 Found 201 hits with Last Name = 'yang' and Initial = 'sg'
Found 201 hits with Last Name = 'yang' and Initial = 'sg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50056112
(CHEMBL3326702)Show SMILES COc1cc2cc(C(=O)NCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2cc1OC Show InChI InChI=1S/C30H33N3O5/c1-36-26-17-19-16-22(30(35)38-25(19)18-27(26)37-2)29(34)32-15-9-3-8-14-31-28-20-10-4-6-12-23(20)33-24-13-7-5-11-21(24)28/h4,6,10,12,16-18H,3,5,7-9,11,13-15H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056107
(CHEMBL3326704)Show SMILES O=C(NCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C29H31N3O3/c33-28(23-19-20-11-3-8-16-26(20)35-29(23)34)31-18-10-2-1-9-17-30-27-21-12-4-6-14-24(21)32-25-15-7-5-13-22(25)27/h3-4,6,8,11-12,14,16,19H,1-2,5,7,9-10,13,15,17-18H2,(H,30,32)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056107

(CHEMBL3326704)Show SMILES O=C(NCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C29H31N3O3/c33-28(23-19-20-11-3-8-16-26(20)35-29(23)34)31-18-10-2-1-9-17-30-27-21-12-4-6-14-24(21)32-25-15-7-5-13-22(25)27/h3-4,6,8,11-12,14,16,19H,1-2,5,7,9-10,13,15,17-18H2,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056109

(CHEMBL3326709)Show SMILES FC(F)(F)Oc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H30F3N3O4/c31-30(32,33)40-20-13-14-26-19(17-20)18-23(29(38)39-26)28(37)35-16-8-2-1-7-15-34-27-21-9-3-5-11-24(21)36-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,1-2,4,6-8,10,12,15-16H2,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056119
(CHEMBL3326710)Show SMILES O=C(NCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C30H33N3O3/c34-29(24-20-21-12-4-9-17-27(21)36-30(24)35)32-19-11-3-1-2-10-18-31-28-22-13-5-7-15-25(22)33-26-16-8-6-14-23(26)28/h4-5,7,9,12-13,15,17,20H,1-3,6,8,10-11,14,16,18-19H2,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056122
(CHEMBL3326705)Show SMILES COc1ccc2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C30H33N3O4/c1-36-21-15-14-20-18-24(30(35)37-27(20)19-21)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056108

(CHEMBL3326707)Show SMILES Cc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N3O3/c1-20-14-15-27-21(18-20)19-24(30(35)36-27)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056121
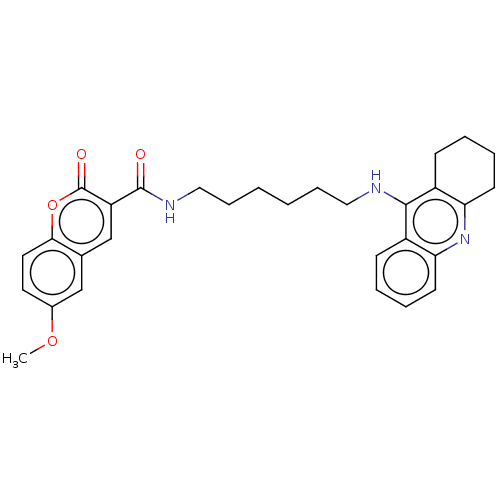
(CHEMBL3326706)Show SMILES COc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N3O4/c1-36-21-14-15-27-20(18-21)19-24(30(35)37-27)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056121

(CHEMBL3326706)Show SMILES COc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N3O4/c1-36-21-14-15-27-20(18-21)19-24(30(35)37-27)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056108

(CHEMBL3326707)Show SMILES Cc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N3O3/c1-20-14-15-27-21(18-20)19-24(30(35)36-27)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056115
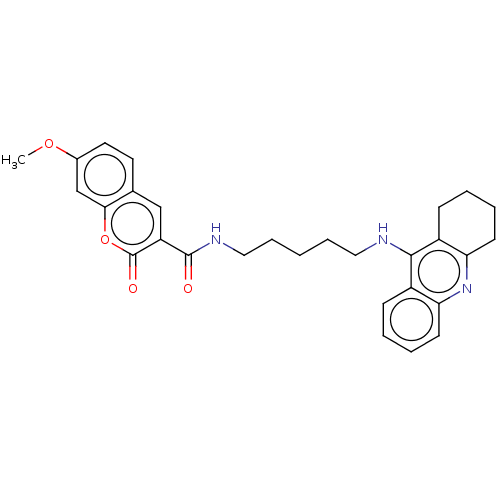
(CHEMBL3326699)Show SMILES COc1ccc2cc(C(=O)NCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C29H31N3O4/c1-35-20-14-13-19-17-23(29(34)36-26(19)18-20)28(33)31-16-8-2-7-15-30-27-21-9-3-5-11-24(21)32-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,2,4,6-8,10,12,15-16H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase, chloroplastic
(Nicotiana tabacum) | BDBM50486212
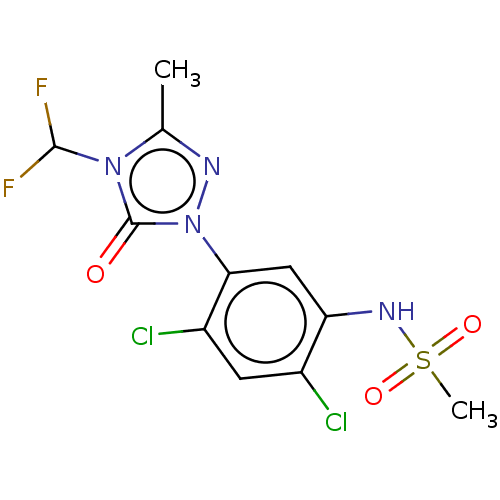
(CHEBI:9339 | SULFENTRAZONE)Show SMILES Cc1nn(-c2cc(NS(C)(=O)=O)c(Cl)cc2Cl)c(=O)n1C(F)F Show InChI InChI=1S/C11H10Cl2F2N4O3S/c1-5-16-19(11(20)18(5)10(14)15)9-4-8(17-23(2,21)22)6(12)3-7(9)13/h3-4,10,17H,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay |
Bioorg Med Chem 21: 3245-55 (2013)
Article DOI: 10.1016/j.bmc.2013.03.056
BindingDB Entry DOI: 10.7270/Q2ZW1PTP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056120
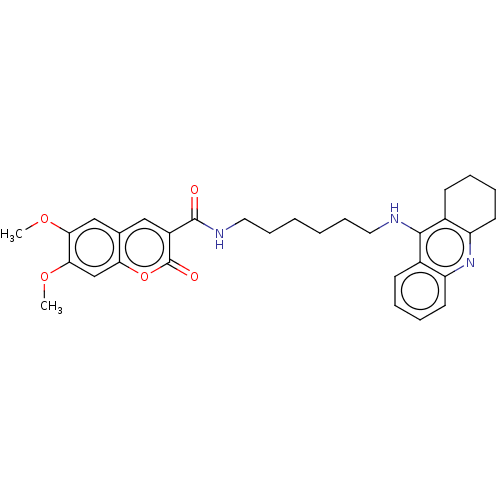
(CHEMBL3326708)Show SMILES COc1cc2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2cc1OC Show InChI InChI=1S/C31H35N3O5/c1-37-27-18-20-17-23(31(36)39-26(20)19-28(27)38-2)30(35)33-16-10-4-3-9-15-32-29-21-11-5-7-13-24(21)34-25-14-8-6-12-22(25)29/h5,7,11,13,17-19H,3-4,6,8-10,12,14-16H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056122

(CHEMBL3326705)Show SMILES COc1ccc2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C30H33N3O4/c1-36-21-15-14-20-18-24(30(35)37-27(20)19-21)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056106
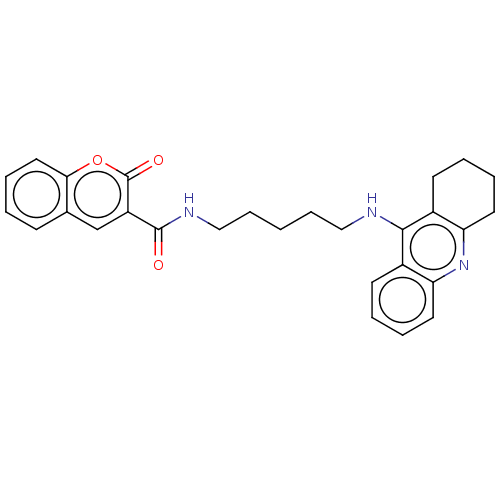
(CHEMBL3326698)Show SMILES O=C(NCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C28H29N3O3/c32-27(22-18-19-10-2-7-15-25(19)34-28(22)33)30-17-9-1-8-16-29-26-20-11-3-5-13-23(20)31-24-14-6-4-12-21(24)26/h2-3,5,7,10-11,13,15,18H,1,4,6,8-9,12,14,16-17H2,(H,29,31)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056113

(CHEMBL3326701)Show SMILES Cc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H31N3O3/c1-19-13-14-26-20(17-19)18-23(29(34)35-26)28(33)31-16-8-2-7-15-30-27-21-9-3-5-11-24(21)32-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,2,4,6-8,10,12,15-16H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056123
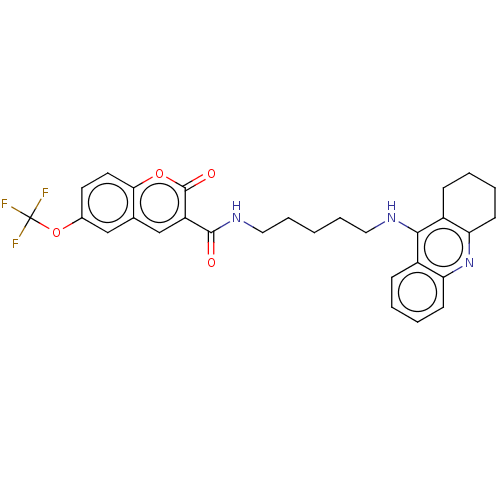
(CHEMBL3326703)Show SMILES FC(F)(F)Oc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H28F3N3O4/c30-29(31,32)39-19-12-13-25-18(16-19)17-22(28(37)38-25)27(36)34-15-7-1-6-14-33-26-20-8-2-4-10-23(20)35-24-11-5-3-9-21(24)26/h2,4,8,10,12-13,16-17H,1,3,5-7,9,11,14-15H2,(H,33,35)(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056114
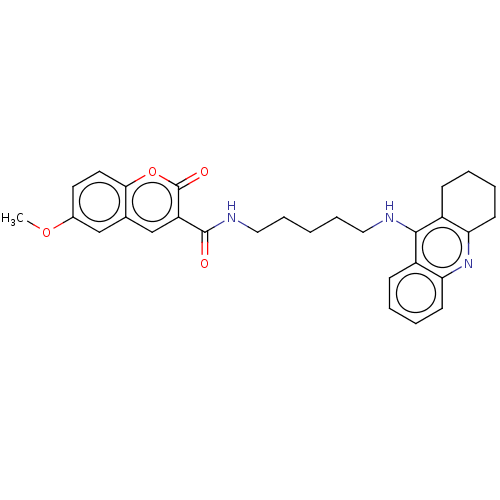
(CHEMBL3326700)Show SMILES COc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H31N3O4/c1-35-20-13-14-26-19(17-20)18-23(29(34)36-26)28(33)31-16-8-2-7-15-30-27-21-9-3-5-11-24(21)32-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,2,4,6-8,10,12,15-16H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056106

(CHEMBL3326698)Show SMILES O=C(NCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C28H29N3O3/c32-27(22-18-19-10-2-7-15-25(19)34-28(22)33)30-17-9-1-8-16-29-26-20-11-3-5-13-23(20)31-24-14-6-4-12-21(24)26/h2-3,5,7,10-11,13,15,18H,1,4,6,8-9,12,14,16-17H2,(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056118
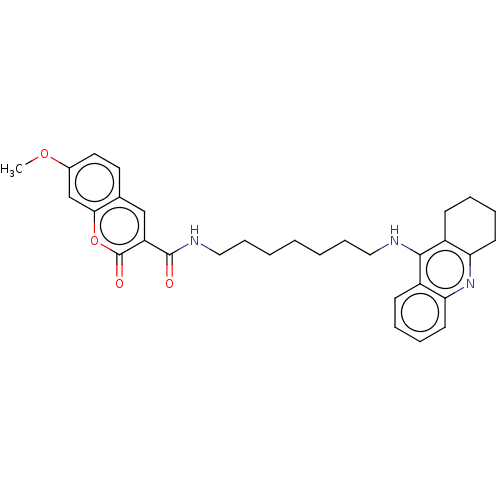
(CHEMBL3326711)Show SMILES COc1ccc2cc(C(=O)NCCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C31H35N3O4/c1-37-22-16-15-21-19-25(31(36)38-28(21)20-22)30(35)33-18-10-4-2-3-9-17-32-29-23-11-5-7-13-26(23)34-27-14-8-6-12-24(27)29/h5,7,11,13,15-16,19-20H,2-4,6,8-10,12,14,17-18H2,1H3,(H,32,34)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056110
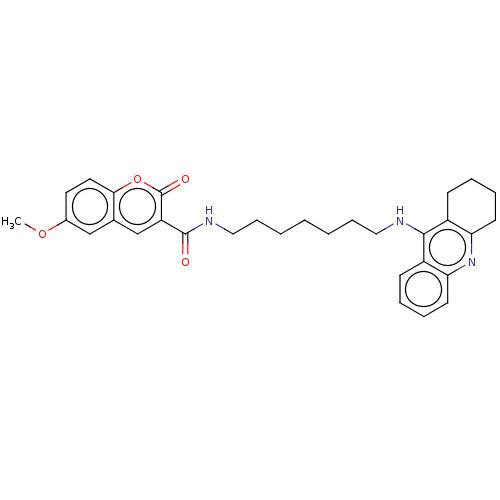
(CHEMBL3327246)Show SMILES COc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H35N3O4/c1-37-22-15-16-28-21(19-22)20-25(31(36)38-28)30(35)33-18-10-4-2-3-9-17-32-29-23-11-5-7-13-26(23)34-27-14-8-6-12-24(27)29/h5,7,11,13,15-16,19-20H,2-4,6,8-10,12,14,17-18H2,1H3,(H,32,34)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056113

(CHEMBL3326701)Show SMILES Cc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H31N3O3/c1-19-13-14-26-20(17-19)18-23(29(34)35-26)28(33)31-16-8-2-7-15-30-27-21-9-3-5-11-24(21)32-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,2,4,6-8,10,12,15-16H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056117
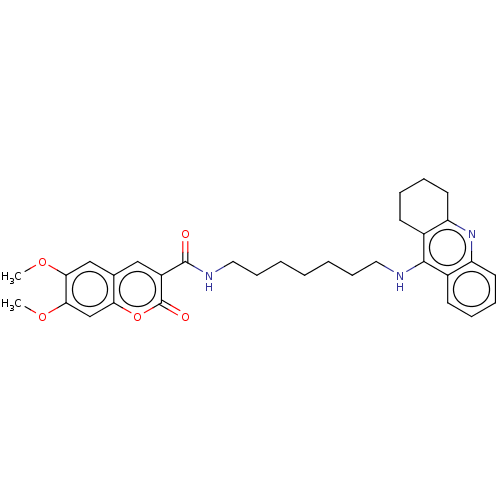
(CHEMBL3327248)Show SMILES COc1cc2cc(C(=O)NCCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2cc1OC Show InChI InChI=1S/C32H37N3O5/c1-38-28-19-21-18-24(32(37)40-27(21)20-29(28)39-2)31(36)34-17-11-5-3-4-10-16-33-30-22-12-6-8-14-25(22)35-26-15-9-7-13-23(26)30/h6,8,12,14,18-20H,3-5,7,9-11,13,15-17H2,1-2H3,(H,33,35)(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056114

(CHEMBL3326700)Show SMILES COc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H31N3O4/c1-35-20-13-14-26-19(17-20)18-23(29(34)36-26)28(33)31-16-8-2-7-15-30-27-21-9-3-5-11-24(21)32-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,2,4,6-8,10,12,15-16H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase
(Homo sapiens (Human)) | BDBM50359944

(CHEMBL1926835)Show SMILES CCOC(=O)Sc1nc2cc(c(F)cc2s1)-n1nc(sc1=O)C(C)(C)C Show InChI InChI=1S/C16H16FN3O3S3/c1-5-23-15(22)26-13-18-9-7-10(8(17)6-11(9)24-13)20-14(21)25-12(19-20)16(2,3)4/h6-7H,5H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human PPO by capillary electrophoresis method |
Bioorg Med Chem 20: 296-304 (2011)
Article DOI: 10.1016/j.bmc.2011.10.079
BindingDB Entry DOI: 10.7270/Q2FJ2H6T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056116
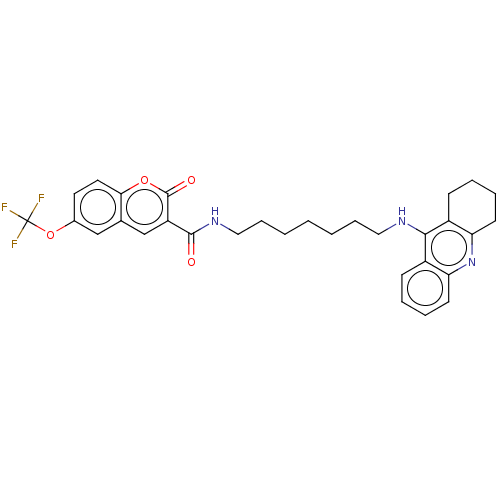
(CHEMBL3327249)Show SMILES FC(F)(F)Oc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H32F3N3O4/c32-31(33,34)41-21-14-15-27-20(18-21)19-24(30(39)40-27)29(38)36-17-9-3-1-2-8-16-35-28-22-10-4-6-12-25(22)37-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,1-3,5,7-9,11,13,16-17H2,(H,35,37)(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056119

(CHEMBL3326710)Show SMILES O=C(NCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C30H33N3O3/c34-29(24-20-21-12-4-9-17-27(21)36-30(24)35)32-19-11-3-1-2-10-18-31-28-22-13-5-7-15-25(22)33-26-16-8-6-14-23(26)28/h4-5,7,9,12-13,15,17,20H,1-3,6,8,10-11,14,16,18-19H2,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056115

(CHEMBL3326699)Show SMILES COc1ccc2cc(C(=O)NCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C29H31N3O4/c1-35-20-14-13-19-17-23(29(34)36-26(19)18-20)28(33)31-16-8-2-7-15-30-27-21-9-3-5-11-24(21)32-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,2,4,6-8,10,12,15-16H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056111

(CHEMBL3327247)Show SMILES Cc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H35N3O3/c1-21-15-16-28-22(19-21)20-25(31(36)37-28)30(35)33-18-10-4-2-3-9-17-32-29-23-11-5-7-13-26(23)34-27-14-8-6-12-24(27)29/h5,7,11,13,15-16,19-20H,2-4,6,8-10,12,14,17-18H2,1H3,(H,32,34)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056110

(CHEMBL3327246)Show SMILES COc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H35N3O4/c1-37-22-15-16-28-21(19-22)20-25(31(36)38-28)30(35)33-18-10-4-2-3-9-17-32-29-23-11-5-7-13-26(23)34-27-14-8-6-12-24(27)29/h5,7,11,13,15-16,19-20H,2-4,6,8-10,12,14,17-18H2,1H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056118

(CHEMBL3326711)Show SMILES COc1ccc2cc(C(=O)NCCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C31H35N3O4/c1-37-22-16-15-21-19-25(31(36)38-28(21)20-22)30(35)33-18-10-4-2-3-9-17-32-29-23-11-5-7-13-26(23)34-27-14-8-6-12-24(27)29/h5,7,11,13,15-16,19-20H,2-4,6,8-10,12,14,17-18H2,1H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056120

(CHEMBL3326708)Show SMILES COc1cc2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2cc1OC Show InChI InChI=1S/C31H35N3O5/c1-37-27-18-20-17-23(31(36)39-26(20)19-28(27)38-2)30(35)33-16-10-4-3-9-15-32-29-21-11-5-7-13-24(21)34-25-14-8-6-12-22(25)29/h5,7,11,13,17-19H,3-4,6,8-10,12,14-16H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase, chloroplastic
(Nicotiana tabacum) | BDBM50488401
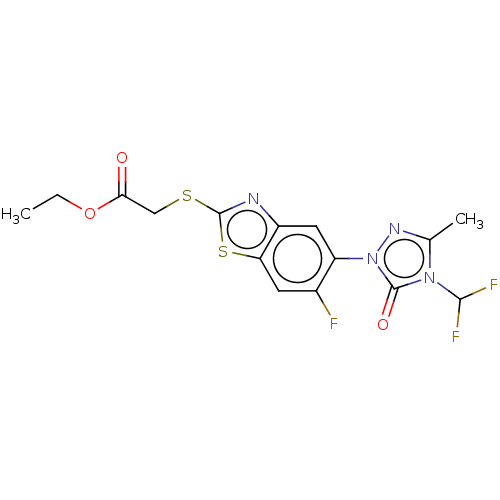
(CHEMBL2286233)Show SMILES CCOC(=O)CSc1nc2cc(c(F)cc2s1)-n1nc(C)n(C(F)F)c1=O Show InChI InChI=1S/C15H13F3N4O3S2/c1-3-25-12(23)6-26-14-19-9-5-10(8(16)4-11(9)27-14)22-15(24)21(13(17)18)7(2)20-22/h4-5,13H,3,6H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay |
Bioorg Med Chem 21: 3245-55 (2013)
Article DOI: 10.1016/j.bmc.2013.03.056
BindingDB Entry DOI: 10.7270/Q2ZW1PTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056109

(CHEMBL3326709)Show SMILES FC(F)(F)Oc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H30F3N3O4/c31-30(32,33)40-20-13-14-26-19(17-20)18-23(29(38)39-26)28(37)35-16-8-2-1-7-15-34-27-21-9-3-5-11-24(21)36-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,1-2,4,6-8,10,12,15-16H2,(H,34,36)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056111

(CHEMBL3327247)Show SMILES Cc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H35N3O3/c1-21-15-16-28-22(19-21)20-25(31(36)37-28)30(35)33-18-10-4-2-3-9-17-32-29-23-11-5-7-13-26(23)34-27-14-8-6-12-24(27)29/h5,7,11,13,15-16,19-20H,2-4,6,8-10,12,14,17-18H2,1H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056112

(CHEMBL3326702)Show SMILES COc1cc2cc(C(=O)NCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2cc1OC Show InChI InChI=1S/C30H33N3O5/c1-36-26-17-19-16-22(30(35)38-25(19)18-27(26)37-2)29(34)32-15-9-3-8-14-31-28-20-10-4-6-12-23(20)33-24-13-7-5-11-21(24)28/h4,6,10,12,16-18H,3,5,7-9,11,13-15H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase, mitochondrial
(Nicotiana tabacum) | BDBM50359942
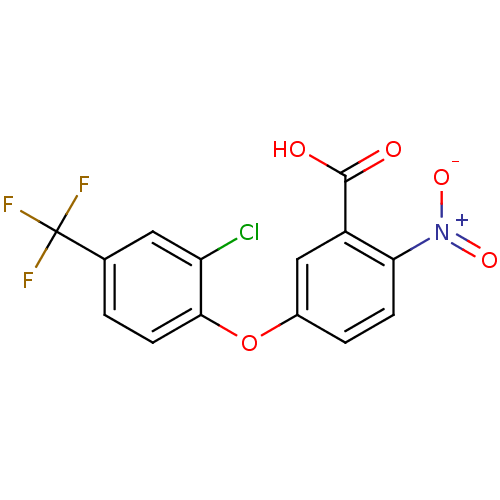
(ACIFLUORFEN)Show SMILES OC(=O)c1cc(Oc2ccc(cc2Cl)C(F)(F)F)ccc1[N+]([O-])=O Show InChI InChI=1S/C14H7ClF3NO5/c15-10-5-7(14(16,17)18)1-4-12(10)24-8-2-3-11(19(22)23)9(6-8)13(20)21/h1-6H,(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Nicotiana tobacum PPO |
Bioorg Med Chem 20: 296-304 (2011)
Article DOI: 10.1016/j.bmc.2011.10.079
BindingDB Entry DOI: 10.7270/Q2FJ2H6T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056123

(CHEMBL3326703)Show SMILES FC(F)(F)Oc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H28F3N3O4/c30-29(31,32)39-19-12-13-25-18(16-19)17-22(28(37)38-25)27(36)34-15-7-1-6-14-33-26-20-8-2-4-10-23(20)35-24-11-5-3-9-21(24)26/h2,4,8,10,12-13,16-17H,1,3,5-7,9,11,14-15H2,(H,33,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056116

(CHEMBL3327249)Show SMILES FC(F)(F)Oc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H32F3N3O4/c32-31(33,34)41-21-14-15-27-20(18-21)19-24(30(39)40-27)29(38)36-17-9-3-1-2-8-16-35-28-22-10-4-6-12-25(22)37-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,1-3,5,7-9,11,13,16-17H2,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056117

(CHEMBL3327248)Show SMILES COc1cc2cc(C(=O)NCCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2cc1OC Show InChI InChI=1S/C32H37N3O5/c1-38-28-19-21-18-24(32(37)40-27(21)20-29(28)39-2)31(36)34-17-11-5-3-4-10-16-33-30-22-12-6-8-14-25(22)35-26-15-9-7-13-23(26)30/h6,8,12,14,18-20H,3-5,7,9-11,13,15-17H2,1-2H3,(H,33,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase
(Homo sapiens (Human)) | BDBM50359953

(CHEMBL1926845)Show SMILES CCOC(=O)Sc1nc2cc(c(Cl)cc2s1)-n1nc(sc1=O)C(C)(C)C Show InChI InChI=1S/C16H16ClN3O3S3/c1-5-23-15(22)26-13-18-9-7-10(8(17)6-11(9)24-13)20-14(21)25-12(19-20)16(2,3)4/h6-7H,5H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human PPO by capillary electrophoresis method |
Bioorg Med Chem 20: 296-304 (2011)
Article DOI: 10.1016/j.bmc.2011.10.079
BindingDB Entry DOI: 10.7270/Q2FJ2H6T |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase, chloroplastic
(Nicotiana tabacum) | BDBM50488398

(CHEMBL2286231)Show InChI InChI=1S/C14H11F3N4OS2/c1-3-4-23-13-18-9-6-10(8(15)5-11(9)24-13)21-14(22)20(12(16)17)7(2)19-21/h3,5-6,12H,1,4H2,2H3 | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay |
Bioorg Med Chem 21: 3245-55 (2013)
Article DOI: 10.1016/j.bmc.2013.03.056
BindingDB Entry DOI: 10.7270/Q2ZW1PTP |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase, chloroplastic
(Nicotiana tabacum) | BDBM50488385
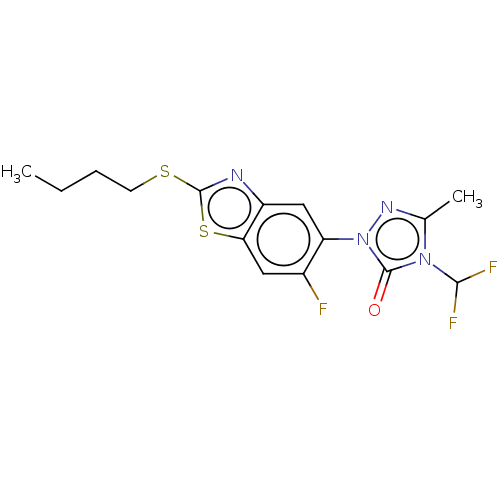
(CHEMBL2286232)Show SMILES CCCCSc1nc2cc(c(F)cc2s1)-n1nc(C)n(C(F)F)c1=O Show InChI InChI=1S/C15H15F3N4OS2/c1-3-4-5-24-14-19-10-7-11(9(16)6-12(10)25-14)22-15(23)21(13(17)18)8(2)20-22/h6-7,13H,3-5H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay |
Bioorg Med Chem 21: 3245-55 (2013)
Article DOI: 10.1016/j.bmc.2013.03.056
BindingDB Entry DOI: 10.7270/Q2ZW1PTP |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase, chloroplastic
(Nicotiana tabacum) | BDBM50488397
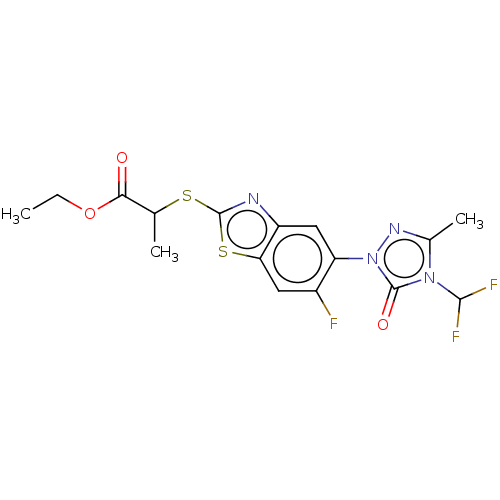
(CHEMBL2286234)Show SMILES CCOC(=O)C(C)Sc1nc2cc(c(F)cc2s1)-n1nc(C)n(C(F)F)c1=O Show InChI InChI=1S/C16H15F3N4O3S2/c1-4-26-13(24)7(2)27-15-20-10-6-11(9(17)5-12(10)28-15)23-16(25)22(14(18)19)8(3)21-23/h5-7,14H,4H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay |
Bioorg Med Chem 21: 3245-55 (2013)
Article DOI: 10.1016/j.bmc.2013.03.056
BindingDB Entry DOI: 10.7270/Q2ZW1PTP |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase
(Homo sapiens (Human)) | BDBM50359932

(CHEMBL1926967)Show SMILES CCOC(=O)CCSc1nc2cc(c(Cl)cc2s1)-n1nc(oc1=O)C(C)(C)C Show InChI InChI=1S/C18H20ClN3O4S2/c1-5-25-14(23)6-7-27-16-20-11-9-12(10(19)8-13(11)28-16)22-17(24)26-15(21-22)18(2,3)4/h8-9H,5-7H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human PPO by capillary electrophoresis method |
Bioorg Med Chem 20: 296-304 (2011)
Article DOI: 10.1016/j.bmc.2011.10.079
BindingDB Entry DOI: 10.7270/Q2FJ2H6T |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase, chloroplastic
(Nicotiana tabacum) | BDBM50488382
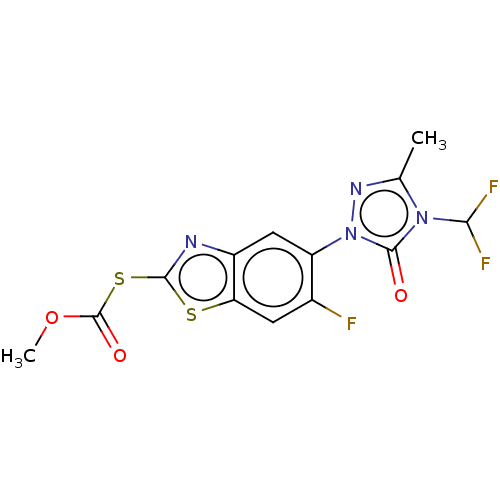
(CHEMBL2286235)Show SMILES COC(=O)Sc1nc2cc(c(F)cc2s1)-n1nc(C)n(C(F)F)c1=O Show InChI InChI=1S/C13H9F3N4O3S2/c1-5-18-20(12(21)19(5)10(15)16)8-4-7-9(3-6(8)14)24-11(17-7)25-13(22)23-2/h3-4,10H,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay |
Bioorg Med Chem 21: 3245-55 (2013)
Article DOI: 10.1016/j.bmc.2013.03.056
BindingDB Entry DOI: 10.7270/Q2ZW1PTP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data