

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069330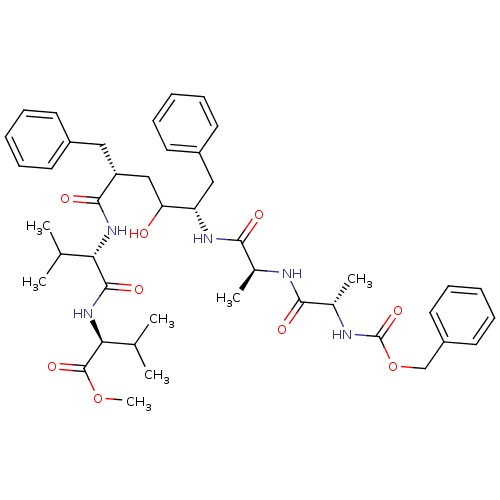 ((S)-2-((S)-2-{(2R,5S)-2-Benzyl-5-[(S)-2-((S)-2-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease was determined | Bioorg Med Chem Lett 8: 699-704 (1999) BindingDB Entry DOI: 10.7270/Q20K27QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50148827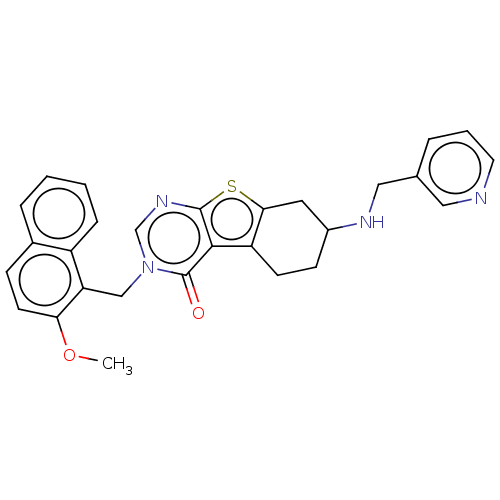 (CHEMBL3769975) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using tubulin-K40 peptide in prese... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/nucleoside cotransporter 1 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 28 member 3 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069332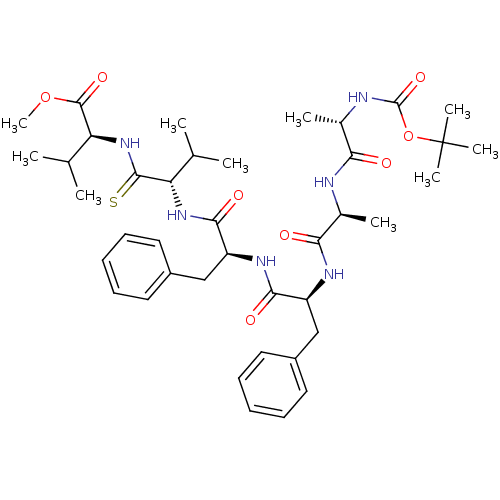 ((S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-tert-Buto...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity was evaluated for competitive inhibition of HIV-1 Protease | Bioorg Med Chem Lett 8: 699-704 (1999) BindingDB Entry DOI: 10.7270/Q20K27QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 28 member 3 (Homo sapiens (Human)) | BDBM50088517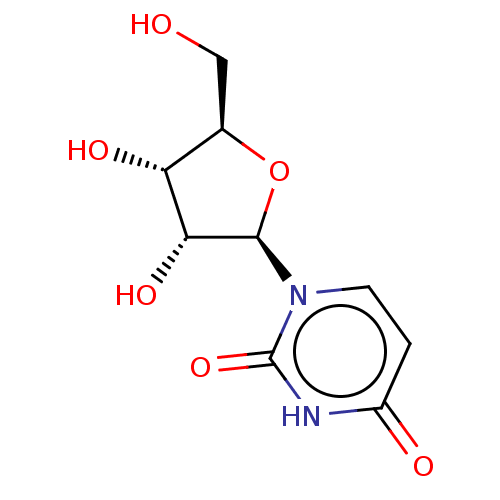 (CHEBI:16704 | Uridine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/nucleoside cotransporter 1 (Homo sapiens (Human)) | BDBM50088517 (CHEBI:16704 | Uridine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/nucleoside cotransporter 2 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50138530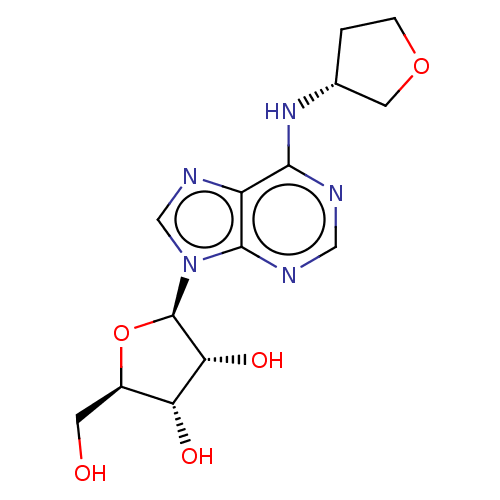 ((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50326469 (3,4'-Dihydroxy-3',5'-dimethoxybiphenyl-4-carboxyli...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 30 mins by Lineweaver-Burk double reciprocal plot analysis | Bioorg Med Chem 18: 6708-14 (2010) Article DOI: 10.1016/j.bmc.2010.07.062 BindingDB Entry DOI: 10.7270/Q2JS9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/nucleoside cotransporter 2 (Homo sapiens (Human)) | BDBM50088517 (CHEBI:16704 | Uridine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50326455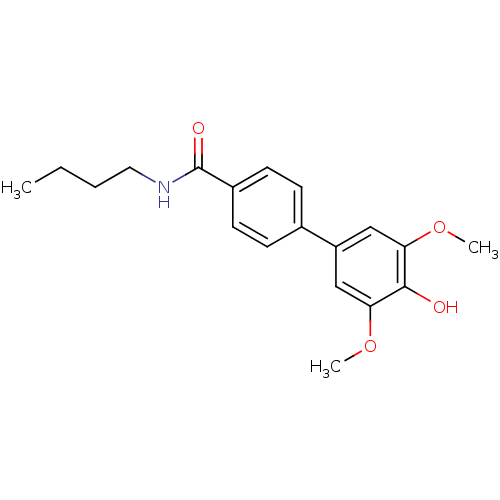 (CHEMBL1243344 | N-Butyl-4'-hydroxy-3',5'-dimethoxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 30 mins by Lineweaver-Burk double reciprocal plot analysis | Bioorg Med Chem 18: 6708-14 (2010) Article DOI: 10.1016/j.bmc.2010.07.062 BindingDB Entry DOI: 10.7270/Q2JS9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/nucleoside cotransporter 1 (Homo sapiens (Human)) | BDBM50138530 ((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50326456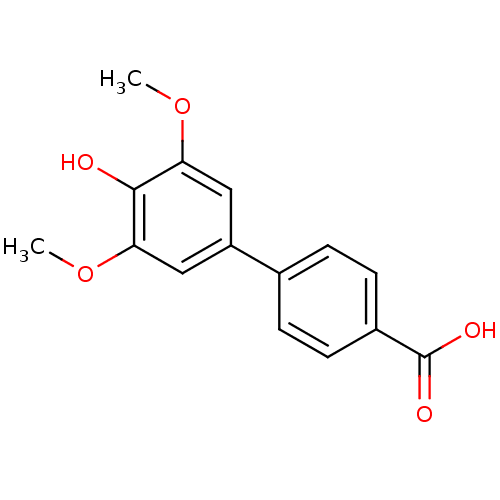 (4'-Hydroxy-3',5'-dimethoxybiphenyl-4-carboxylic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 30 mins by Lineweaver-Burk double reciprocal plot analysis | Bioorg Med Chem 18: 6708-14 (2010) Article DOI: 10.1016/j.bmc.2010.07.062 BindingDB Entry DOI: 10.7270/Q2JS9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50088517 (CHEBI:16704 | Uridine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50326467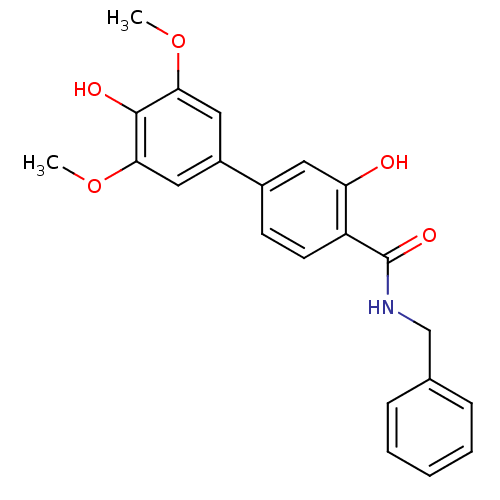 (CHEMBL1243315 | N-Benzyl-3,4'-dihydroxy-3',5'-dime...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 30 mins by Lineweaver-Burk double reciprocal plot analysis | Bioorg Med Chem 18: 6708-14 (2010) Article DOI: 10.1016/j.bmc.2010.07.062 BindingDB Entry DOI: 10.7270/Q2JS9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 2 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 28 member 3 (Homo sapiens (Human)) | BDBM50138530 ((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 2 (Homo sapiens (Human)) | BDBM50138530 ((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/nucleoside cotransporter 2 (Homo sapiens (Human)) | BDBM50138530 ((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 2 (Homo sapiens (Human)) | BDBM50088517 (CHEBI:16704 | Uridine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... | Drug Metab Dispos 41: 916-22 (2013) Article DOI: 10.1124/dmd.112.049858 BindingDB Entry DOI: 10.7270/Q21N82VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165469 (CHEMBL3797911) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165465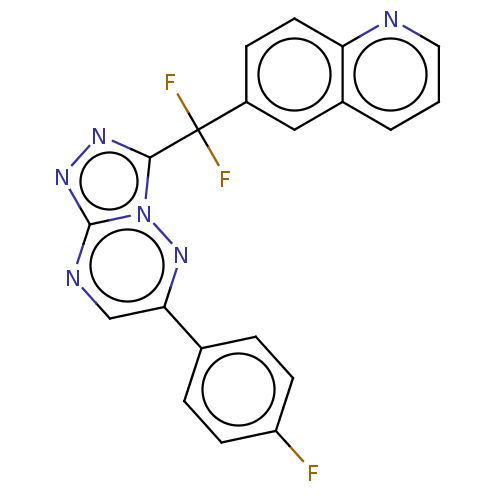 (CHEMBL3797579) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165571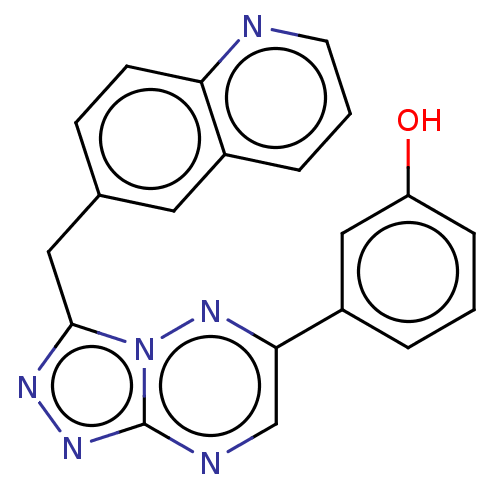 (CHEMBL3800471) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165466 (CHEMBL3800420) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of c-Met in human EBC-1 cells assessed as reduction in cell proliferation after 72 hrs by SRB or MTT assay | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165465 (CHEMBL3797579) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of c-Met in human MKN45 cells assessed as reduction in cell proliferation after 72 hrs by SRB or MTT assay | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165567 (CHEMBL3797914) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165578 (CHEMBL3799192) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165467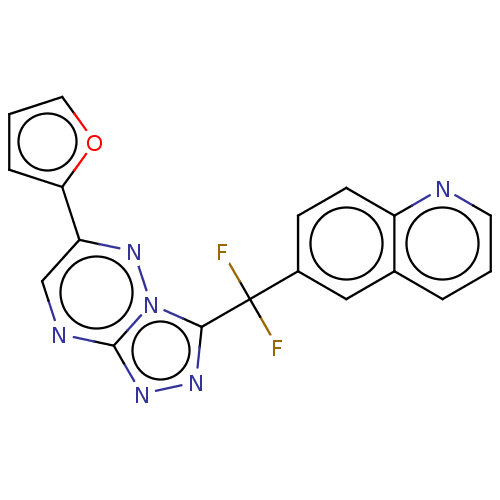 (CHEMBL3798905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165466 (CHEMBL3800420) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165572 (CHEMBL3800559) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165468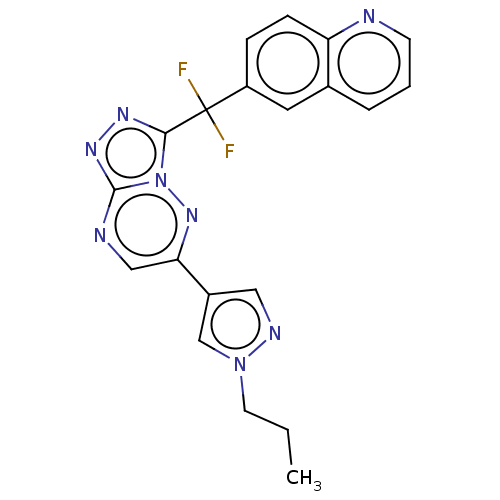 (CHEMBL3799263) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of c-Met in human EBC-1 cells assessed as reduction in cell proliferation after 72 hrs by SRB or MTT assay | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165465 (CHEMBL3797579) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of c-Met in human EBC-1 cells assessed as reduction in cell proliferation after 72 hrs by SRB or MTT assay | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165468 (CHEMBL3799263) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM163243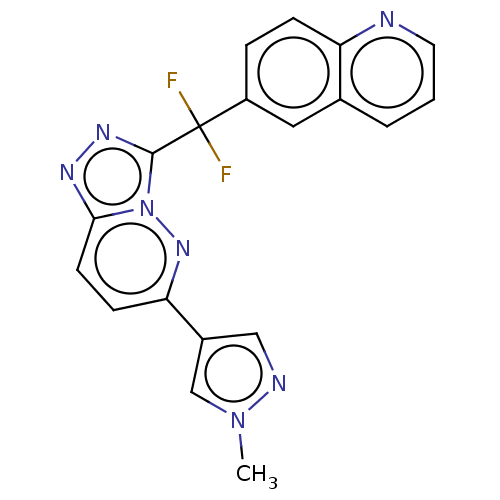 (US9062045, Comparator No. 1 (JNJ-38877605)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50549813 (CHEMBL4776177) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged FGFR3 V555M mutant (unknown origin) expressed in insect cells using poly Glu-Tyr preincubated for 1 hr followed by substrate... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00517 BindingDB Entry DOI: 10.7270/Q2RV0S9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165565 (CHEMBL3800295) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165576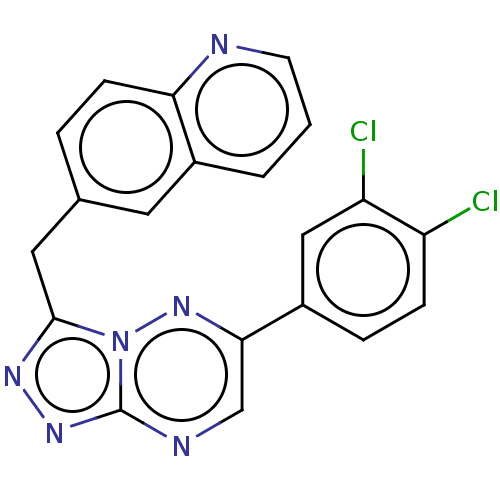 (CHEMBL3800427) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleoprotein TPR (Mus musculus) | BDBM50165465 (CHEMBL3797579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of TPR-MET in mouse BAF3 cells assessed as reduction in cell proliferation after 72 hrs by SRB or MTT assay | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165470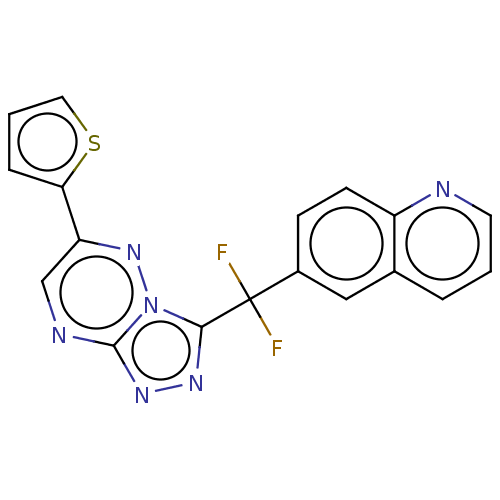 (CHEMBL3797871) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165575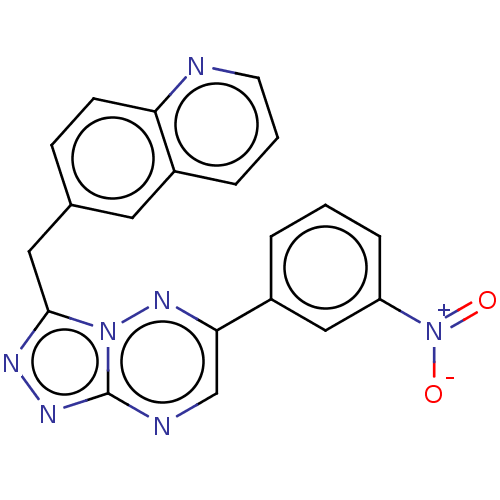 (CHEMBL3798623) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165569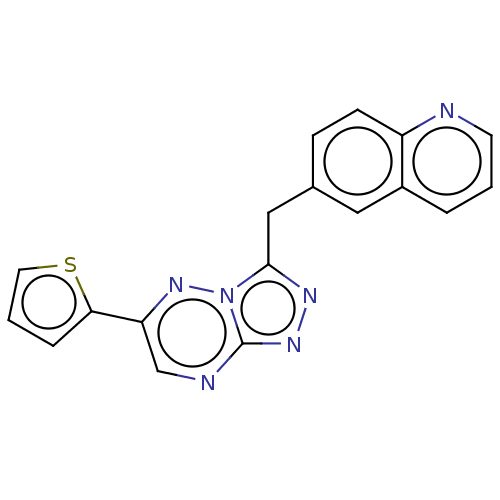 (CHEMBL3797547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165570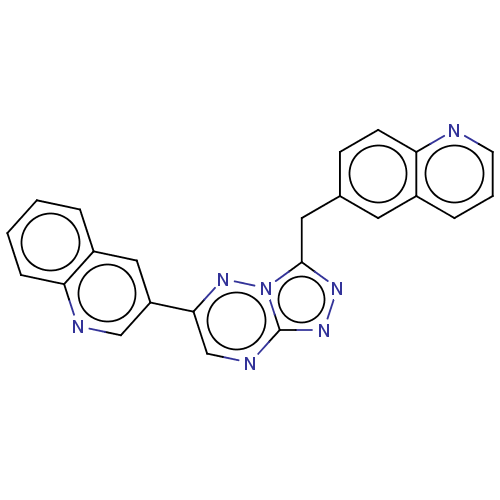 (CHEMBL3798174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165467 (CHEMBL3798905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of c-Met in human EBC-1 cells assessed as reduction in cell proliferation after 72 hrs by SRB or MTT assay | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165574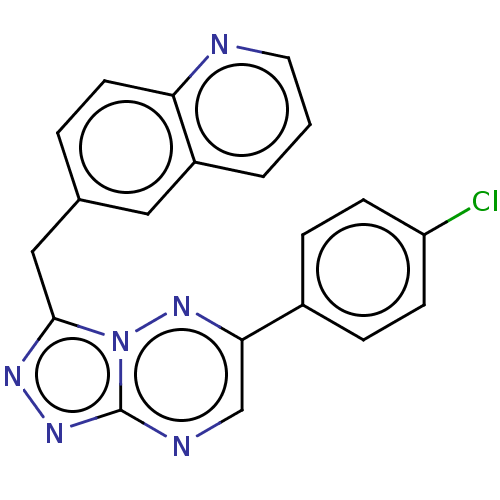 (CHEMBL3797307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165476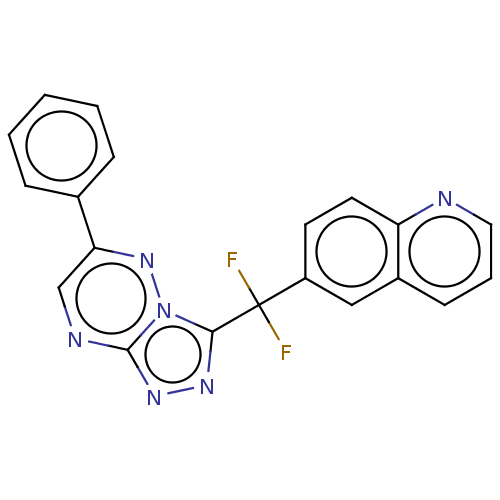 (CHEMBL3800061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165568 (CHEMBL3798660) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165566 (CHEMBL3799924) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50165573 (CHEMBL3798130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS) Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISA | Eur J Med Chem 116: 239-251 (2016) Article DOI: 10.1016/j.ejmech.2016.03.076 BindingDB Entry DOI: 10.7270/Q2P27111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 651 total ) | Next | Last >> |