

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50035208 (1-Pyridin-4-yl-1a,2,3,7b-tetrahydro-1H-cyclopropa[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035206 (4-(5-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049763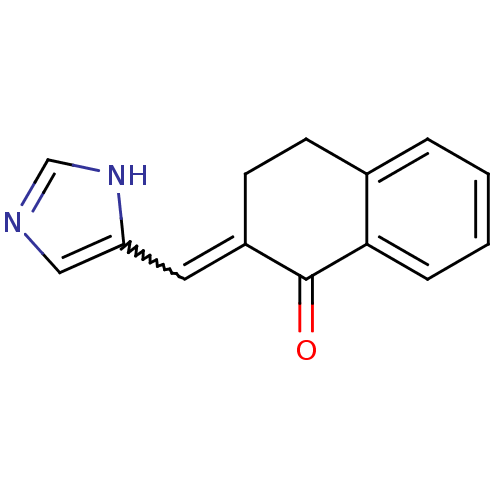 (2-[1-(3H-Imidazol-4-yl)-meth-(E)-ylidene]-3,4-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035209 (4-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049763 (2-[1-(3H-Imidazol-4-yl)-meth-(E)-ylidene]-3,4-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of rat ovarian microsomal Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035211 (1-Pyridin-4-yl-1a,2,3,7b-tetrahydro-1H-cyclopropa[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035209 (4-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035213 (4-(6-Methoxy-1-methyl-1a,2,3,7b-tetrahydro-1H-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049774 (5-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035205 (4-(5-Bromo-6-methoxy-1a,2,3,7b-tetrahydro-1H-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035205 (4-(5-Bromo-6-methoxy-1a,2,3,7b-tetrahydro-1H-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049777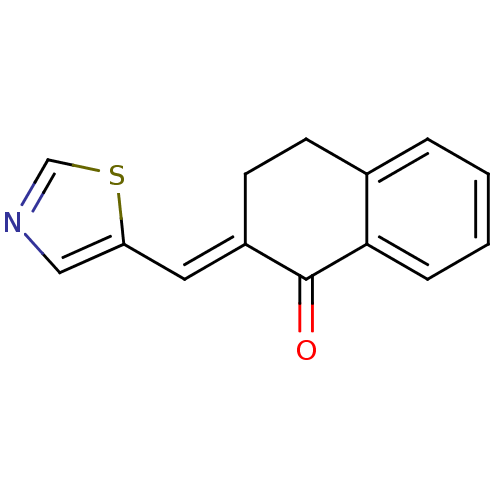 (2-[1-Thiazol-5-yl-meth-(E)-ylidene]-3,4-dihydro-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50035209 (4-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035212 (4-(4-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035210 (4-(7-Bromo-4-methoxy-1a,2,3,7b-tetrahydro-1H-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50009605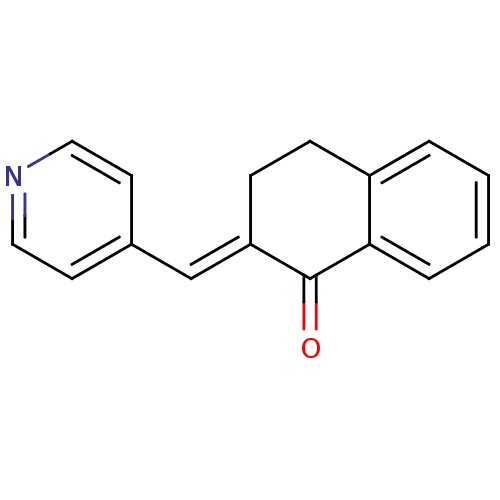 (2-(pyridin-4-ylmethylene)-3,4-dihydronaphthalen-1(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035207 (1-(pyridin-4-yl)-1a,2,3,7b-tetrahydro-1H-cycloprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of rat ovarian microsomal Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50035208 (1-Pyridin-4-yl-1a,2,3,7b-tetrahydro-1H-cyclopropa[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049765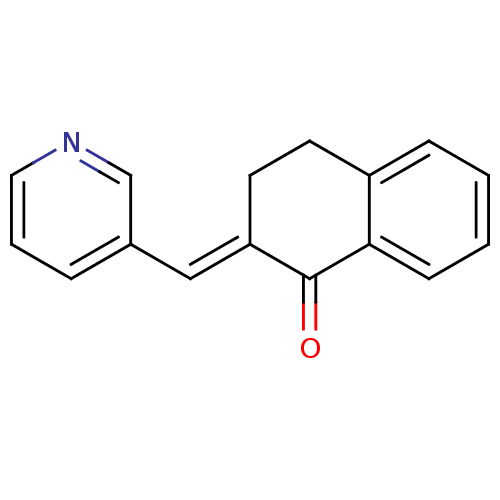 (2-[1-Pyridin-3-yl-meth-(E)-ylidene]-3,4-dihydro-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50049768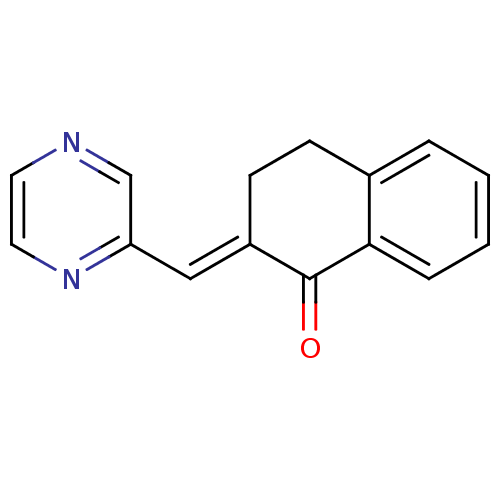 (2-[1-Pyrazin-2-yl-meth-(E)-ylidene]-3,4-dihydro-2H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50378780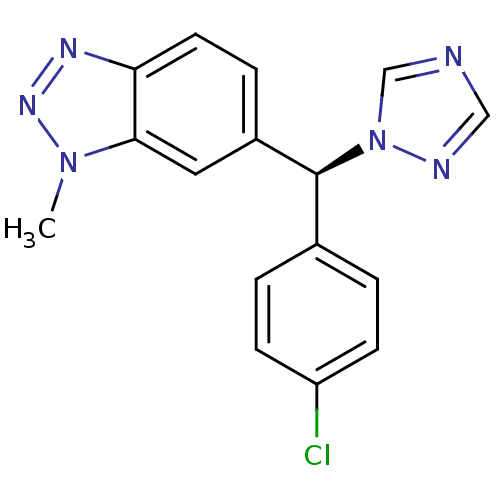 ((S)-VOROZOLE) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50009605 (2-(pyridin-4-ylmethylene)-3,4-dihydronaphthalen-1(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049776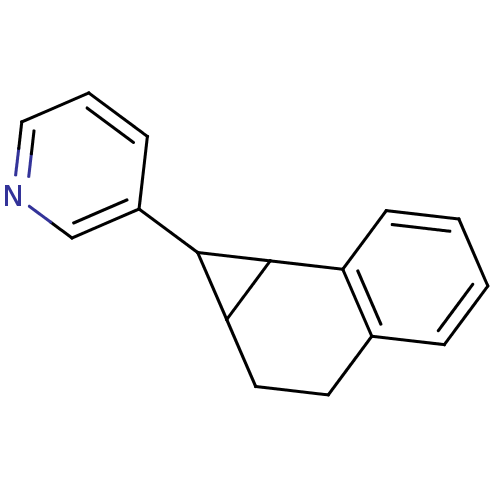 (3-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50049766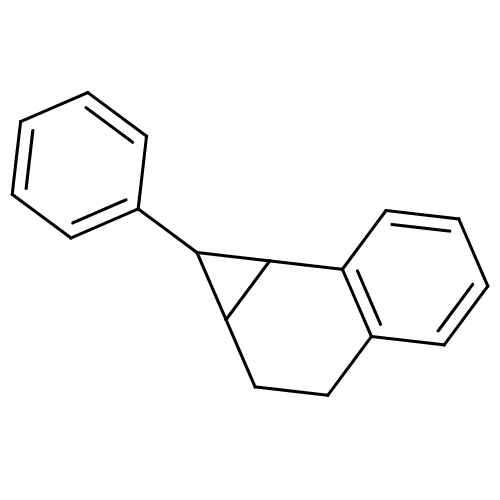 (1-Phenyl-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]naph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049778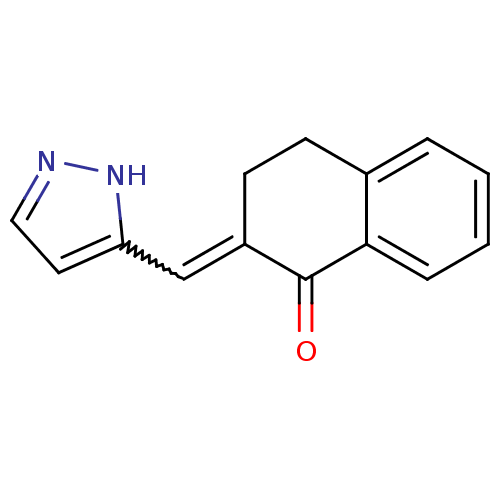 (2-[1-(2H-Pyrazol-3-yl)-meth-(E)-ylidene]-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description Inhibition of Human placental Cytochrome P450 19A1 | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50049762 (3-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50049778 (2-[1-(2H-Pyrazol-3-yl)-meth-(E)-ylidene]-3,4-dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50049777 (2-[1-Thiazol-5-yl-meth-(E)-ylidene]-3,4-dihydro-2H...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50049764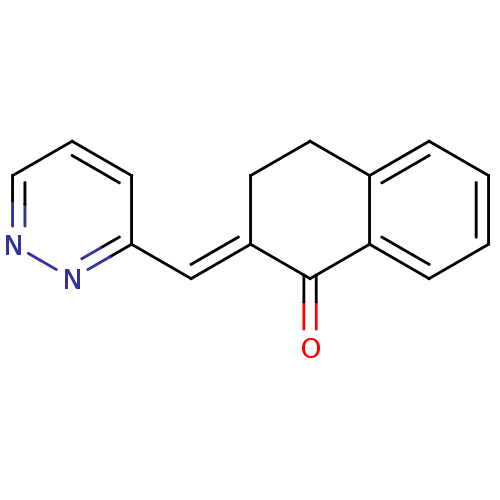 (2-[1-Pyridazin-3-yl-meth-(E)-ylidene]-3,4-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50049776 (3-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049775 (2-(Hydroxy-pyrimidin-4-yl-methyl)-3,4-dihydro-2H-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated corticosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated corticosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50049763 (2-[1-(3H-Imidazol-4-yl)-meth-(E)-ylidene]-3,4-dihy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50035209 (4-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50009605 (2-(pyridin-4-ylmethylene)-3,4-dihydronaphthalen-1(...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049768 (2-[1-Pyrazin-2-yl-meth-(E)-ylidene]-3,4-dihydro-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50049763 (2-[1-(3H-Imidazol-4-yl)-meth-(E)-ylidene]-3,4-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50049765 (2-[1-Pyridin-3-yl-meth-(E)-ylidene]-3,4-dihydro-2H...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50049762 (3-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50035209 (4-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität de Saarlandes Curated by ChEMBL | Assay Description Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria. | J Med Chem 39: 834-41 (1996) Article DOI: 10.1021/jm950377t BindingDB Entry DOI: 10.7270/Q29K499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |