 Found 190 hits with Last Name = 'kobrlova' and Initial = 't'
Found 190 hits with Last Name = 'kobrlova' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM412060
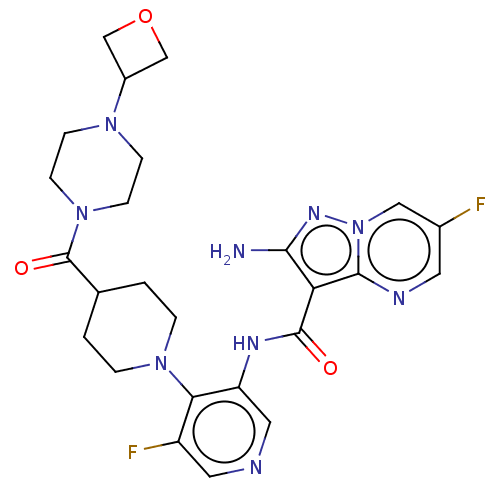
(2-amino-6-fluoro-N-[5-fluoro-4-[4-[4-(oxetan-3-yl)...)Show SMILES Nc1nn2cc(F)cnc2c1C(=O)Nc1cncc(F)c1N1CCC(CC1)C(=O)N1CCN(CC1)C1COC1 Show InChI InChI=1S/C25H29F2N9O3/c26-16-9-30-23-20(22(28)32-36(23)12-16)24(37)31-19-11-29-10-18(27)21(19)34-3-1-15(2-4-34)25(38)35-7-5-33(6-8-35)17-13-39-14-17/h9-12,15,17H,1-8,13-14H2,(H2,28,32)(H,31,37) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| Article
PubMed
| <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114580
BindingDB Entry DOI: 10.7270/Q261149W |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50555835
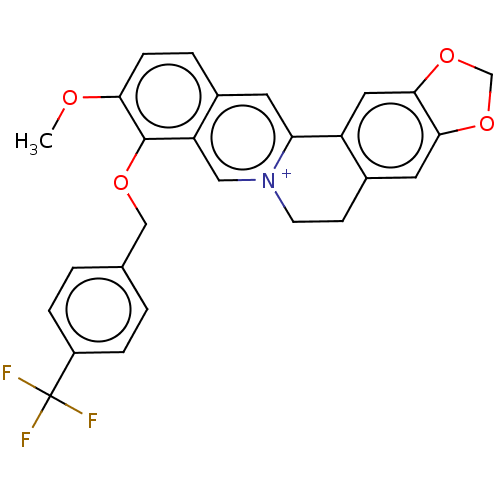
(CHEMBL4752175)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc(cc1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Noncompetitive inhibition of human acetylcholinesterase assessed as affinity towards free enzyme using acetylthiocholine iodide as substrate measured... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... |
J Med Chem 62: 11416-11422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00937
BindingDB Entry DOI: 10.7270/Q2736V9G |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50000483
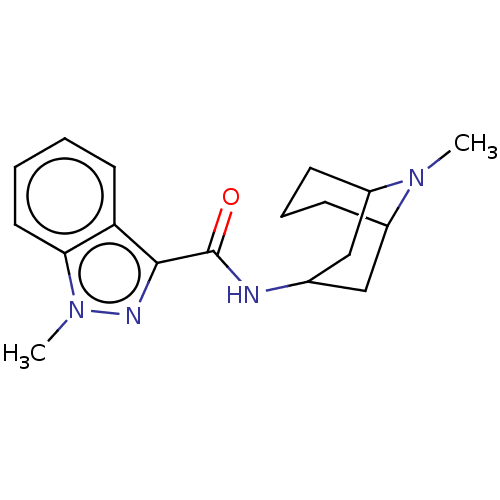
((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...)Show SMILES CN1C2CCCC1CC(C2)NC(=O)c1nn(C)c2ccccc12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM268079

(2-[(3R)-3-methylmorpholin-4-yl]-4-(1-methyl-1H-pyr...)Show SMILES C[C@@H]1COCCN1c1cc(-c2ccnn2C)c2ccnc(-c3ccn[nH]3)c2n1 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114580
BindingDB Entry DOI: 10.7270/Q261149W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50555837
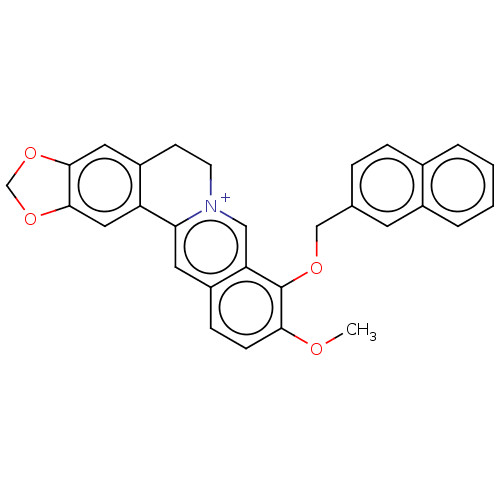
(CHEMBL4747577)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc2ccccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of human butyrylcholinesterase assessed as affinity towards free enzyme using butyrylthiocholine iodide as substrate measured by Ell... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599917

(CHEMBL5172819)Show SMILES O=C(N1CCN(CCCCOc2ccc3CCC(=O)Nc3c2)CC1)c1ccccn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599900
(CHEMBL5190788)Show SMILES Clc1cccc(N2CCN(CCCN3CCN(CC3)c3ncccc3C#N)CC2)c1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50599903
(CHEMBL5182877)Show SMILES COc1ccccc1N1CCN(CCCN2CCN(CC2)c2ncccc2C#N)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50599900

(CHEMBL5190788)Show SMILES Clc1cccc(N2CCN(CCCN3CCN(CC3)c3ncccc3C#N)CC2)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50519742
(CHEMBL4469017)Show SMILES CCOC(=O)C1=C(C)NC(C)=C(C1c1ccc(OCCCN2CCCCC2)cc1)C(=O)OCC |c:5,10| Show InChI InChI=1S/C27H38N2O5/c1-5-32-26(30)23-19(3)28-20(4)24(27(31)33-6-2)25(23)21-11-13-22(14-12-21)34-18-10-17-29-15-8-7-9-16-29/h11-14,25,28H,5-10,15-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... |
J Med Chem 62: 11416-11422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00937
BindingDB Entry DOI: 10.7270/Q2736V9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50519739
(CHEMBL4443523)Show SMILES CCOC(=O)C1=C(C)NC(C)=C(C1c1ccc(OCCCCN2CCCC2)cc1)C(=O)OCC |c:5,10| Show InChI InChI=1S/C27H38N2O5/c1-5-32-26(30)23-19(3)28-20(4)24(27(31)33-6-2)25(23)21-11-13-22(14-12-21)34-18-10-9-17-29-15-7-8-16-29/h11-14,25,28H,5-10,15-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... |
J Med Chem 62: 11416-11422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00937
BindingDB Entry DOI: 10.7270/Q2736V9G |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50555837

(CHEMBL4747577)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc2ccccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of human butyrylcholinesterase assessed as affinity towards enzyme-substrate complex using butyrylthiocholine iodide as substrate me... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599915
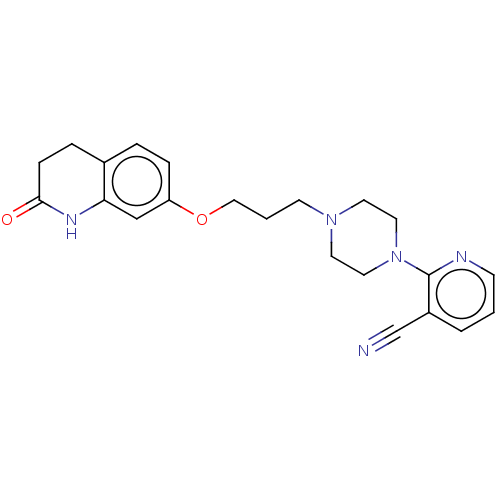
(CHEMBL5170927)Show SMILES O=C1CCc2ccc(OCCCN3CCN(CC3)c3ncccc3C#N)cc2N1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599909
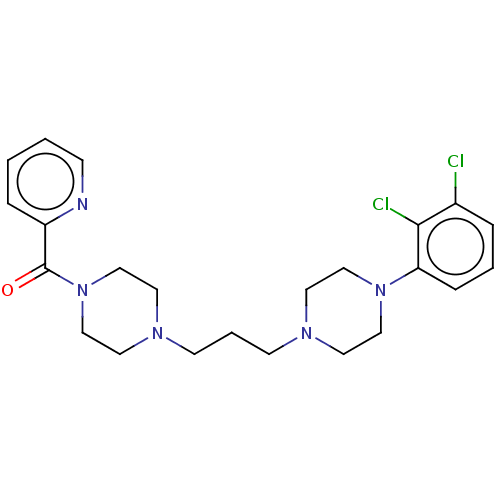
(CHEMBL5188923)Show SMILES Clc1cccc(N2CCN(CCCN3CCN(CC3)C(=O)c3ccccn3)CC2)c1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50519746
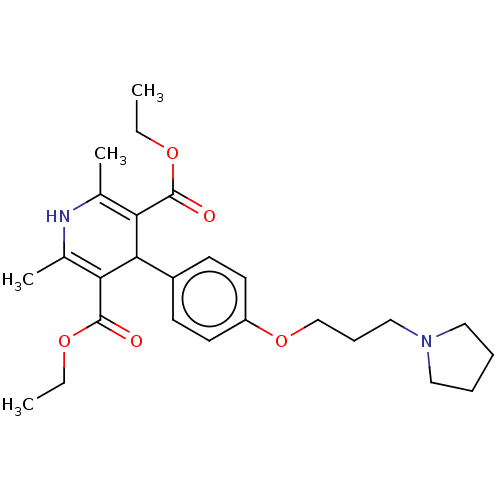
(CHEMBL4455698)Show SMILES CCOC(=O)C1=C(C)NC(C)=C(C1c1ccc(OCCCN2CCCC2)cc1)C(=O)OCC |c:5,10| Show InChI InChI=1S/C26H36N2O5/c1-5-31-25(29)22-18(3)27-19(4)23(26(30)32-6-2)24(22)20-10-12-21(13-11-20)33-17-9-16-28-14-7-8-15-28/h10-13,24,27H,5-9,14-17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... |
J Med Chem 62: 11416-11422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00937
BindingDB Entry DOI: 10.7270/Q2736V9G |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599903

(CHEMBL5182877)Show SMILES COc1ccccc1N1CCN(CCCN2CCN(CC2)c2ncccc2C#N)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599907
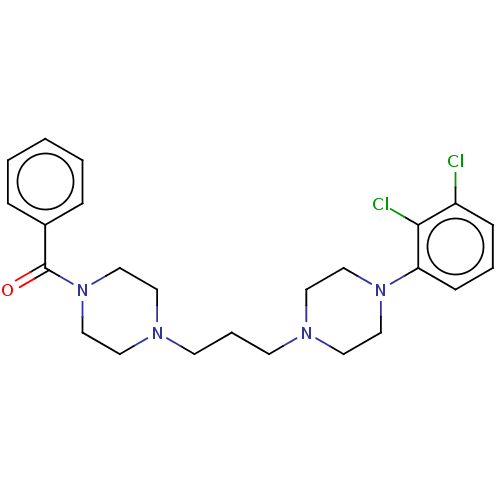
(CHEMBL5195091)Show SMILES Clc1cccc(N2CCN(CCCN3CCN(CC3)C(=O)c3ccccc3)CC2)c1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599910

(CHEMBL5196828)Show SMILES COc1ccccc1N1CCN(CCN2CCN(CC2)c2ncccc2C#N)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599913
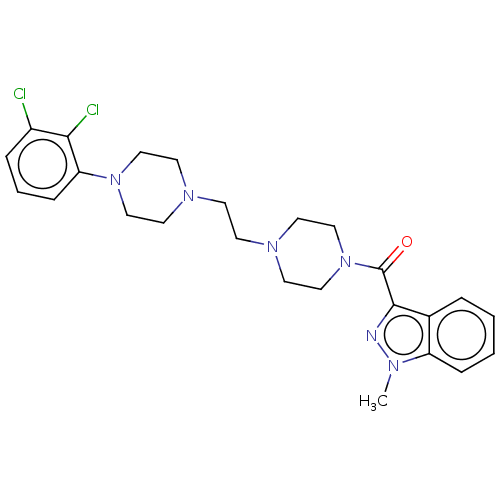
(CHEMBL5200023)Show SMILES Cn1nc(C(=O)N2CCN(CCN3CCN(CC3)c3cccc(Cl)c3Cl)CC2)c2ccccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599911
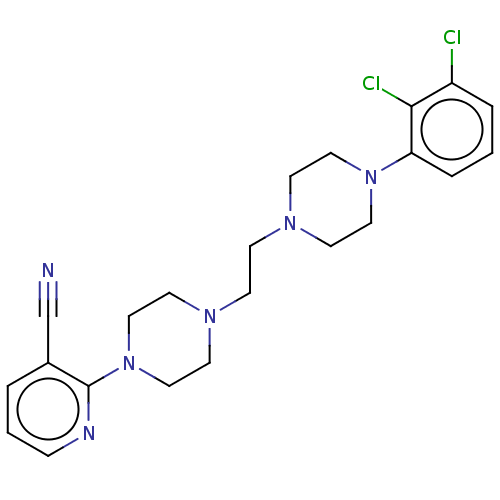
(CHEMBL5205697)Show SMILES Clc1cccc(N2CCN(CCN3CCN(CC3)c3ncccc3C#N)CC2)c1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599904
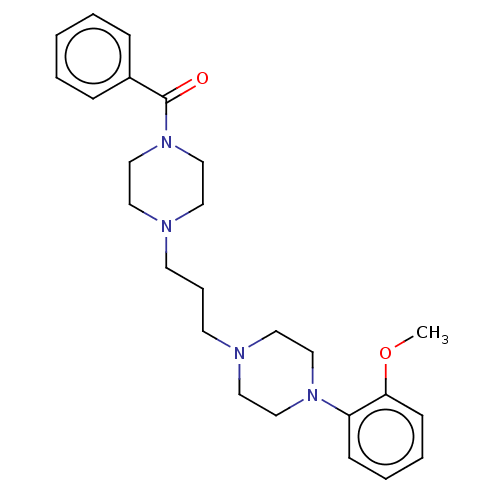
(CHEMBL5178994)Show SMILES COc1ccccc1N1CCN(CCCN2CCN(CC2)C(=O)c2ccccc2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50519740

(CHEMBL4528980)Show SMILES CCOC(=O)C1=C(C)NC(C)=C(C1c1ccc(OCCCCCN2CCCC2)cc1)C(=O)OCC |c:5,10| Show InChI InChI=1S/C28H40N2O5/c1-5-33-27(31)24-20(3)29-21(4)25(28(32)34-6-2)26(24)22-12-14-23(15-13-22)35-19-11-7-8-16-30-17-9-10-18-30/h12-15,26,29H,5-11,16-19H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... |
J Med Chem 62: 11416-11422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00937
BindingDB Entry DOI: 10.7270/Q2736V9G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50599900

(CHEMBL5190788)Show SMILES Clc1cccc(N2CCN(CCCN3CCN(CC3)c3ncccc3C#N)CC2)c1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50519735

(CHEMBL4573309)Show SMILES CCOC(=O)C1=C(C)NC(C)=C(C1c1ccc(OCCCCCN2CCCCC2)cc1)C(=O)OCC |c:5,10| Show InChI InChI=1S/C29H42N2O5/c1-5-34-28(32)25-21(3)30-22(4)26(29(33)35-6-2)27(25)23-13-15-24(16-14-23)36-20-12-8-11-19-31-17-9-7-10-18-31/h13-16,27,30H,5-12,17-20H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... |
J Med Chem 62: 11416-11422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00937
BindingDB Entry DOI: 10.7270/Q2736V9G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50599900

(CHEMBL5190788)Show SMILES Clc1cccc(N2CCN(CCCN3CCN(CC3)c3ncccc3C#N)CC2)c1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599908
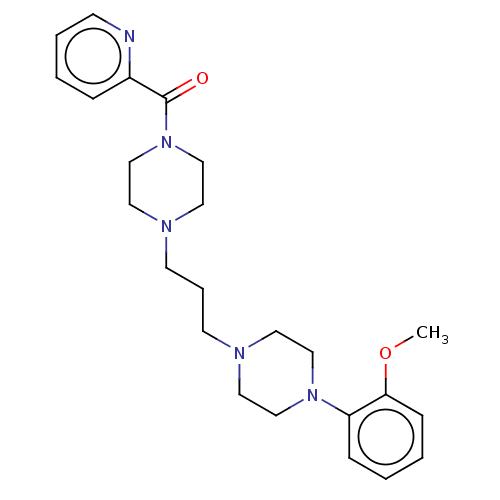
(CHEMBL5178688)Show SMILES COc1ccccc1N1CCN(CCCN2CCN(CC2)C(=O)c2ccccn2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599912
(CHEMBL5209381)Show SMILES COc1ccccc1N1CCN(CCN2CCN(CC2)C(=O)c2nn(C)c3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599901

(CHEMBL5176374) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599914
(CHEMBL5202003) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM85330

(CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...)Show InChI InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50599911

(CHEMBL5205697)Show SMILES Clc1cccc(N2CCN(CCN3CCN(CC3)c3ncccc3C#N)CC2)c1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599902
(CHEMBL5193798)Show SMILES COc1ccc(cc1)N1CCN(CCCN2CCN(CC2)c2ncccc2C#N)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50519745

(CHEMBL4518949)Show SMILES CCOC(=O)C1=C(C)NC(C)=C(C1c1ccc(OCCCN2CCCCC2)c(OCC)c1)C(=O)OCC |c:5,10| Show InChI InChI=1S/C29H42N2O6/c1-6-34-24-19-22(13-14-23(24)37-18-12-17-31-15-10-9-11-16-31)27-25(28(32)35-7-2)20(4)30-21(5)26(27)29(33)36-8-3/h13-14,19,27,30H,6-12,15-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... |
J Med Chem 62: 11416-11422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00937
BindingDB Entry DOI: 10.7270/Q2736V9G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50599907

(CHEMBL5195091)Show SMILES Clc1cccc(N2CCN(CCCN3CCN(CC3)C(=O)c3ccccc3)CC2)c1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50599913

(CHEMBL5200023)Show SMILES Cn1nc(C(=O)N2CCN(CCN3CCN(CC3)c3cccc(Cl)c3Cl)CC2)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599905
(CHEMBL5173564)Show SMILES O=C(N1CCN(CCCN2CCN(CC2)c2ccccc2)CC1)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50599916
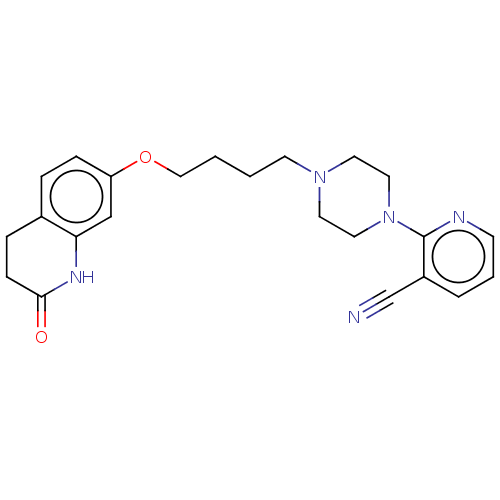
(CHEMBL5201798)Show SMILES O=C1CCc2ccc(OCCCCN3CCN(CC3)c3ncccc3C#N)cc2N1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50599909

(CHEMBL5188923)Show SMILES Clc1cccc(N2CCN(CCCN3CCN(CC3)C(=O)c3ccccn3)CC2)c1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50599903

(CHEMBL5182877)Show SMILES COc1ccccc1N1CCN(CCCN2CCN(CC2)c2ncccc2C#N)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114193
BindingDB Entry DOI: 10.7270/Q2WS8Z8T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data