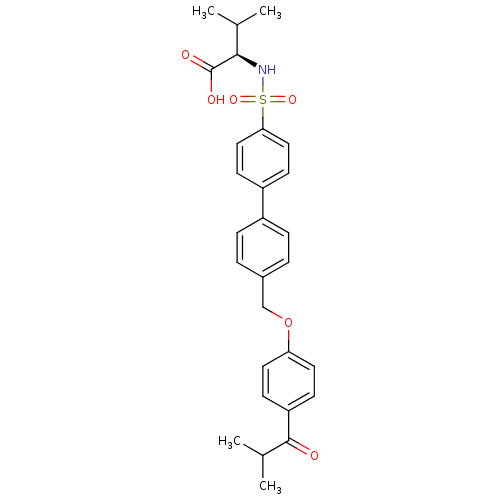
((R)-2-(4'-((4-isobutyrylphenoxy)methyl)biphenyl-4-...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C(C)C)cc1)C(O)=O |r| Show InChI InChI=1S/C28H31NO6S/c1-18(2)26(28(31)32)29-36(33,34)25-15-11-22(12-16-25)21-7-5-20(6-8-21)17-35-24-13-9-23(10-14-24)27(30)19(3)4/h5-16,18-19,26,29H,17H2,1-4H3,(H,31,32)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM395062

((2-{[(6- methylpyridin-3- yl)methyl]amino} pyrimid...)Show SMILES Cc1ccc(CNc2ncc(cn2)C(=O)c2cccc(c2)C(F)(F)F)cn1 Show InChI InChI=1S/C19H15F3N4O/c1-12-5-6-13(8-23-12)9-24-18-25-10-15(11-26-18)17(27)14-3-2-4-16(7-14)19(20,21)22/h2-8,10-11H,9H2,1H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50259007

((R)-2-(4'-((4-isobutyrylphenoxy)methyl)biphenyl-4-...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C(C)C)cc1)C(O)=O |r| Show InChI InChI=1S/C28H31NO6S/c1-18(2)26(28(31)32)29-36(33,34)25-15-11-22(12-16-25)21-7-5-20(6-8-21)17-35-24-13-9-23(10-14-24)27(30)19(3)4/h5-16,18-19,26,29H,17H2,1-4H3,(H,31,32)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM395062

((2-{[(6- methylpyridin-3- yl)methyl]amino} pyrimid...)Show SMILES Cc1ccc(CNc2ncc(cn2)C(=O)c2cccc(c2)C(F)(F)F)cn1 Show InChI InChI=1S/C19H15F3N4O/c1-12-5-6-13(8-23-12)9-24-18-25-10-15(11-26-18)17(27)14-3-2-4-16(7-14)19(20,21)22/h2-8,10-11H,9H2,1H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM394659
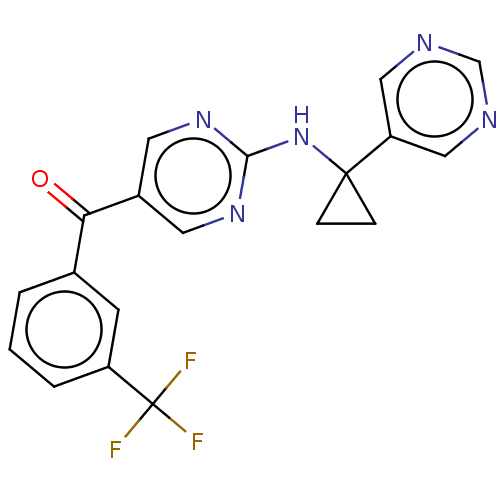
(US10308615, Example 16 | preparation of (2-{[1-(py...)Show SMILES FC(F)(F)c1cccc(c1)C(=O)c1cnc(NC2(CC2)c2cncnc2)nc1 Show InChI InChI=1S/C19H14F3N5O/c20-19(21,22)14-3-1-2-12(6-14)16(28)13-7-25-17(26-8-13)27-18(4-5-18)15-9-23-11-24-10-15/h1-3,6-11H,4-5H2,(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185140
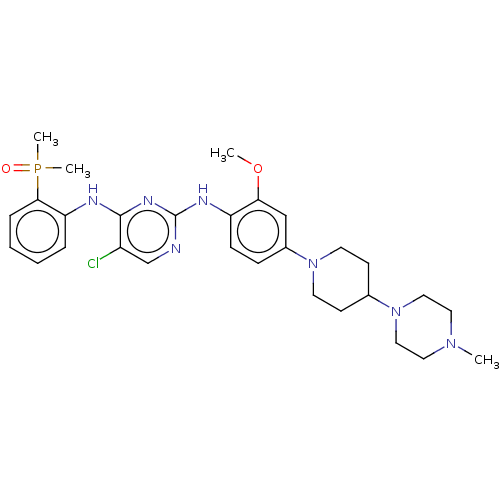
(AP-26113 | Brigatinib | US11248003, Example Brigat...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pantetheinase
(Homo sapiens (Human)) | BDBM394660

(US10308615, Example 17 | preparation of [3-(methyl...)Show SMILES CS(=O)(=O)c1cccc(c1)C(=O)c1cnc(NC2(CC2)c2cncnc2)nc1 Show InChI InChI=1S/C19H17N5O3S/c1-28(26,27)16-4-2-3-13(7-16)17(25)14-8-22-18(23-9-14)24-19(5-6-19)15-10-20-12-21-11-15/h2-4,7-12H,5-6H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM394655

(US10308615, Example 12 | preparation of {2-[(pyrid...)Show SMILES FC(F)(F)c1cccc(c1)C(=O)c1cnc(NCc2cccnc2)nc1 Show InChI InChI=1S/C18H13F3N4O/c19-18(20,21)15-5-1-4-13(7-15)16(26)14-10-24-17(25-11-14)23-9-12-3-2-6-22-8-12/h1-8,10-11H,9H2,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173220

((S)-2-{4'-[(5-Chloro-4-methoxy-3-methyl-benzofuran...)Show SMILES COc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27ClN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50189781

(CHEMBL3828009)Show SMILES C[C@@H](Oc1ccc2[nH]nc(\C=C\c3cnn(CCO)c3)c2c1)c1c(Cl)cncc1Cl |r| Show InChI InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Pantetheinase
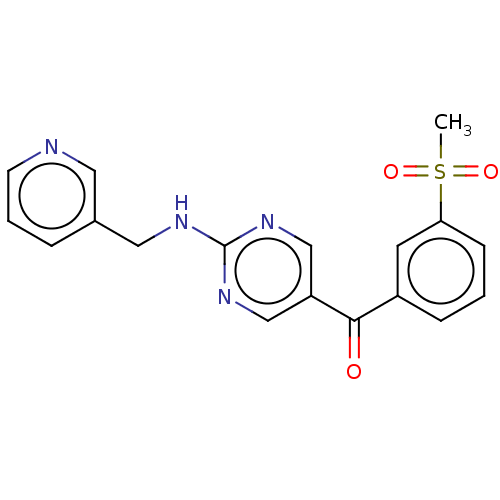
(Homo sapiens (Human)) | BDBM394656
(US10308615, Example 13 | [3-(methylsulfonyl)phenyl...)Show SMILES CS(=O)(=O)c1cccc(c1)C(=O)c1cnc(NCc2cccnc2)nc1 Show InChI InChI=1S/C18H16N4O3S/c1-26(24,25)16-6-2-5-14(8-16)17(23)15-11-21-18(22-12-15)20-10-13-4-3-7-19-9-13/h2-9,11-12H,10H2,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50507492

(Loxo-195 | Selitrectinib | US10966985, Compound 33...)Show SMILES C[C@@H]1CCc2ncc(F)cc2[C@H]2CCCN2c2ccn3ncc(C(=O)N1)c3n2 Show InChI InChI=1S/C20H21FN6O/c1-12-4-5-16-14(9-13(21)10-22-16)17-3-2-7-26(17)18-6-8-27-19(25-18)15(11-23-27)20(28)24-12/h6,8-12,17H,2-5,7H2,1H3,(H,24,28)/t12-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00308
BindingDB Entry DOI: 10.7270/Q2KP868W |
More data for this
Ligand-Target Pair | |
Pantetheinase
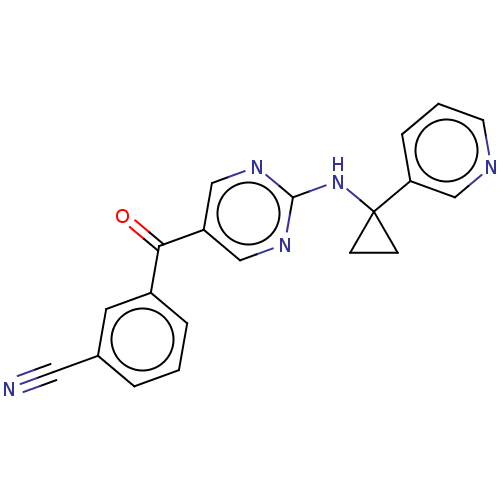
(Homo sapiens (Human)) | BDBM394645
(3-[(2-{[1-(pyridin-3-yl)cyclopropyl]amino}pyrimidi...)Show SMILES O=C(c1cnc(NC2(CC2)c2cccnc2)nc1)c1cccc(c1)C#N Show InChI InChI=1S/C20H15N5O/c21-10-14-3-1-4-15(9-14)18(26)16-11-23-19(24-12-16)25-20(6-7-20)17-5-2-8-22-13-17/h1-5,8-9,11-13H,6-7H2,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50189781

(CHEMBL3828009)Show SMILES C[C@@H](Oc1ccc2[nH]nc(\C=C\c3cnn(CCO)c3)c2c1)c1c(Cl)cncc1Cl |r| Show InChI InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
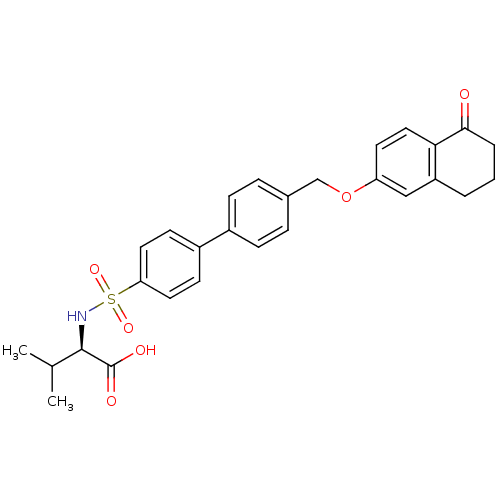
(Homo sapiens (Human)) | BDBM50259009
((R)-3-methyl-2-(4'-((5-oxo-5,6,7,8-tetrahydronapht...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc3C(=O)CCCc3c2)cc1)C(O)=O |r| Show InChI InChI=1S/C28H29NO6S/c1-18(2)27(28(31)32)29-36(33,34)24-13-10-21(11-14-24)20-8-6-19(7-9-20)17-35-23-12-15-25-22(16-23)4-3-5-26(25)30/h6-16,18,27,29H,3-5,17H2,1-2H3,(H,31,32)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
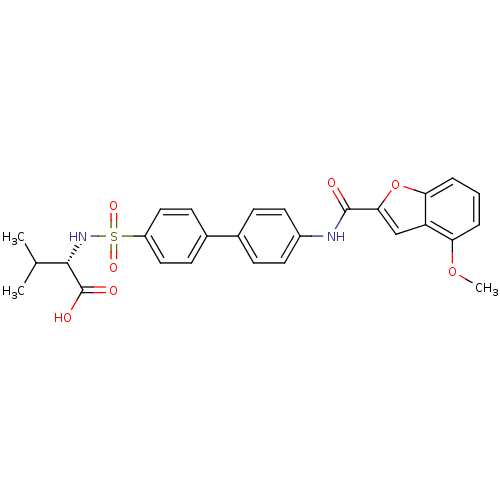
(Homo sapiens (Human)) | BDBM50170298
((S)-2-{4'-[(4-Methoxy-benzofuran-2-carbonyl)-amino...)Show SMILES COc1cccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H26N2O7S/c1-16(2)25(27(31)32)29-37(33,34)20-13-9-18(10-14-20)17-7-11-19(12-8-17)28-26(30)24-15-21-22(35-3)5-4-6-23(21)36-24/h4-16,25,29H,1-3H3,(H,28,30)(H,31,32)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50507492

(Loxo-195 | Selitrectinib | US10966985, Compound 33...)Show SMILES C[C@@H]1CCc2ncc(F)cc2[C@H]2CCCN2c2ccn3ncc(C(=O)N1)c3n2 Show InChI InChI=1S/C20H21FN6O/c1-12-4-5-16-14(9-13(21)10-22-16)17-3-2-7-26(17)18-6-8-27-19(25-18)15(11-23-27)20(28)24-12/h6,8-12,17H,2-5,7H2,1H3,(H,24,28)/t12-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00308
BindingDB Entry DOI: 10.7270/Q2KP868W |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM28485
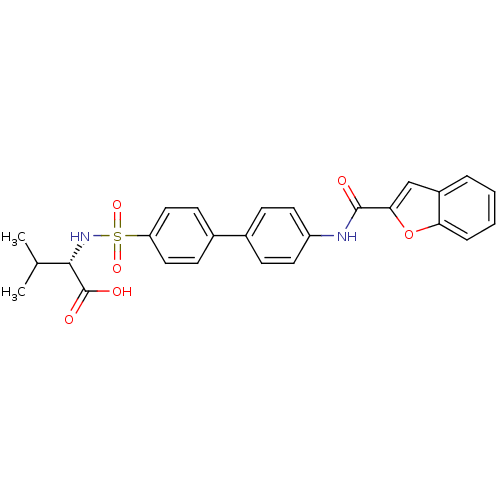
((2S)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM24043
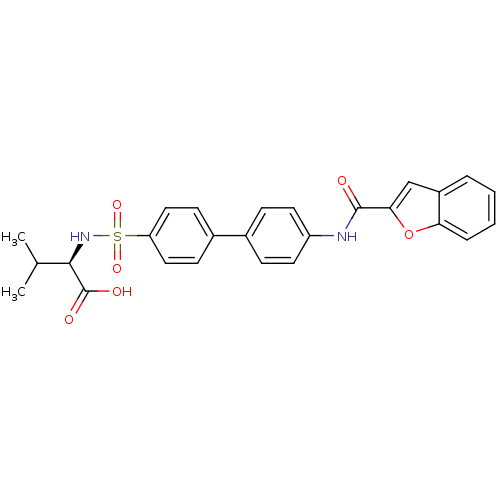
((2R)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM394659

(US10308615, Example 16 | preparation of (2-{[1-(py...)Show SMILES FC(F)(F)c1cccc(c1)C(=O)c1cnc(NC2(CC2)c2cncnc2)nc1 Show InChI InChI=1S/C19H14F3N5O/c20-19(21,22)14-3-1-2-12(6-14)16(28)13-7-25-17(26-8-13)27-18(4-5-18)15-9-23-11-24-10-15/h1-3,6-11H,4-5H2,(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM136597
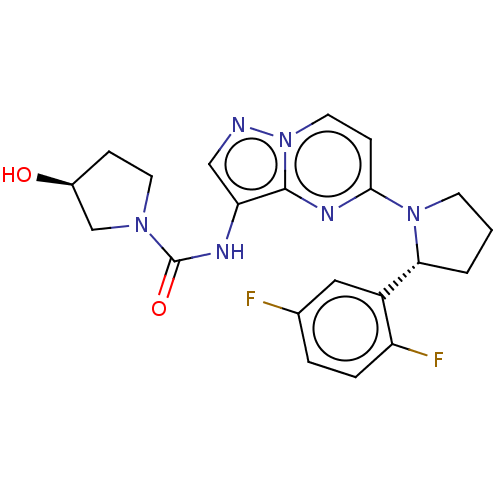
(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00308
BindingDB Entry DOI: 10.7270/Q2KP868W |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50605160

(CHEMBL5184321)Show SMILES CN1CCN(Cc2cc(NC(=O)c3cc4OCCCCC(=O)NCCN(C)c5ccc6ncc(C#Cc(c4)c3C)n6n5)cc(c2)C(F)(F)F)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00308
BindingDB Entry DOI: 10.7270/Q2KP868W |
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM394655

(US10308615, Example 12 | preparation of {2-[(pyrid...)Show SMILES FC(F)(F)c1cccc(c1)C(=O)c1cnc(NCc2cccnc2)nc1 Show InChI InChI=1S/C18H13F3N4O/c19-18(20,21)15-5-1-4-13(7-15)16(26)14-10-24-17(25-11-14)23-9-12-3-2-6-22-8-12/h1-8,10-11H,9H2,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
BindingDB Entry DOI: 10.7270/Q22809Z8 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173229
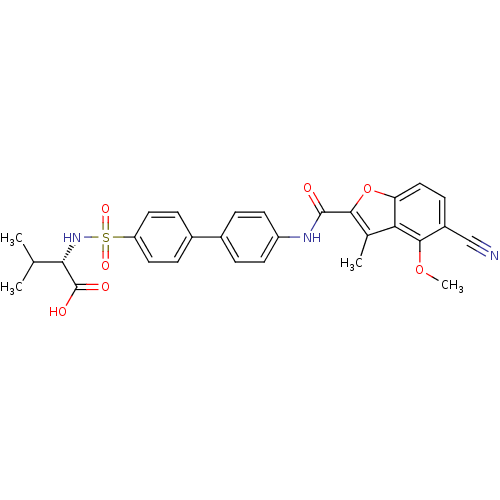
((S)-2-{4'-[(5-Cyano-4-methoxy-3-methyl-benzofuran-...)Show SMILES COc1c(ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12)C#N Show InChI InChI=1S/C29H27N3O7S/c1-16(2)25(29(34)35)32-40(36,37)22-12-7-19(8-13-22)18-5-10-21(11-6-18)31-28(33)26-17(3)24-23(39-26)14-9-20(15-30)27(24)38-4/h5-14,16,25,32H,1-4H3,(H,31,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50459416

(CHEMBL4216473)Show SMILES COCCOc1cc(F)c2c(Nc3ccc(NC(=O)Cn4cc(nn4)C(C)C)cc3)ncnc2c1 Show InChI InChI=1S/C24H26FN7O3/c1-15(2)21-12-32(31-30-21)13-22(33)28-16-4-6-17(7-5-16)29-24-23-19(25)10-18(35-9-8-34-3)11-20(23)26-14-27-24/h4-7,10-12,14-15H,8-9,13H2,1-3H3,(H,28,33)(H,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173224
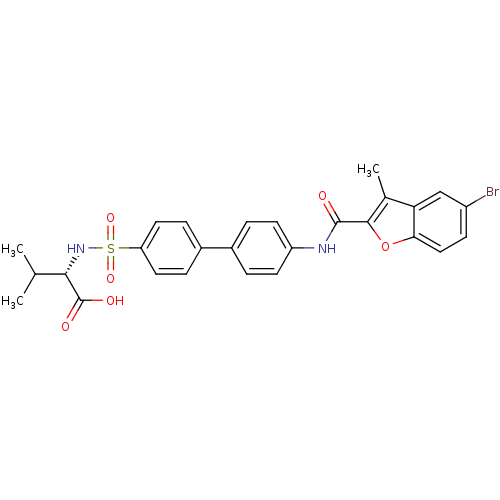
((S)-2-{4'-[(5-Bromo-3-methyl-benzofuran-2-carbonyl...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccc(Br)cc3c2C)cc1)C(O)=O Show InChI InChI=1S/C27H25BrN2O6S/c1-15(2)24(27(32)33)30-37(34,35)21-11-6-18(7-12-21)17-4-9-20(10-5-17)29-26(31)25-16(3)22-14-19(28)8-13-23(22)36-25/h4-15,24,30H,1-3H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50507492

(Loxo-195 | Selitrectinib | US10966985, Compound 33...)Show SMILES C[C@@H]1CCc2ncc(F)cc2[C@H]2CCCN2c2ccn3ncc(C(=O)N1)c3n2 Show InChI InChI=1S/C20H21FN6O/c1-12-4-5-16-14(9-13(21)10-22-16)17-3-2-7-26(17)18-6-8-27-19(25-18)15(11-23-27)20(28)24-12/h6,8-12,17H,2-5,7H2,1H3,(H,24,28)/t12-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00308
BindingDB Entry DOI: 10.7270/Q2KP868W |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50605159
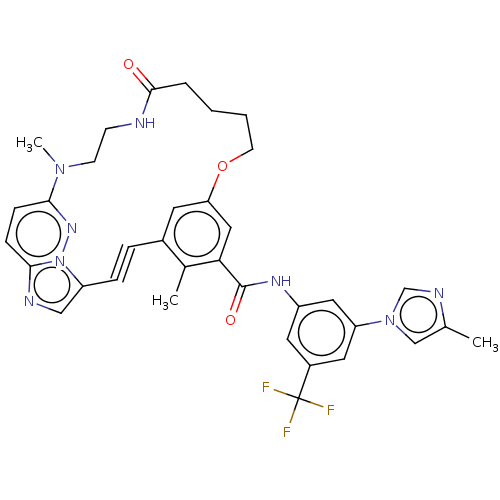
(CHEMBL5195469)Show SMILES CN1CCNC(=O)CCCCOc2cc(C#Cc3cnc4ccc1nn34)c(C)c(c2)C(=O)Nc1cc(cc(c1)C(F)(F)F)-n1cnc(C)c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00308
BindingDB Entry DOI: 10.7270/Q2KP868W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data