

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524981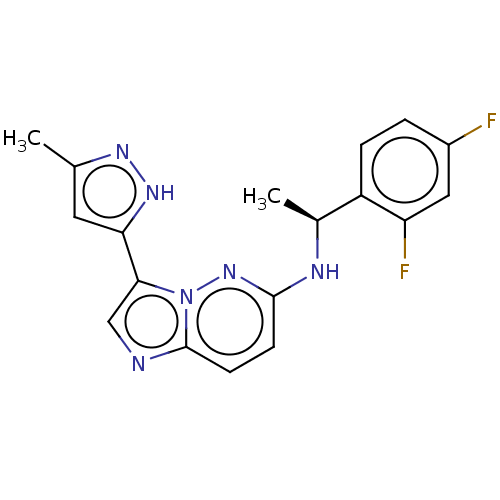 (CHEMBL4562879) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50180268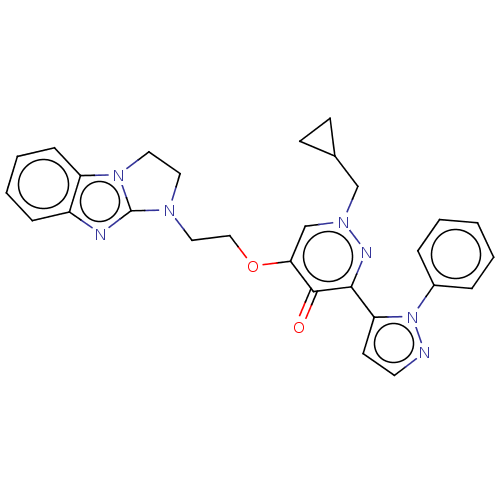 (CHEMBL3814662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... | Bioorg Med Chem 24: 3447-55 (2016) Article DOI: 10.1016/j.bmc.2016.05.049 BindingDB Entry DOI: 10.7270/Q2H9974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50180271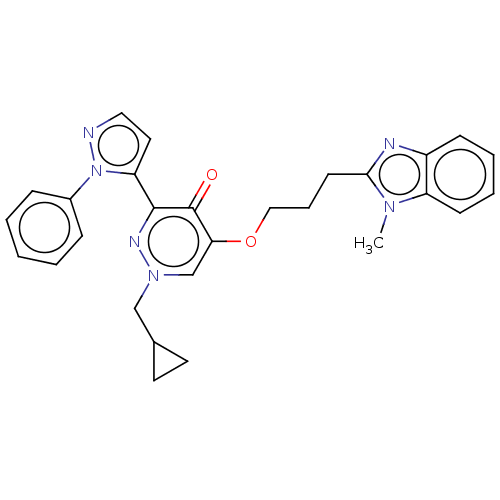 (CHEMBL3814601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... | Bioorg Med Chem 24: 3447-55 (2016) Article DOI: 10.1016/j.bmc.2016.05.049 BindingDB Entry DOI: 10.7270/Q2H9974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50003994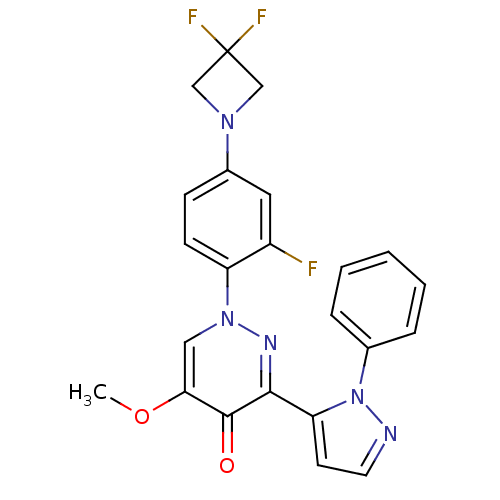 (CHEMBL2180010 | US9579407, Example 00161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Human PDE1A, 3A, 4D2, 5A1, 7B, 8A1, 9A2, and 11A4 enzymes were purchased from BPS Bioscience. Human PDE6AB enzyme was purchased from Scottish Biomedi... | US Patent US9579407 (2017) BindingDB Entry DOI: 10.7270/Q2FB5502 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM290889 (1-[2-fluoro-4- (tetrahydro-2H- pyran-4-yl)phenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Human PDE1A, 3A, 4D2, 5A1, 7B, 8A1, 9A2, and 11A4 enzymes were purchased from BPS Bioscience. Human PDE6AB enzyme was purchased from Scottish Biomedi... | US Patent US9579407 (2017) BindingDB Entry DOI: 10.7270/Q2FB5502 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524985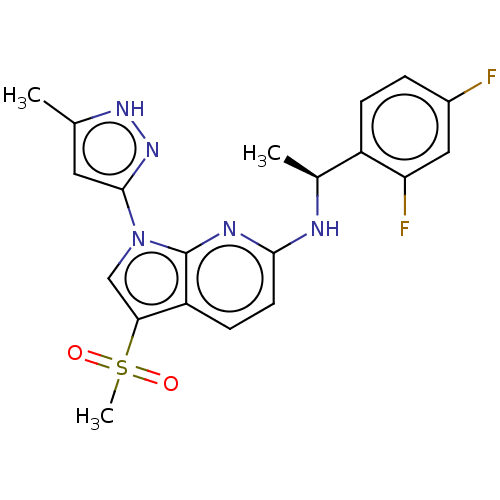 (CHEMBL4460367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524987 (CHEMBL4516801) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524983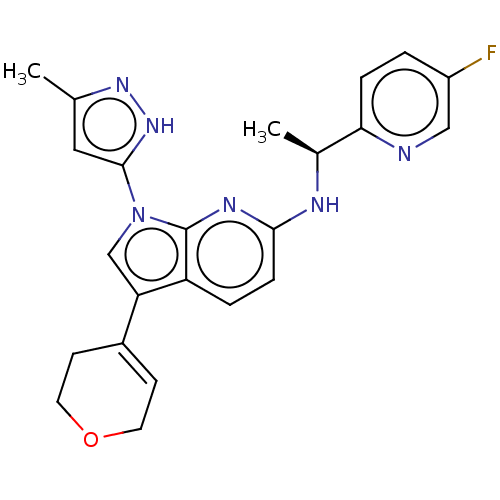 (CHEMBL4458269) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524981 (CHEMBL4562879) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524980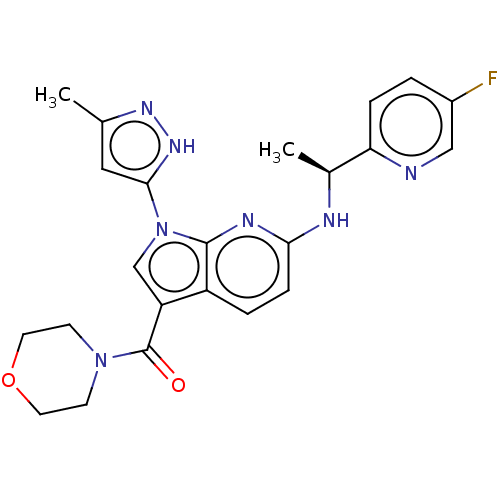 (CHEMBL4437605) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524983 (CHEMBL4458269) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524979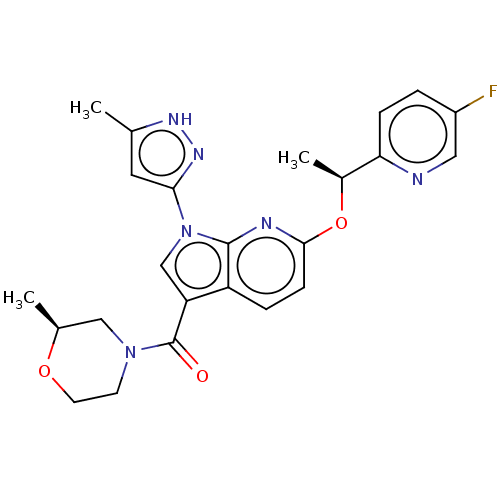 (CHEMBL4440381) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524984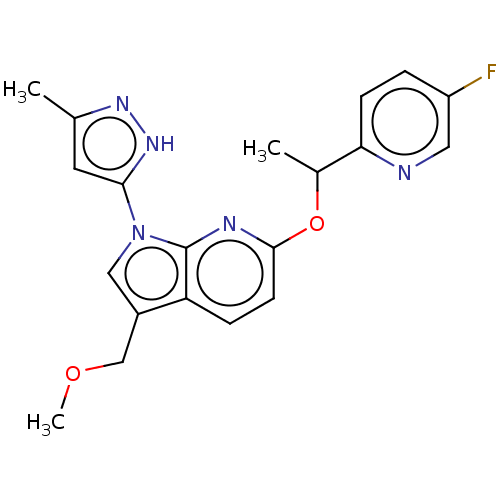 (CHEMBL4434659) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524984 (CHEMBL4434659) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524989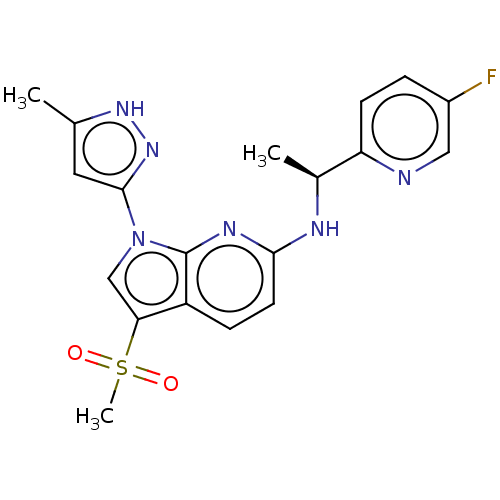 (CHEMBL4535072) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50180269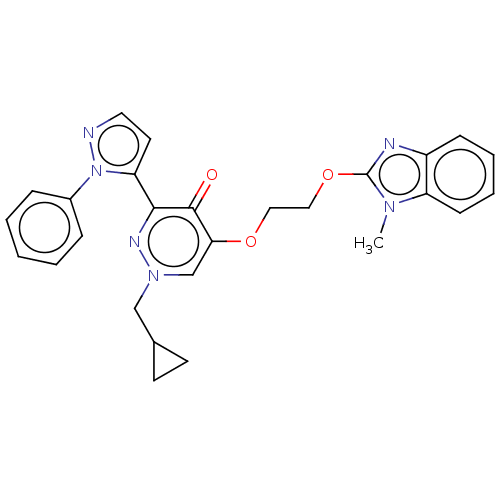 (CHEMBL3814367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... | Bioorg Med Chem 24: 3447-55 (2016) Article DOI: 10.1016/j.bmc.2016.05.049 BindingDB Entry DOI: 10.7270/Q2H9974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524978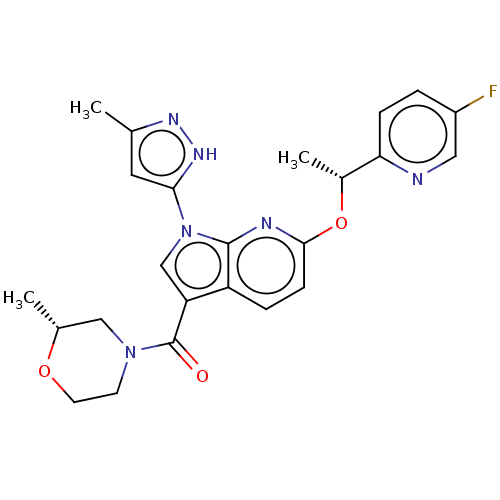 (CHEMBL4573505) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524981 (CHEMBL4562879) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524988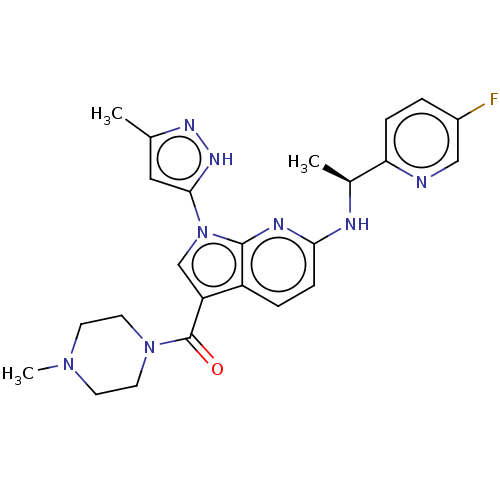 (CHEMBL4586773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524975 (CHEMBL4476859) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50024849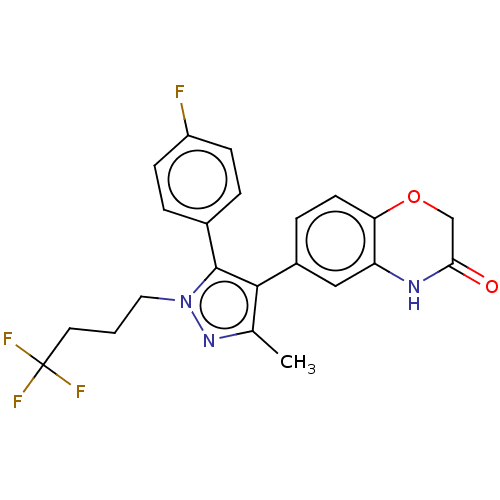 (CHEMBL3337825) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]aldosterone from human mineralocorticoid receptor expressed in 293 cells after 16 hrs by scintillation counting | Bioorg Med Chem 22: 5428-45 (2014) Article DOI: 10.1016/j.bmc.2014.07.038 BindingDB Entry DOI: 10.7270/Q218383G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524983 (CHEMBL4458269) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524977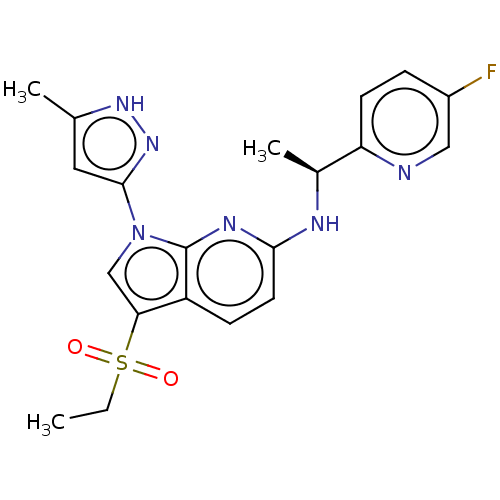 (CHEMBL4443254) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524984 (CHEMBL4434659) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50024859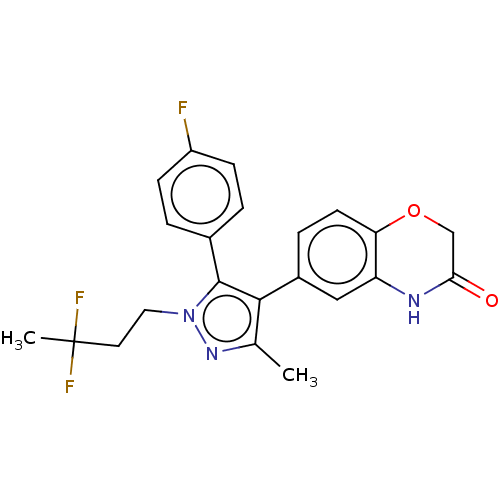 (CHEMBL3337831) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]aldosterone from human mineralocorticoid receptor expressed in 293 cells after 16 hrs by scintillation counting | Bioorg Med Chem 22: 5428-45 (2014) Article DOI: 10.1016/j.bmc.2014.07.038 BindingDB Entry DOI: 10.7270/Q218383G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM290892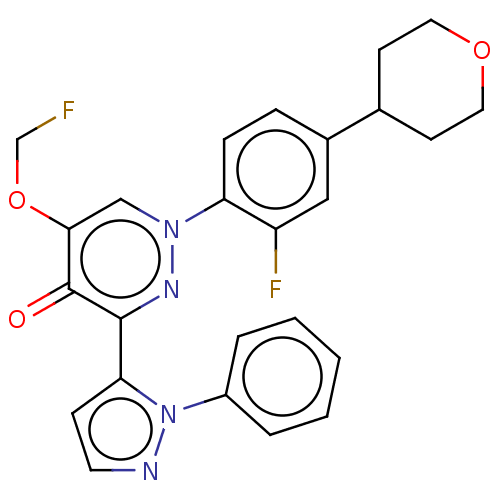 (5-(fluoro-methyloxy- d2)-1-(2-fluoro-4- (tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Human PDE1A, 3A, 4D2, 5A1, 7B, 8A1, 9A2, and 11A4 enzymes were purchased from BPS Bioscience. Human PDE6AB enzyme was purchased from Scottish Biomedi... | US Patent US9579407 (2017) BindingDB Entry DOI: 10.7270/Q2FB5502 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM291105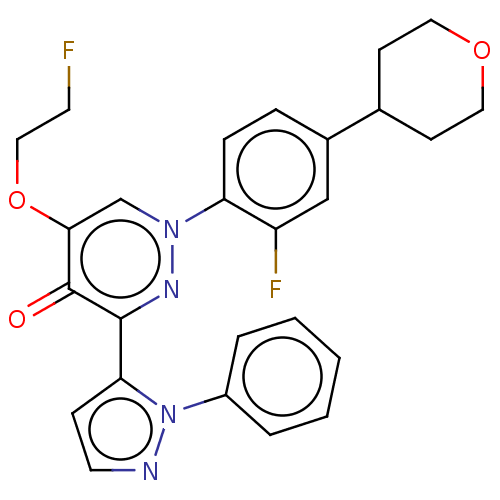 (5-(2-fluoro- ethyloxy-d4)-1-(2- fluoro-4- (tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Human PDE1A, 3A, 4D2, 5A1, 7B, 8A1, 9A2, and 11A4 enzymes were purchased from BPS Bioscience. Human PDE6AB enzyme was purchased from Scottish Biomedi... | US Patent US9579407 (2017) BindingDB Entry DOI: 10.7270/Q2FB5502 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524982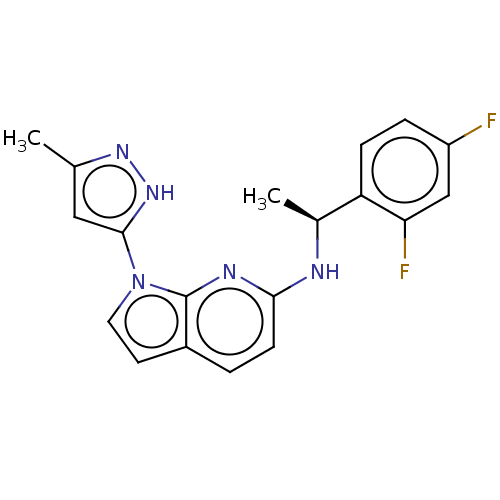 (CHEMBL4448434) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50359651 (CHEMBL1929041) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]aldosterone from cytosolic human MR expressed in HEK293 cells after 16 hrs by scintillation counting | J Med Chem 54: 8616-31 (2011) Article DOI: 10.1021/jm2011645 BindingDB Entry DOI: 10.7270/Q21G0MP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524989 (CHEMBL4535072) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524982 (CHEMBL4448434) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50359652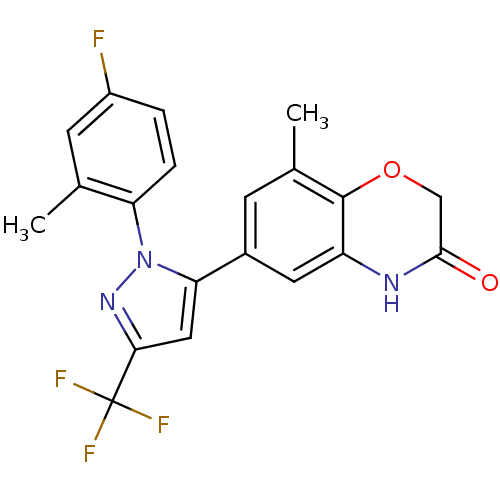 (CHEMBL1929042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human MR transfected in human COS1 cells after 1 day by luciferase reporter gene assay | J Med Chem 54: 8616-31 (2011) Article DOI: 10.1021/jm2011645 BindingDB Entry DOI: 10.7270/Q21G0MP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524985 (CHEMBL4460367) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524987 (CHEMBL4516801) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50024859 (CHEMBL3337831) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Antagonist activity at human mineralocorticoid receptor expressed in COS1 cells after 1 day by luciferase reporter gene assay | Bioorg Med Chem 22: 5428-45 (2014) Article DOI: 10.1016/j.bmc.2014.07.038 BindingDB Entry DOI: 10.7270/Q218383G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524976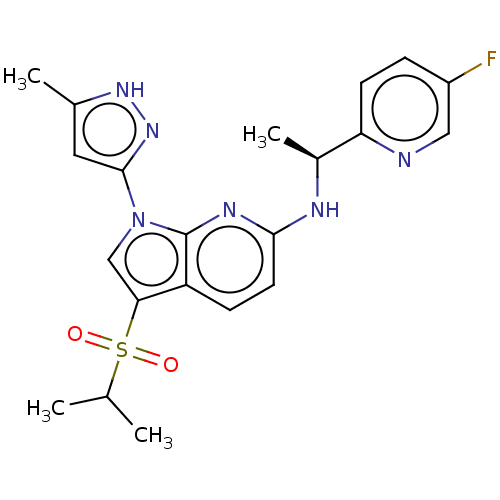 (CHEMBL4435574) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524986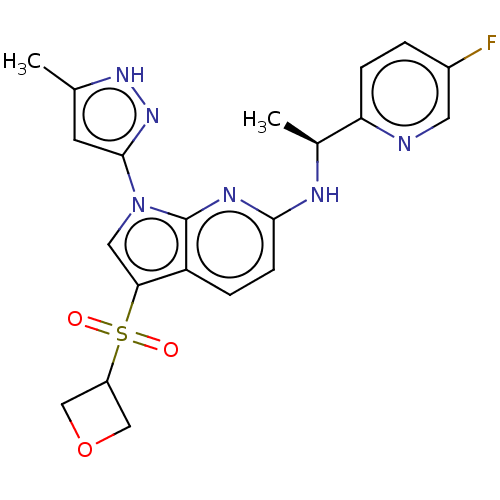 (CHEMBL4516124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50359652 (CHEMBL1929042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]aldosterone from cytosolic human MR expressed in HEK293 cells after 16 hrs by scintillation counting | J Med Chem 54: 8616-31 (2011) Article DOI: 10.1021/jm2011645 BindingDB Entry DOI: 10.7270/Q21G0MP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50024847 (CHEMBL3337823) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]aldosterone from human mineralocorticoid receptor expressed in 293 cells after 16 hrs by scintillation counting | Bioorg Med Chem 22: 5428-45 (2014) Article DOI: 10.1016/j.bmc.2014.07.038 BindingDB Entry DOI: 10.7270/Q218383G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50439608 (CHEMBL2419300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]-Aldosterone from human MR expressed in 293 cells after 16 hrs by scintillation counting | Bioorg Med Chem 21: 5983-94 (2013) Article DOI: 10.1016/j.bmc.2013.07.043 BindingDB Entry DOI: 10.7270/Q2TD9ZSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524980 (CHEMBL4437605) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50524979 (CHEMBL4440381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human FAK kinase domain (411 to 686 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incubate... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50180272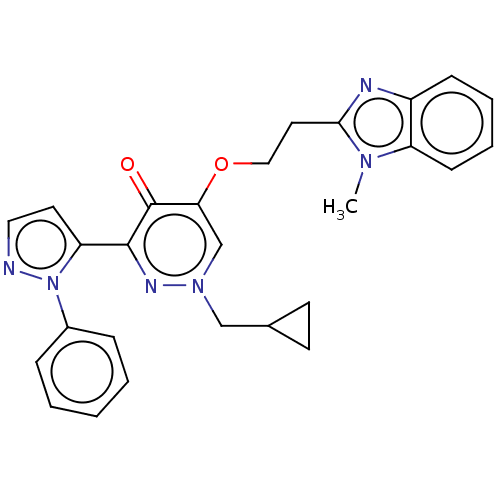 (CHEMBL3814223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... | Bioorg Med Chem 24: 3447-55 (2016) Article DOI: 10.1016/j.bmc.2016.05.049 BindingDB Entry DOI: 10.7270/Q2H9974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524979 (CHEMBL4440381) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524985 (CHEMBL4460367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50439613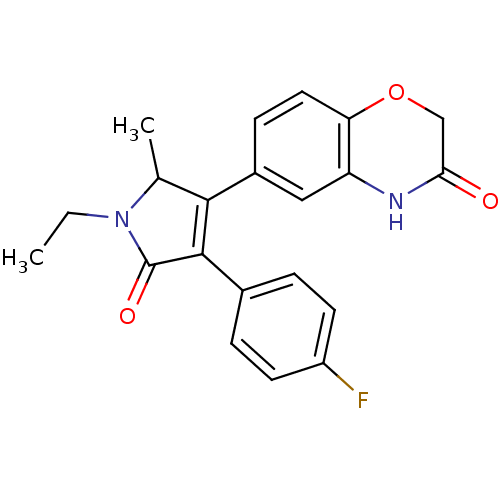 (CHEMBL2419295) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]-Aldosterone from human MR expressed in 293 cells after 16 hrs by scintillation counting | Bioorg Med Chem 21: 5983-94 (2013) Article DOI: 10.1016/j.bmc.2013.07.043 BindingDB Entry DOI: 10.7270/Q2TD9ZSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50024849 (CHEMBL3337825) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Antagonist activity at human mineralocorticoid receptor expressed in COS1 cells after 1 day by luciferase reporter gene assay | Bioorg Med Chem 22: 5428-45 (2014) Article DOI: 10.1016/j.bmc.2014.07.038 BindingDB Entry DOI: 10.7270/Q218383G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50024833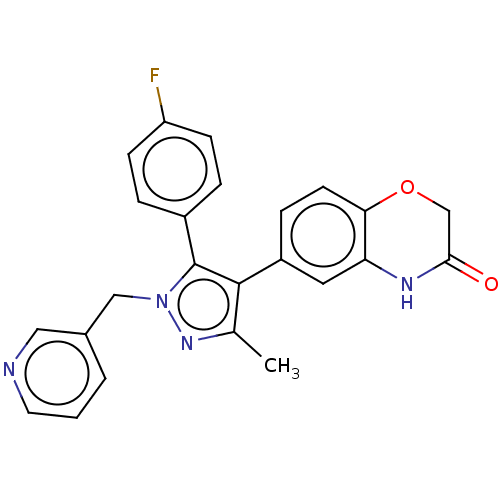 (CHEMBL3337821) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Antagonist activity at human mineralocorticoid receptor expressed in COS1 cells after 1 day by luciferase reporter gene assay | Bioorg Med Chem 22: 5428-45 (2014) Article DOI: 10.1016/j.bmc.2014.07.038 BindingDB Entry DOI: 10.7270/Q218383G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50359644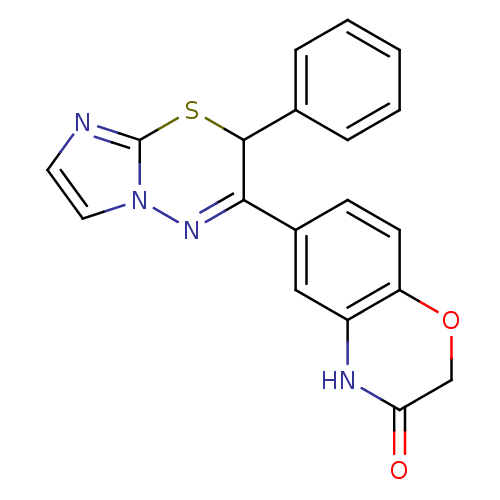 (CHEMBL1928877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]aldosterone from cytosolic human MR expressed in HEK293 cells after 16 hrs by scintillation counting | J Med Chem 54: 8616-31 (2011) Article DOI: 10.1021/jm2011645 BindingDB Entry DOI: 10.7270/Q21G0MP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 441 total ) | Next | Last >> |