

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142519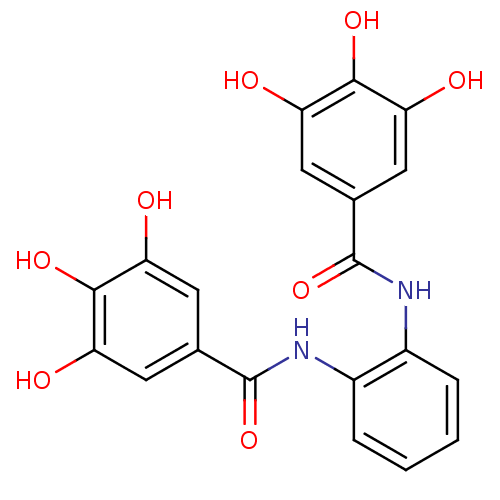 (CHEMBL47027 | N-[2-(3,4,5-triihydroxy-benzoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibition of relaxation activities of DNA topoisomerase I with respect to pBR322 DNA | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50142519 (CHEMBL47027 | N-[2-(3,4,5-triihydroxy-benzoylamino...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibition of relaxation activities of DNA topoisomerase II with respect to pBR322 DNA | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50142519 (CHEMBL47027 | N-[2-(3,4,5-triihydroxy-benzoylamino...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for... | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50350107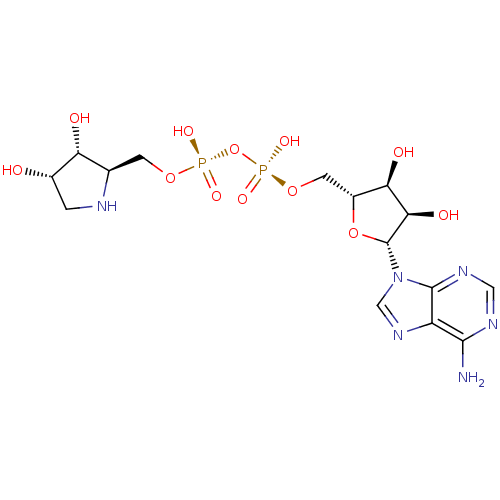 (CHEMBL1230692) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50142517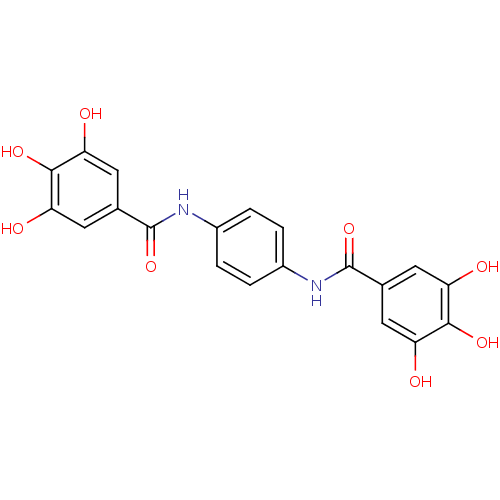 (CHEMBL47885 | N-[4-(3,4,5-triihydroxy-benzoylamino...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for... | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha/2-beta (Homo sapiens (Human)) | BDBM50142507 (CHEMBL47526 | N-[3-(3,4,5-triihydroxy-benzoylamino...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for... | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142519 (CHEMBL47027 | N-[2-(3,4,5-triihydroxy-benzoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052832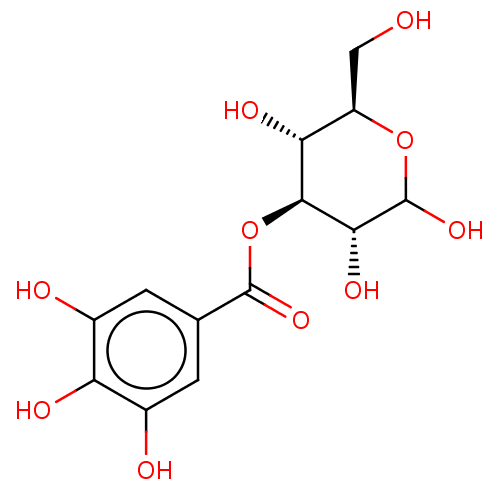 (CHEMBL3318753) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50053036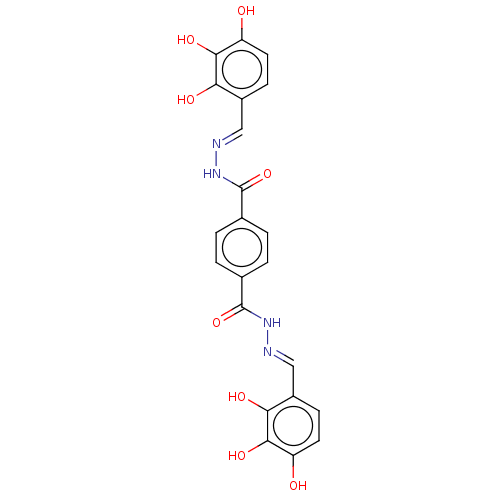 (CHEMBL3318737) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142517 (CHEMBL47885 | N-[4-(3,4,5-triihydroxy-benzoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052838 (CHEMBL3318748) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142507 (CHEMBL47526 | N-[3-(3,4,5-triihydroxy-benzoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50053035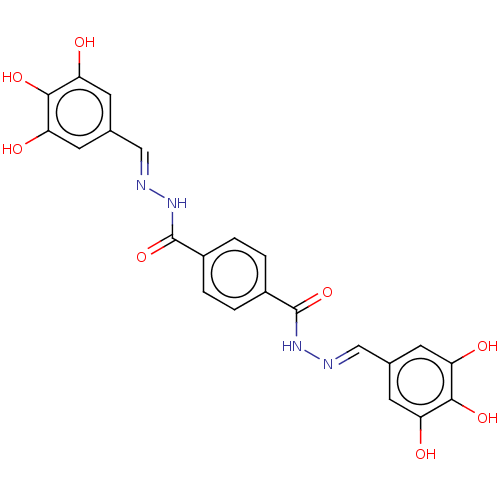 (CHEMBL3318738) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142526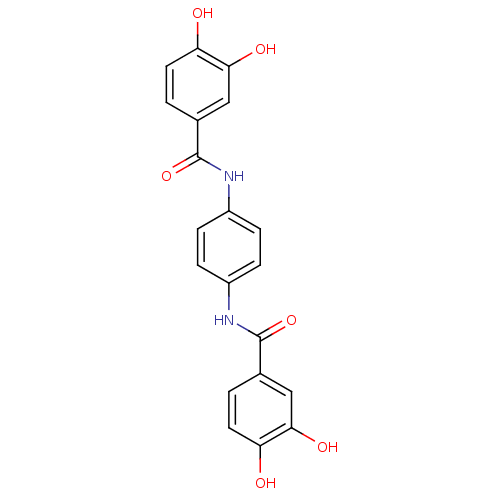 (CHEMBL44765 | N-[4-(3,4-dihydroxy-benzoylamino)-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052839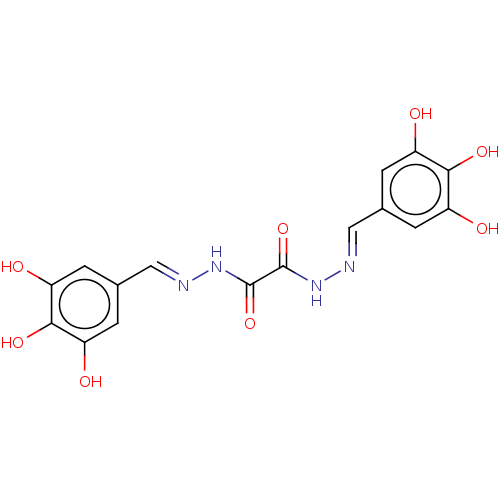 (CHEMBL3318747) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052831 (CHEMBL3318754) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50410165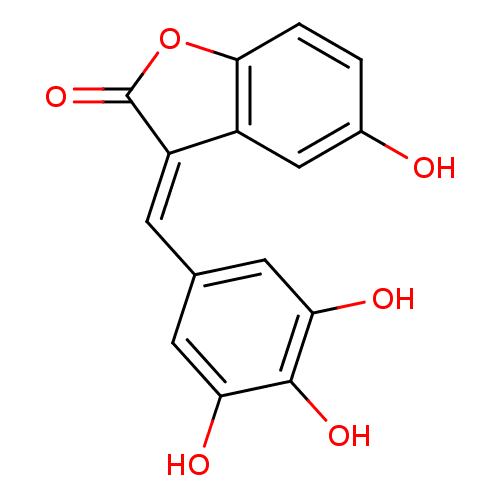 (CHEMBL372661) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against DNA topoisomerase I activity | Bioorg Med Chem Lett 15: 2065-8 (2005) Article DOI: 10.1016/j.bmcl.2005.02.052 BindingDB Entry DOI: 10.7270/Q2WH2R6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052840 (CHEMBL3318746) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052843 (CHEMBL3318743) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052842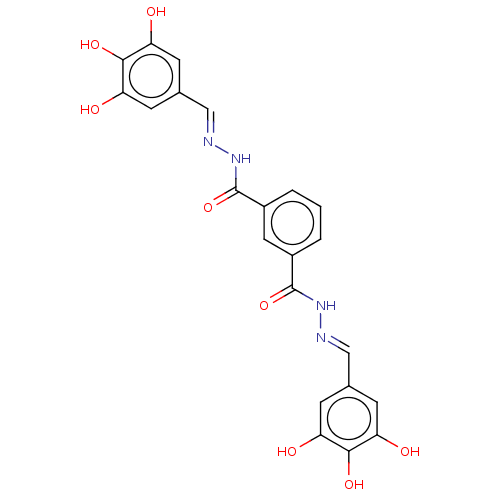 (CHEMBL3318744) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50410153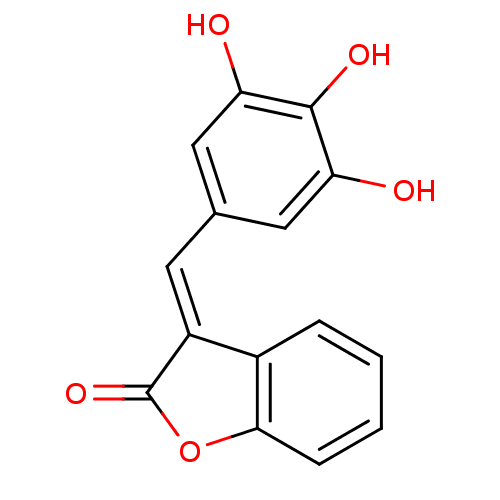 (CHEMBL197216) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against DNA topoisomerase I activity | Bioorg Med Chem Lett 15: 2065-8 (2005) Article DOI: 10.1016/j.bmcl.2005.02.052 BindingDB Entry DOI: 10.7270/Q2WH2R6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142529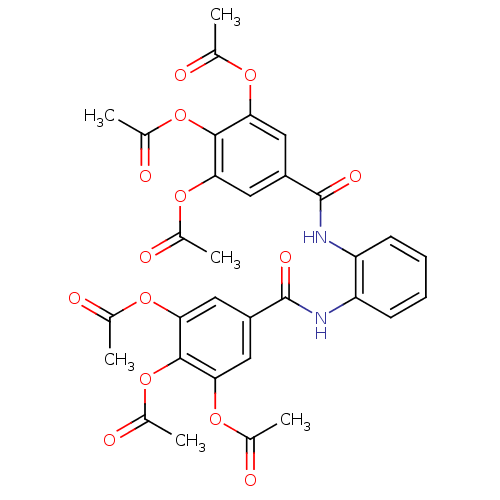 (Acetic acid 2,3-diacetoxy-5-[2-(3,4,5-triacetoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50410158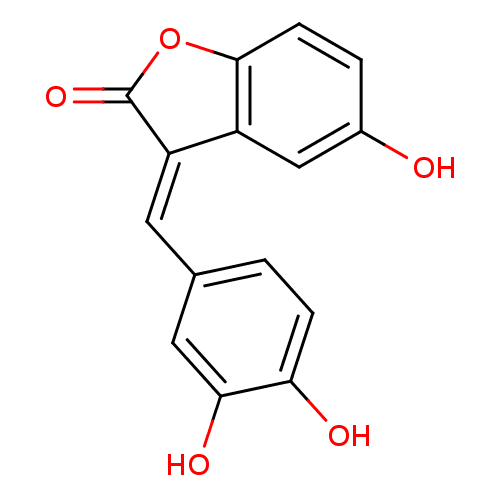 (CHEMBL195195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against DNA topoisomerase I activity | Bioorg Med Chem Lett 15: 2065-8 (2005) Article DOI: 10.1016/j.bmcl.2005.02.052 BindingDB Entry DOI: 10.7270/Q2WH2R6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142509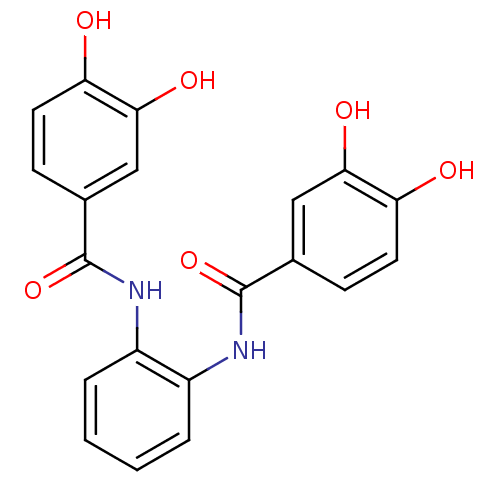 (CHEMBL44441 | N-[2-(3,4-dihydroxy-benzoylamino)-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142528 (CHEMBL296351 | N-Benzothiazol-2-yl-3,4,5-trihydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052835 (CHEMBL3318751) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142514 (Acetic acid 2,3-diacetoxy-5-[4-(3,4,5-triacetoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052836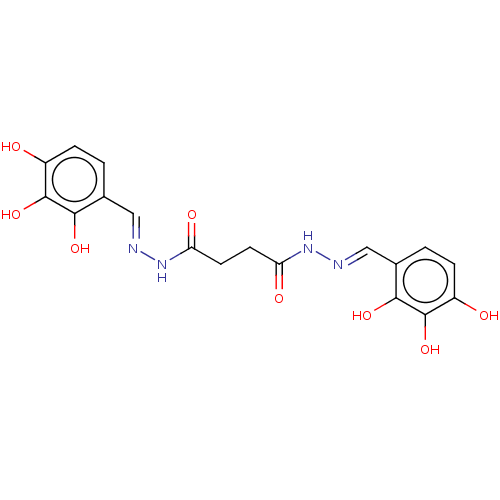 (CHEMBL3318750) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50053034 (CHEMBL3318739) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142523 (CHEMBL297255 | N-[3-(3,4-dihydroxy-benzoylamino)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142510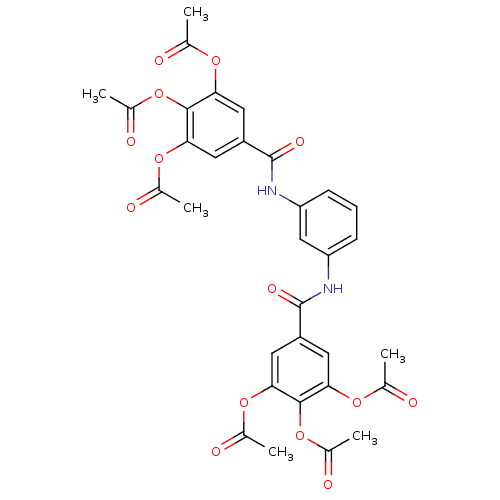 (Acetic acid 2,3-diacetoxy-5-[3-(3,4,5-triacetoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052834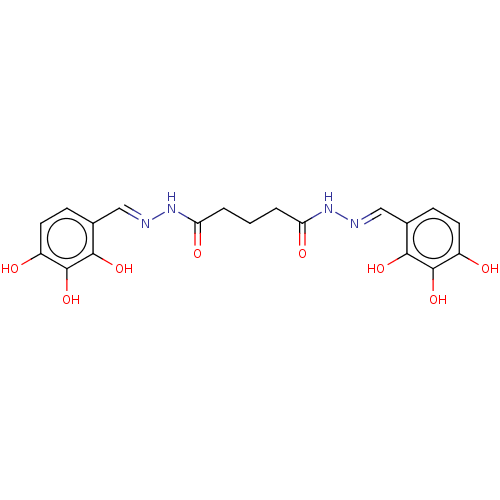 (CHEMBL3317464) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50053033 (CHEMBL3318740) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052837 (CHEMBL3318749) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142515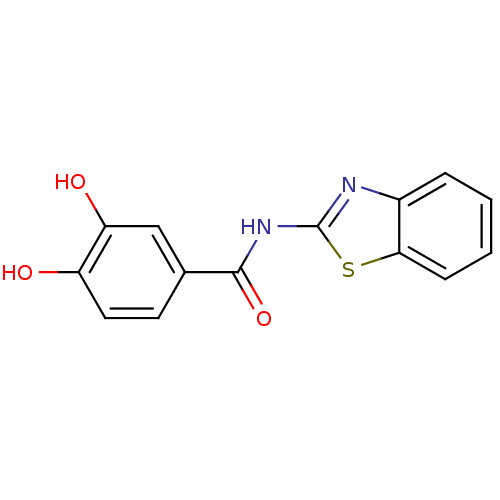 (CHEMBL44747 | N-Benzothiazol-2-yl-3,4-dihydroxy-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052841 (CHEMBL3318745) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052844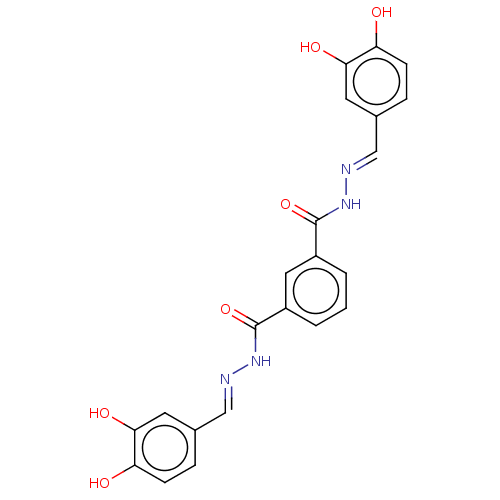 (CHEMBL3318742) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50052833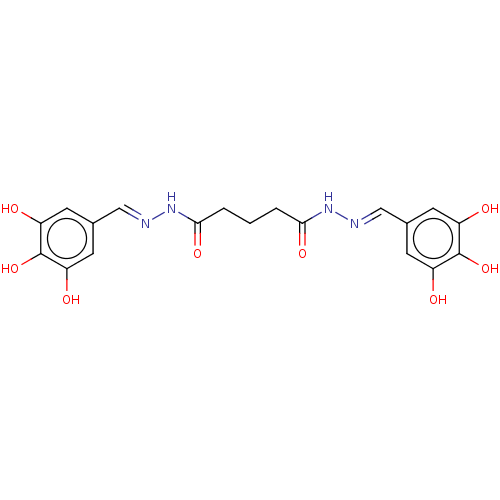 (CHEMBL3318752) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142525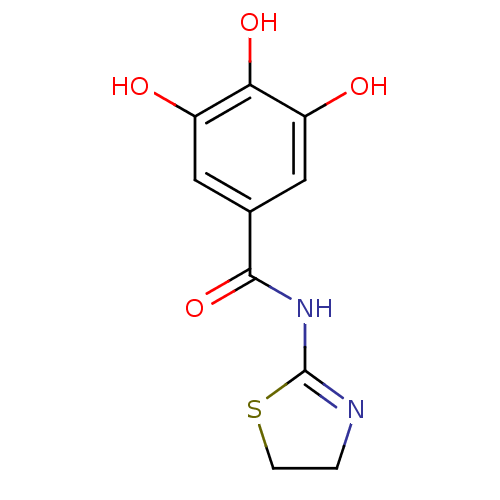 (CHEMBL47524 | N-(4,5-Dihydro-thiazol-2-yl)-3,4,5-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50053032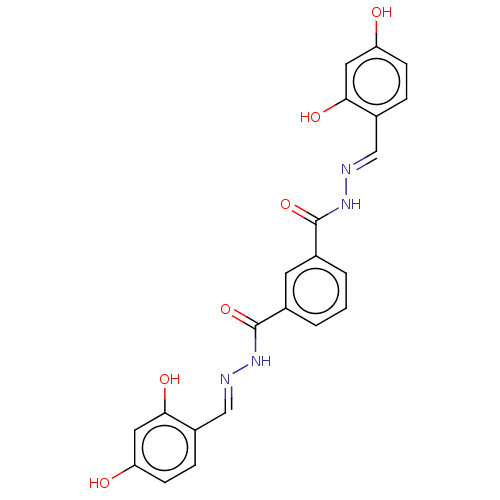 (CHEMBL3318741) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50142513 (Acetic acid 2-acetoxy-5-[3-(3,4-diacetoxy-benzoyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed form | Bioorg Med Chem Lett 14: 1669-72 (2004) Article DOI: 10.1016/j.bmcl.2004.01.060 BindingDB Entry DOI: 10.7270/Q2GX4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50338992 (CHEMBL1687978 | N-[5-(2-Furyl)-1,3,4-oxadiazol-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of STAT3 transcriptional activity in human HeLa cells by luciferase reporter gene assay | ACS Med Chem Lett 1: 371-375 (2010) Article DOI: 10.1021/ml1000273 BindingDB Entry DOI: 10.7270/Q2FJ2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50338996 (2-(4-Chlorophenyl)-N-(5-phenyl-1,3,4-oxadiazol-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of STAT3 transcriptional activity in human HeLa cells by luciferase reporter gene assay | ACS Med Chem Lett 1: 371-375 (2010) Article DOI: 10.1021/ml1000273 BindingDB Entry DOI: 10.7270/Q2FJ2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50338994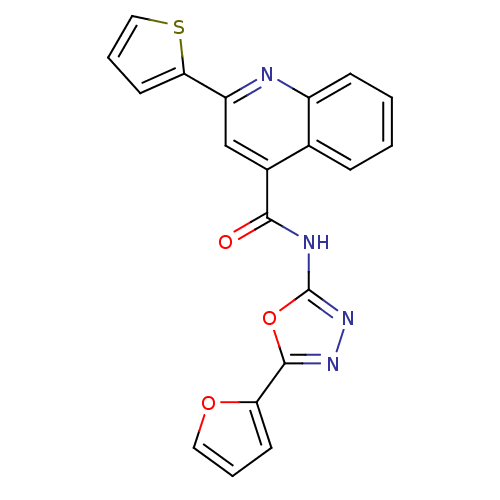 (CHEMBL1687976 | N-[5-(2-Furyl)-1,3,4-oxadiazol-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of STAT3 transcriptional activity in human HeLa cells by luciferase reporter gene assay | ACS Med Chem Lett 1: 371-375 (2010) Article DOI: 10.1021/ml1000273 BindingDB Entry DOI: 10.7270/Q2FJ2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50410153 (CHEMBL197216) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Curated by ChEMBL | Assay Description Inhibitory concentration against DNA topoisomerase II activity | Bioorg Med Chem Lett 15: 2065-8 (2005) Article DOI: 10.1016/j.bmcl.2005.02.052 BindingDB Entry DOI: 10.7270/Q2WH2R6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50338995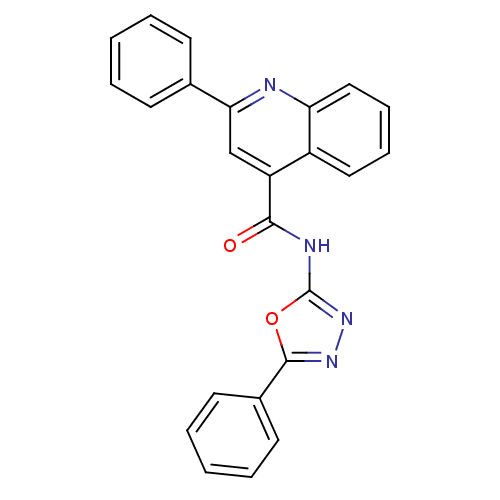 (2-Phenyl-N-(5-phenyl-1,3,4-oxadiazol-2-yl)-4-quino...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of STAT3 transcriptional activity in human HeLa cells by luciferase reporter gene assay | ACS Med Chem Lett 1: 371-375 (2010) Article DOI: 10.1021/ml1000273 BindingDB Entry DOI: 10.7270/Q2FJ2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50338993 (2-(2-Furyl)-N-[5-(2-furyl)-1,3,4-oxadiazol-2-yl]-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of STAT3 transcriptional activity in human HeLa cells by luciferase reporter gene assay | ACS Med Chem Lett 1: 371-375 (2010) Article DOI: 10.1021/ml1000273 BindingDB Entry DOI: 10.7270/Q2FJ2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50338998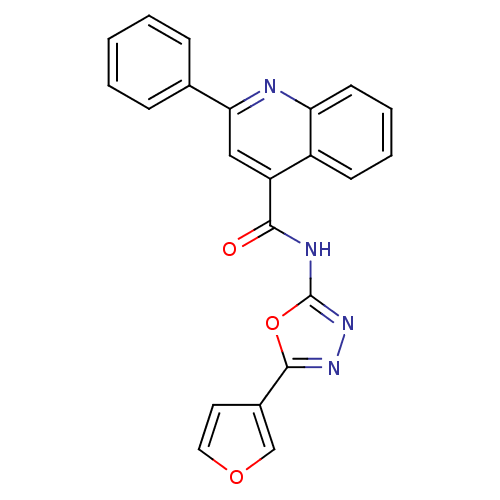 (CHEMBL1687965 | N-[5-(3-Furyl)-1,3,4-oxadiazol-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of STAT3 transcriptional activity in human HeLa cells by luciferase reporter gene assay | ACS Med Chem Lett 1: 371-375 (2010) Article DOI: 10.1021/ml1000273 BindingDB Entry DOI: 10.7270/Q2FJ2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50338991 (CHEMBL1687979 | N-[5-(2-Furyl)-1,3,4-oxadiazol-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of STAT3 transcriptional activity in human HeLa cells by luciferase reporter gene assay | ACS Med Chem Lett 1: 371-375 (2010) Article DOI: 10.1021/ml1000273 BindingDB Entry DOI: 10.7270/Q2FJ2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Rattus norvegicus) | BDBM50053038 (CHEMBL3318735) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto Health Science University Curated by ChEMBL | Assay Description Inhibition of rat testis GST-tagged PARG catalytic activity after 30 mins using [32P]poly(ADP-ribose) by radioassay | Bioorg Med Chem Lett 24: 3802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.065 BindingDB Entry DOI: 10.7270/Q2348N18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |