

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469417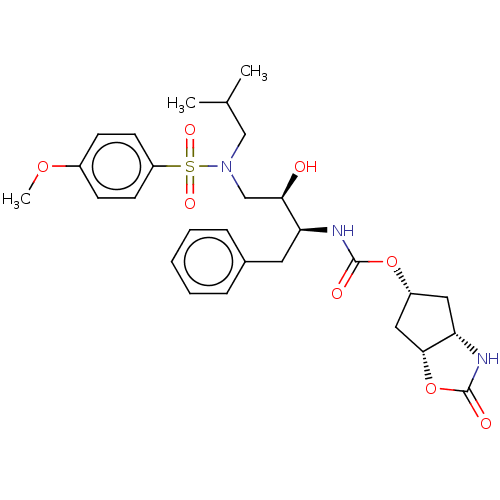 (CHEMBL4293023) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520752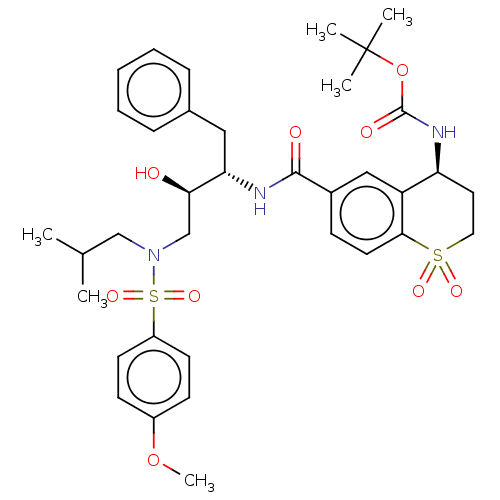 (CHEMBL4474261) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469413 (CHEMBL4286231) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469407 (CHEMBL4286714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561628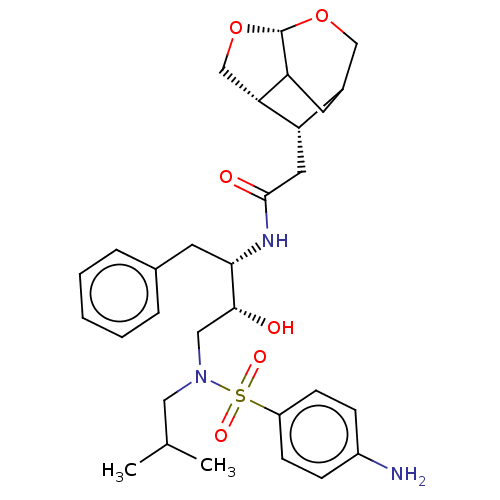 (CHEMBL4800615) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469410 (CHEMBL4277886) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561630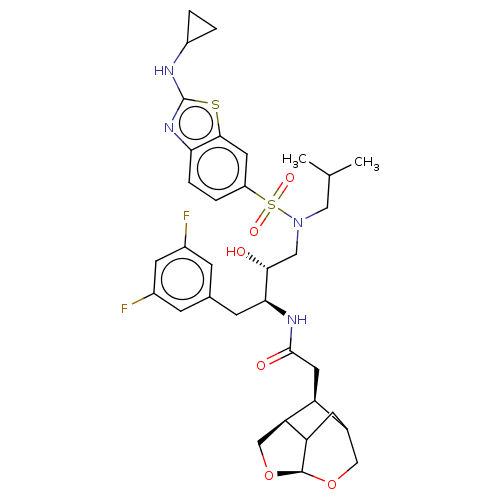 (CHEMBL4743665) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561627 (CHEMBL4744116) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469405 (CHEMBL4292006) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520751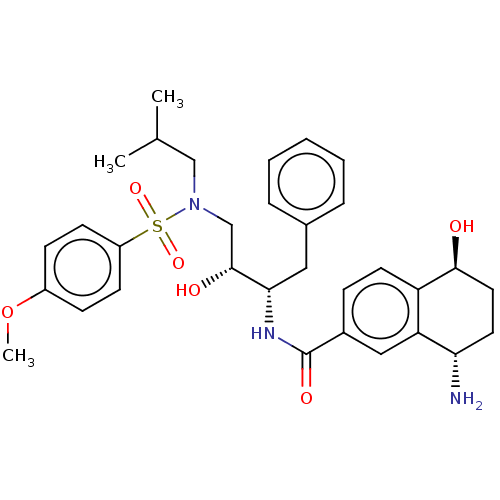 (CHEMBL4473891) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561629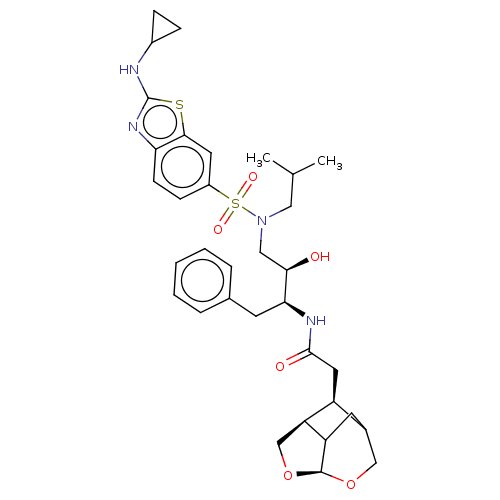 (CHEMBL4740468) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469412 (CHEMBL4277970) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520749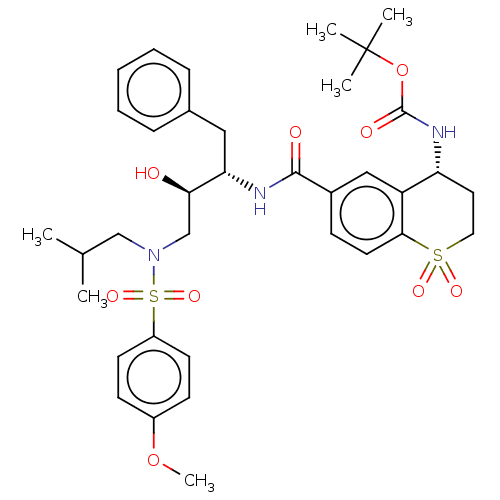 (CHEMBL4459250) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520742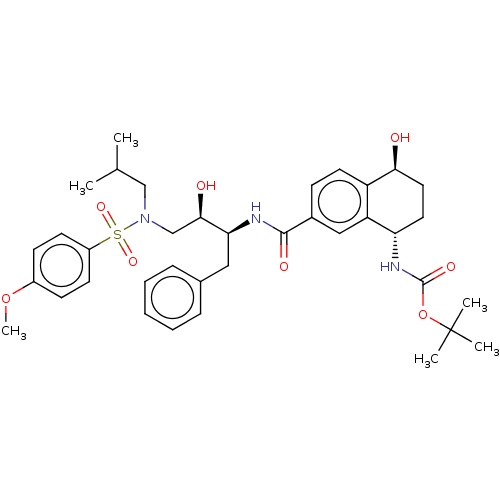 (CHEMBL4526105) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469418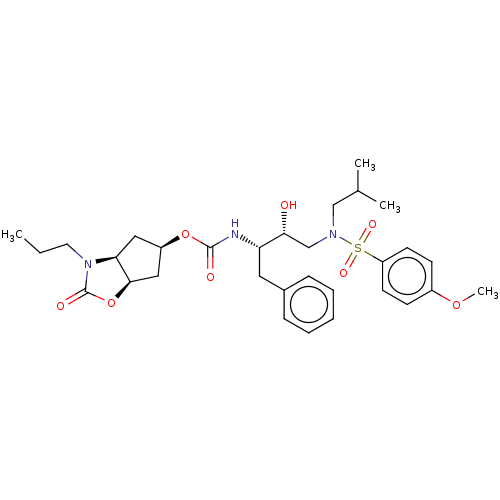 (CHEMBL4283819) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469406 (CHEMBL4277539) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469409 (CHEMBL4278306) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469408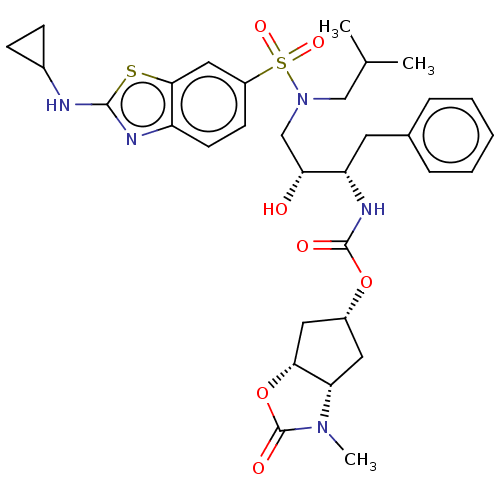 (CHEMBL4278989) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561623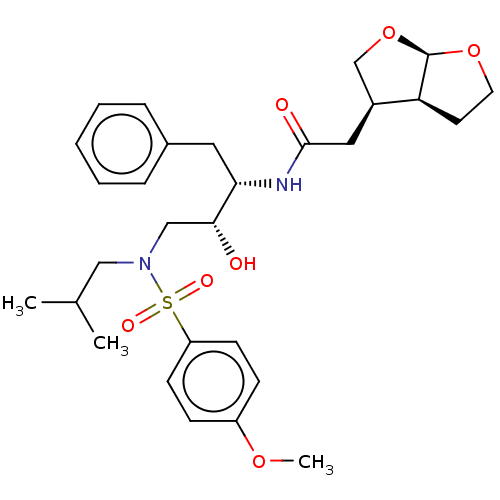 (CHEMBL256107 | GRL-0026A) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520745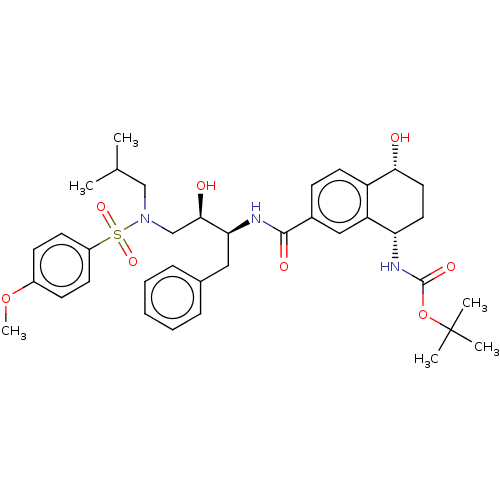 (CHEMBL4444395) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469416 (CHEMBL4286215) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520747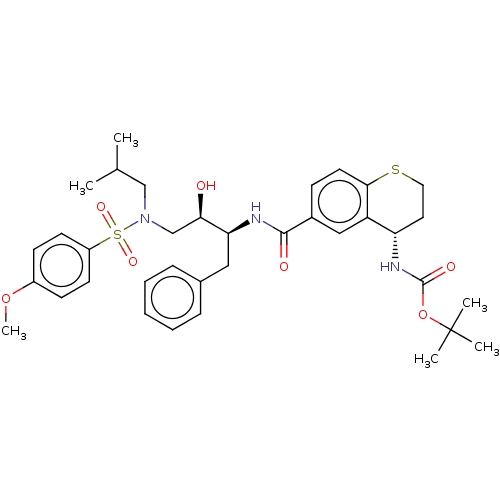 (CHEMBL4532723) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522184 (CHEMBL4444017) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469411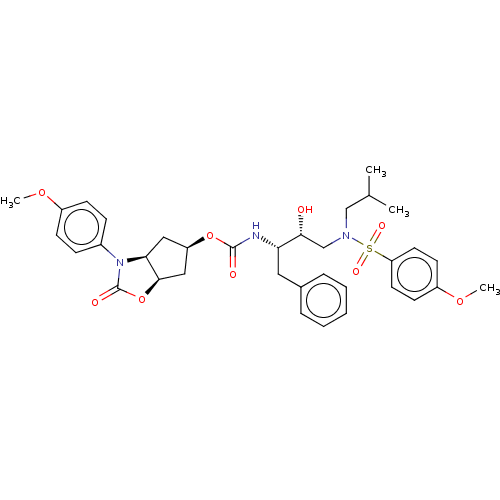 (CHEMBL4279922) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520750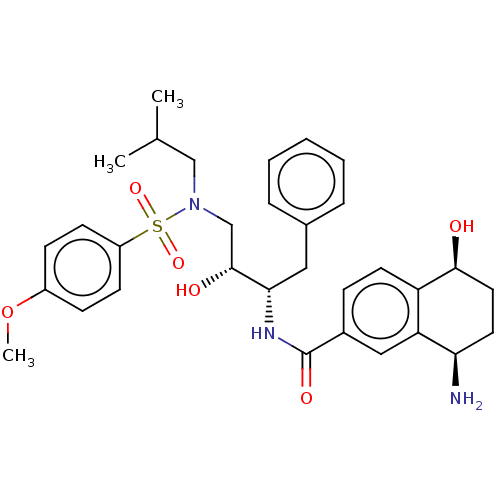 (CHEMBL4452621) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469414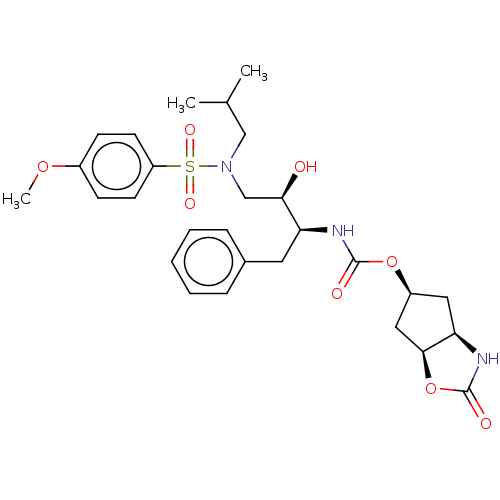 (CHEMBL4288541) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561626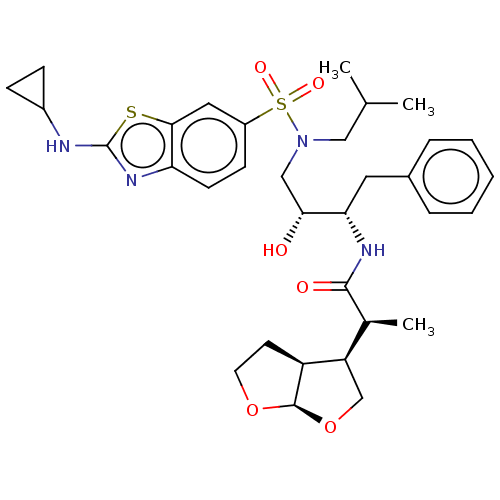 (CHEMBL4763723) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520743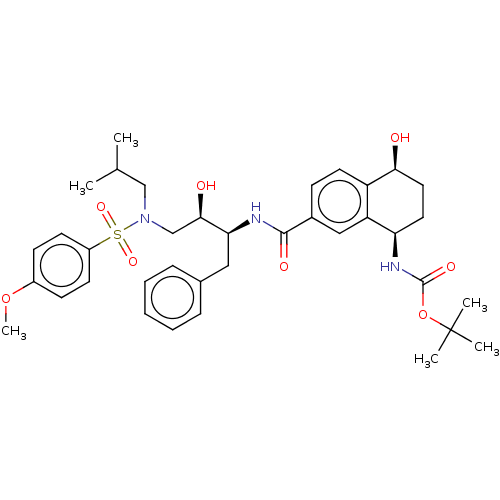 (CHEMBL4444545) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522181 (CHEMBL4456725) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522183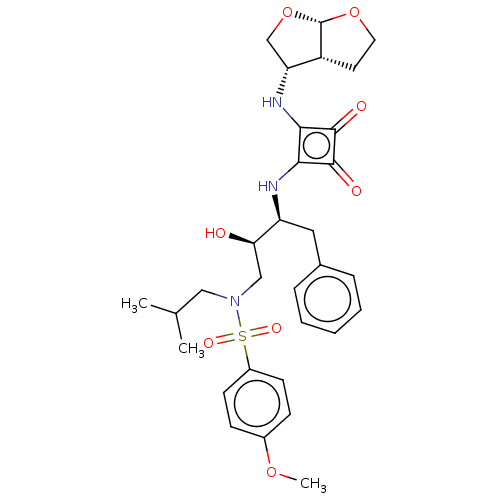 (CHEMBL4467544) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520746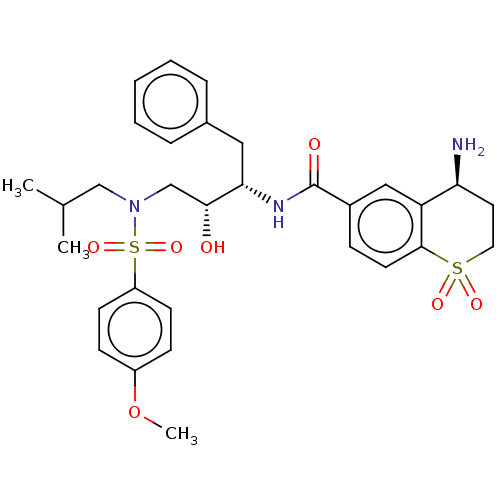 (CHEMBL4569180) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520744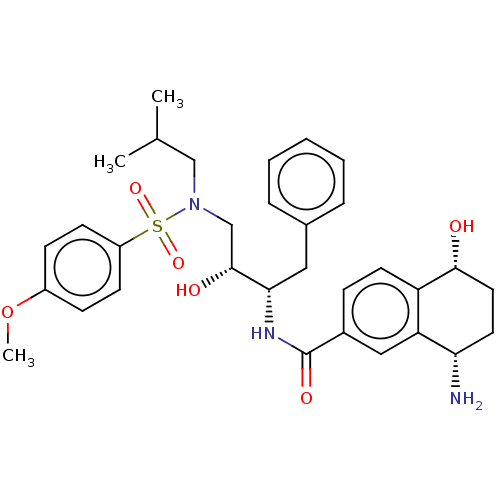 (CHEMBL4444772) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561624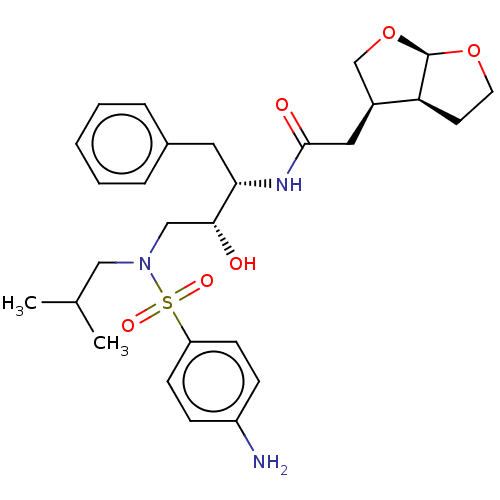 (CHEMBL4764958) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496891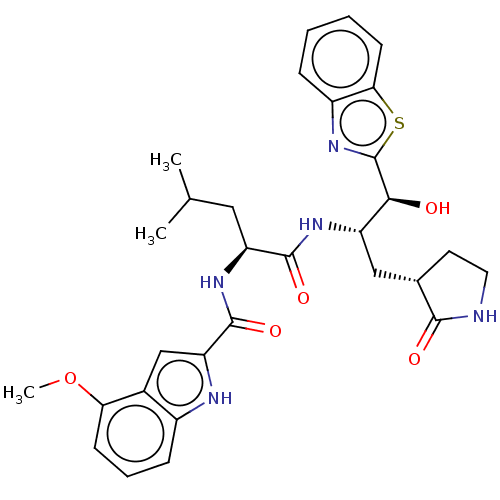 (GRL-2420) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520748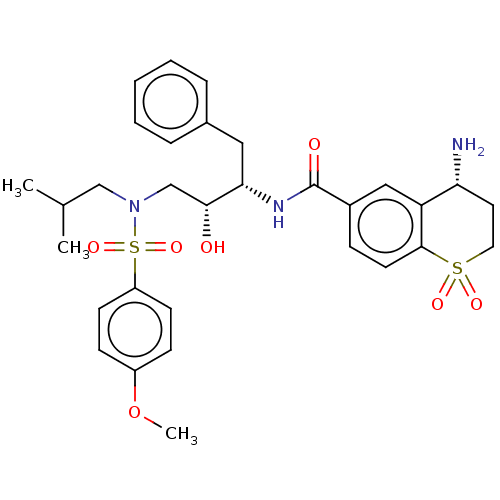 (CHEMBL4588251) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520741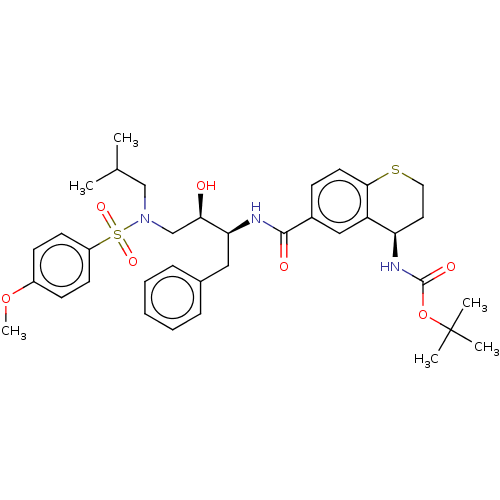 (CHEMBL4561948) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522186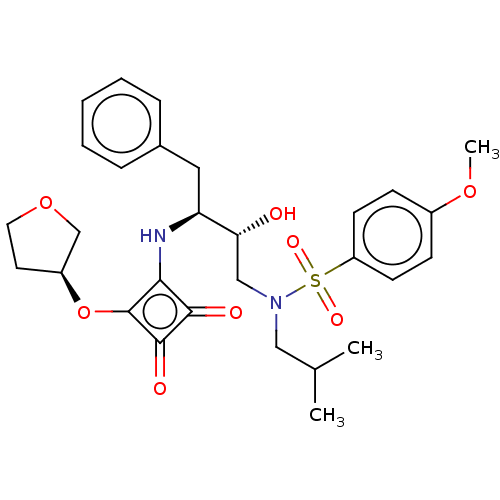 (CHEMBL4445644) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522189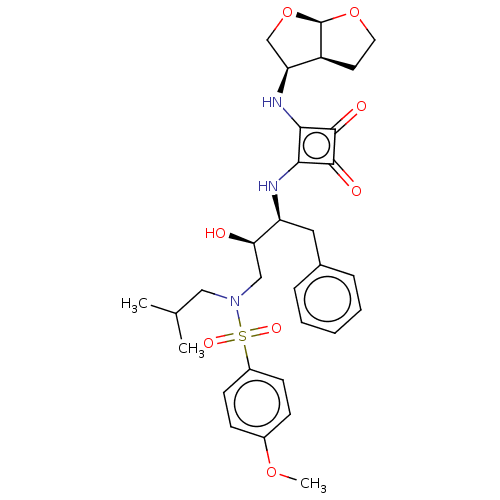 (CHEMBL4469790) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469415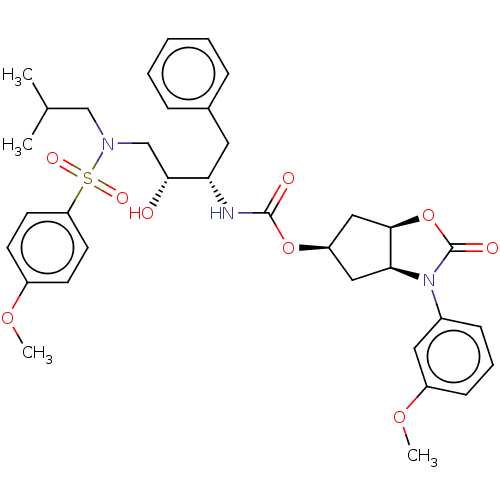 (CHEMBL4284787) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522185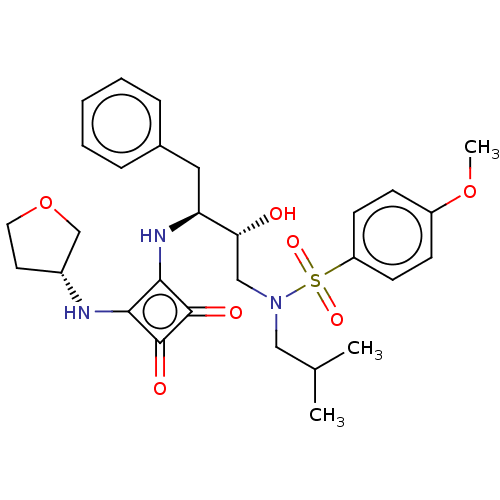 (CHEMBL4474819) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522192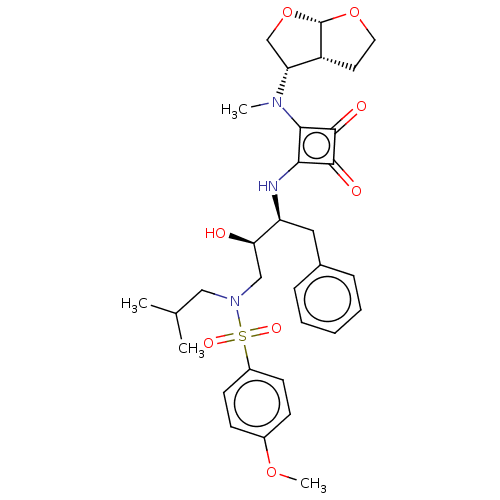 (CHEMBL4570048) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522190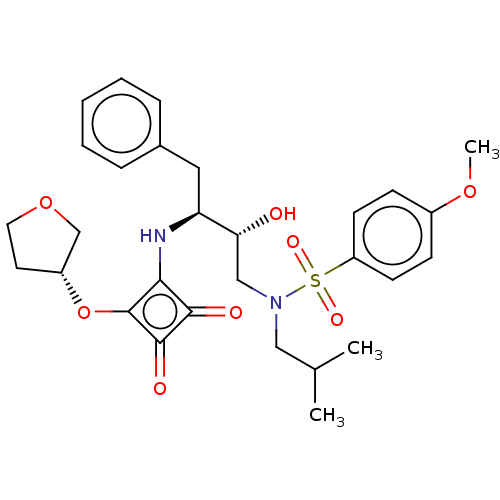 (CHEMBL4440087) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561625 (CHEMBL4784475) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 284 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522191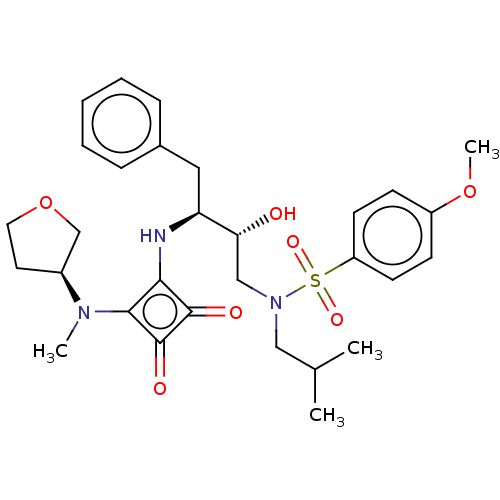 (CHEMBL4558365) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 397 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522187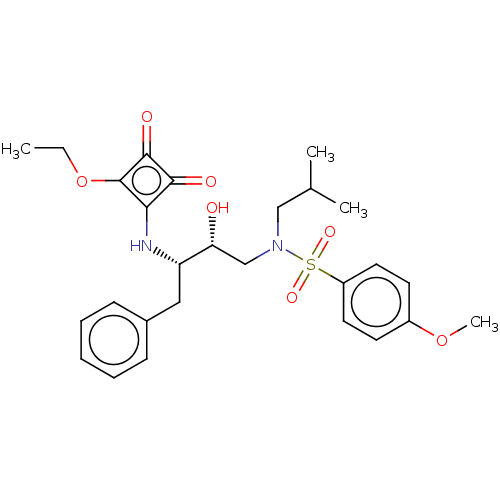 (CHEMBL4437581) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 463 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522182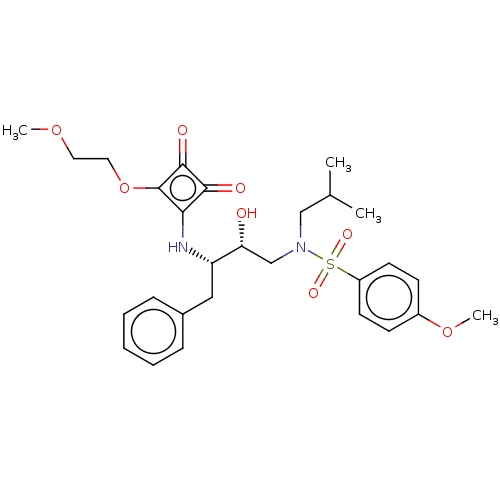 (CHEMBL4556792) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522188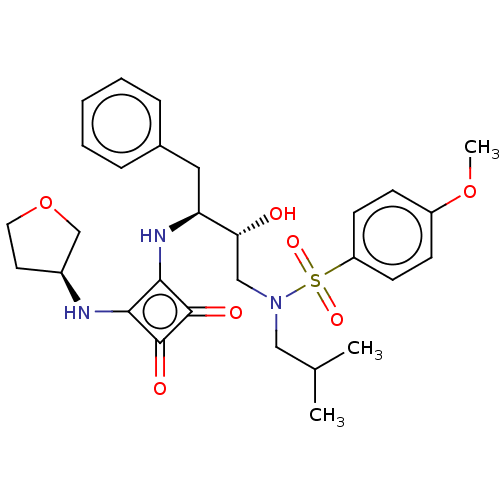 (CHEMBL4467187) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496875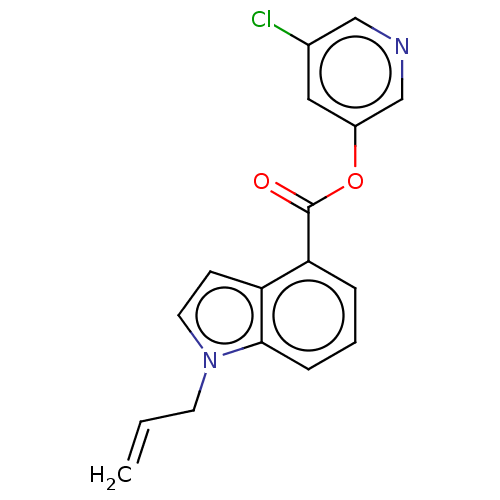 (indole chloropyridinyl-ester derived, 7d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 92 total ) | Next | Last >> |