

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079508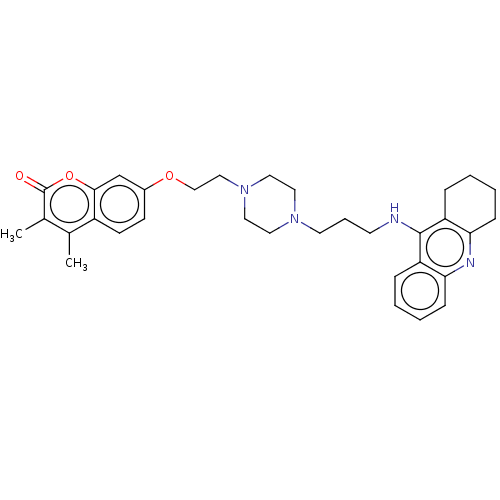 (CHEMBL3417300) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE by Lineweaver-Burk plot analysis | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50295320 (CHEMBL4167524) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibitory binding concentration against VCAM/VLA-4 in Ramos. | Eur J Med Chem 146: 287-298 (2018) Article DOI: 10.1016/j.ejmech.2018.01.055 BindingDB Entry DOI: 10.7270/Q2M61NST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435960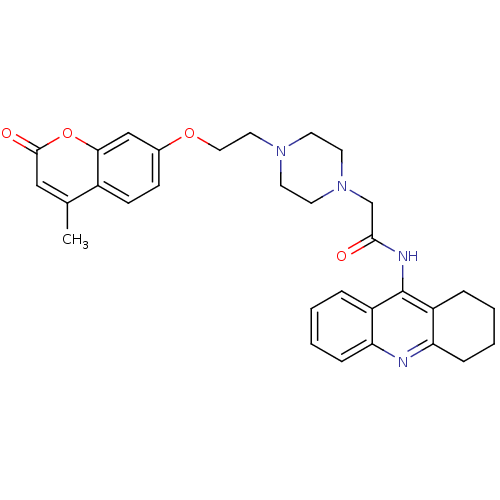 (CHEMBL2391486) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50562841 (CHEMBL4752063) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of equine serum BuChE using varying levels of butylthiocholine iodide as substrate preincubated for 5 mins followed by substrat... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113154 BindingDB Entry DOI: 10.7270/Q2NG4VDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50562841 (CHEMBL4752063) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrat... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113154 BindingDB Entry DOI: 10.7270/Q2NG4VDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50610277 (CHEMBL5270722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456821 (CHEMBL4208961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50251343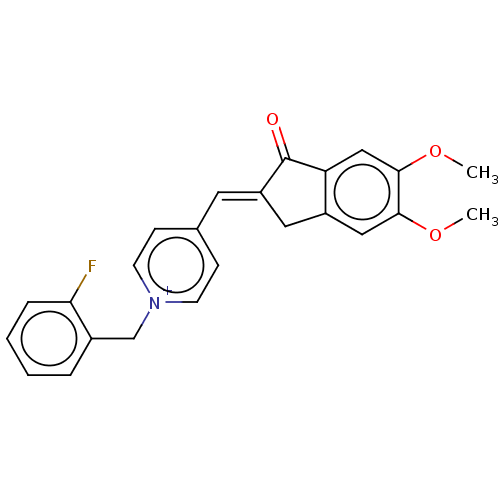 (CHEMBL4076580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50282506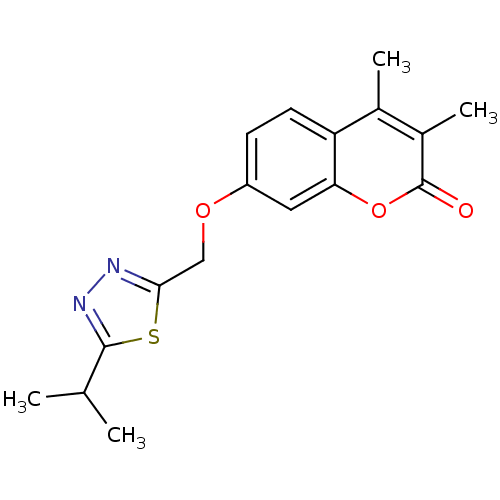 (7-(5-Isopropyl-[1,3,4]thiadiazol-2-ylmethoxy)-3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-B (unknown origin) | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456825 (CHEMBL4210401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456822 (CHEMBL4205764) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50251343 (CHEMBL4076580) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456826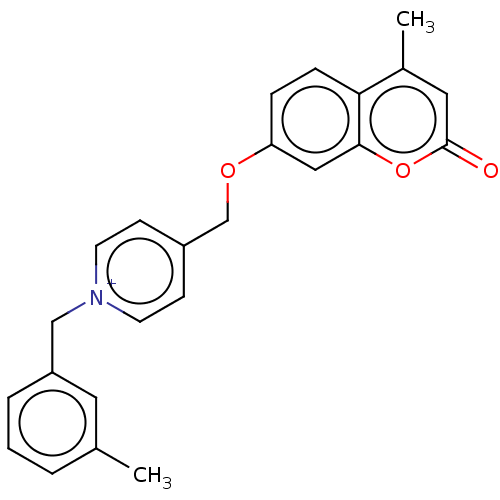 (CHEMBL4205520) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456827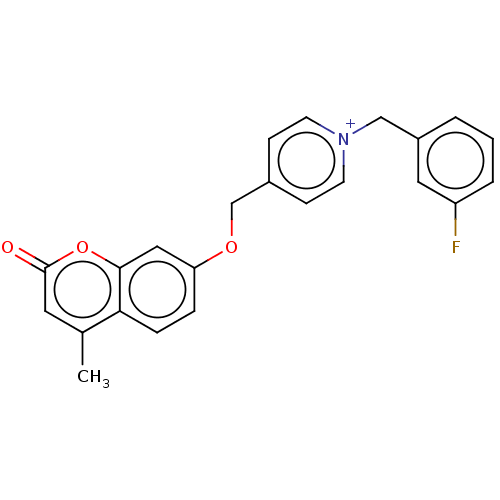 (CHEMBL4206041) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50117587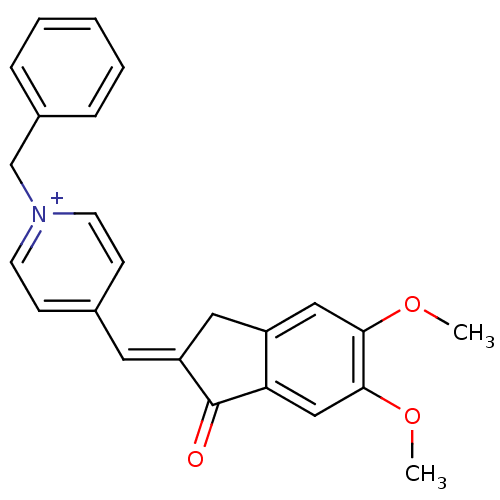 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylidenemet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50251344 (CHEMBL4095035) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50251349 (CHEMBL4063583) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456823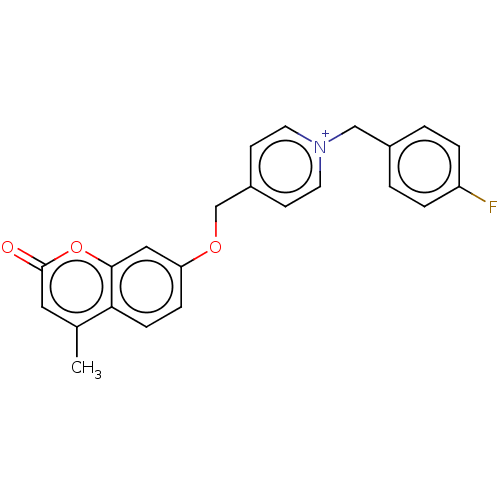 (CHEMBL4217755) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117587 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylidenemet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50251344 (CHEMBL4095035) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50251347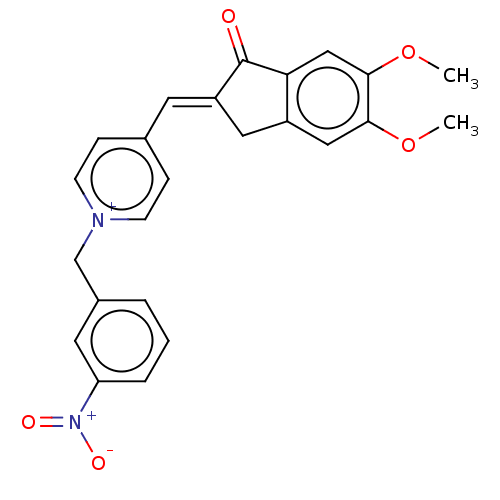 (CHEMBL4101018) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50251349 (CHEMBL4063583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50565155 (CHEMBL4790537) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAOB expressed in baculovirus infected BTI insect cells using p-tyramine as substrate measured after 15 mins by Ample... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112475 BindingDB Entry DOI: 10.7270/Q21Z485N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50251345 (CHEMBL4084842) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50251342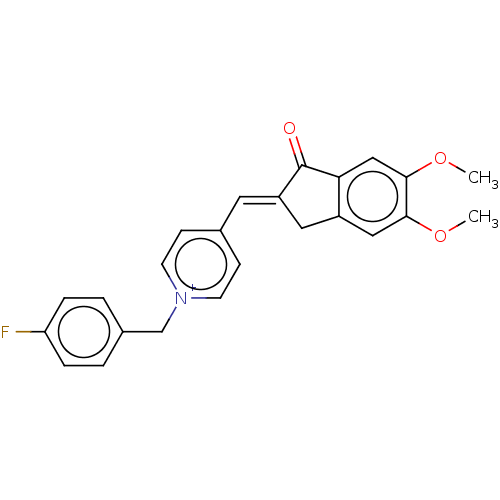 (CHEMBL4073287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456824 (CHEMBL4217466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50251345 (CHEMBL4084842) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099601 (CHEMBL3343930) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456817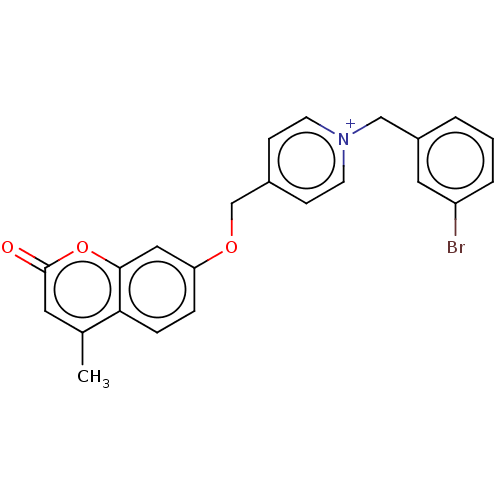 (CHEMBL4218281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456820 (CHEMBL4205110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50251346 (CHEMBL4082048) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAOA expressed in baculovirus infected BTI insect cells using p-tyramine as substrate measured after 15 mins by Ample... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112475 BindingDB Entry DOI: 10.7270/Q21Z485N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50251342 (CHEMBL4073287) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456818 (CHEMBL4213345) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50251348 (CHEMBL4099391) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50251346 (CHEMBL4082048) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073116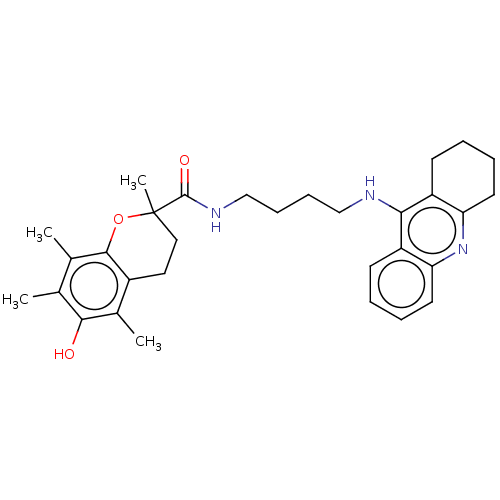 (CHEMBL3410952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50251348 (CHEMBL4099391) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to ... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50251347 (CHEMBL4101018) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Experiment Center of Teaching& Learning, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured up to 180 s... | Eur J Med Chem 133: 184-196 (2017) Article DOI: 10.1016/j.ejmech.2017.02.045 BindingDB Entry DOI: 10.7270/Q2FB55C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099603 (CHEMBL3343932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099597 (CHEMBL3343926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456819 (CHEMBL4213874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441841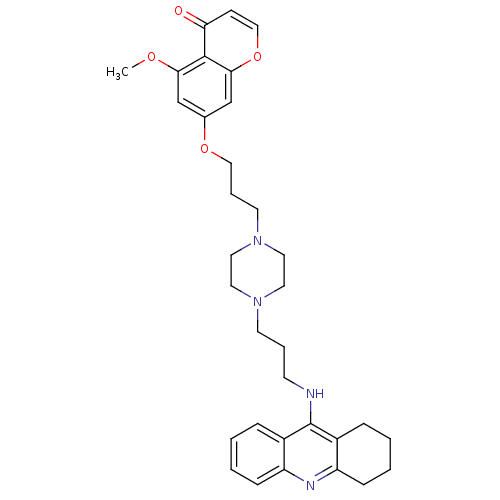 (CHEMBL2436691) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073114 (CHEMBL3410954) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up t... | Bioorg Med Chem 24: 1528-39 (2016) Article DOI: 10.1016/j.bmc.2016.02.023 BindingDB Entry DOI: 10.7270/Q2125VHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073115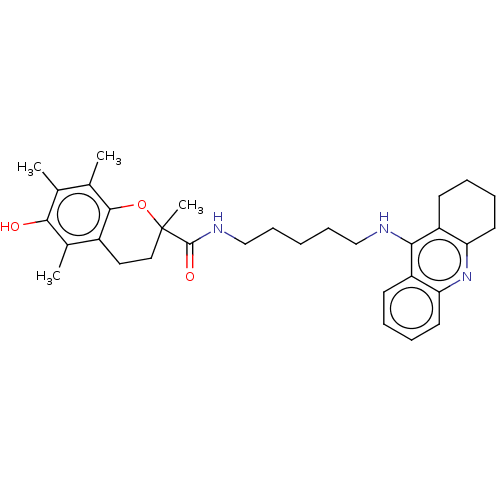 (CHEMBL3410953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441840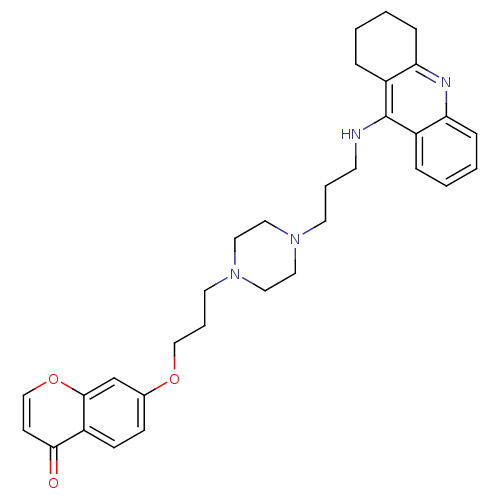 (CHEMBL2436692) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099602 (CHEMBL3343931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441842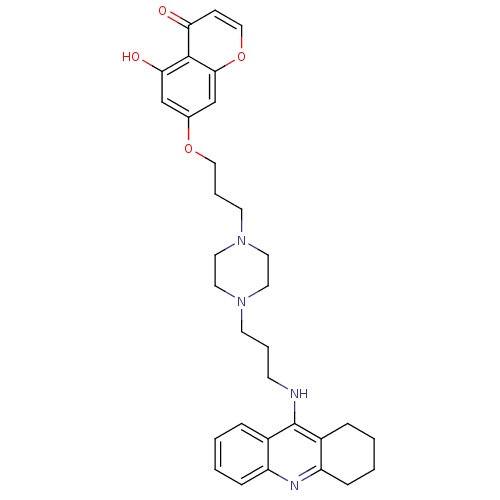 (CHEMBL2436690) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 666 total ) | Next | Last >> |