 Found 74 hits with Last Name = 'lansdell' and Initial = 'ta'
Found 74 hits with Last Name = 'lansdell' and Initial = 'ta' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50025098
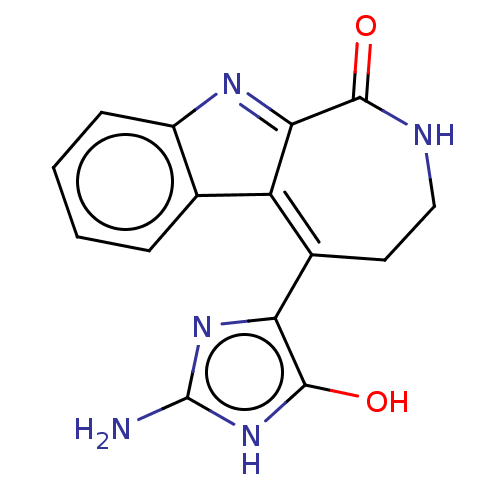
(CHEMBL332551)Show SMILES Nc1nc(c(O)[nH]1)C1=C2C(=Nc3ccccc23)C(=O)NCC1 |c:10,t:8| Show InChI InChI=1S/C15H13N5O2/c16-15-19-11(14(22)20-15)8-5-6-17-13(21)12-10(8)7-3-1-2-4-9(7)18-12/h1-4,22H,5-6H2,(H,17,21)(H3,16,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363647
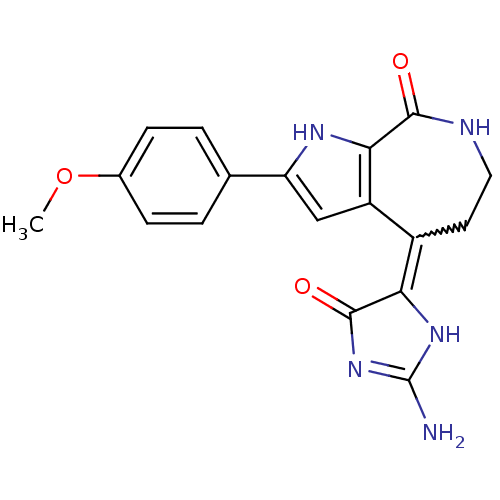
(CHEMBL1947251)Show SMILES COc1ccc(cc1)-c1cc2c([nH]1)C(=O)NCCC2=C1NC(N)=NC1=O |w:18.19,c:25| Show InChI InChI=1S/C18H17N5O3/c1-26-10-4-2-9(3-5-10)13-8-12-11(14-17(25)23-18(19)22-14)6-7-20-16(24)15(12)21-13/h2-5,8,21H,6-7H2,1H3,(H,20,24)(H3,19,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363648
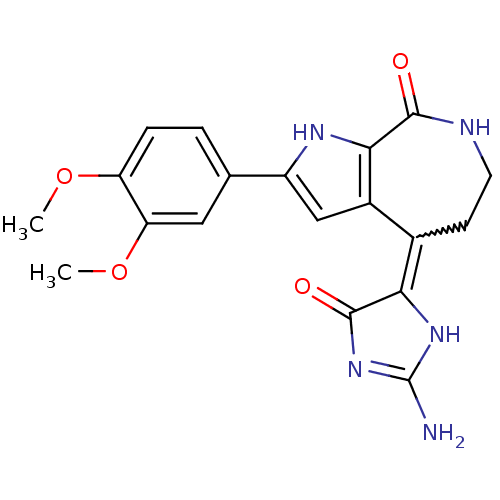
(CHEMBL1947252)Show SMILES COc1ccc(cc1OC)-c1cc2c([nH]1)C(=O)NCCC2=C1NC(N)=NC1=O |w:20.21,c:27| Show InChI InChI=1S/C19H19N5O4/c1-27-13-4-3-9(7-14(13)28-2)12-8-11-10(15-18(26)24-19(20)23-15)5-6-21-17(25)16(11)22-12/h3-4,7-8,22H,5-6H2,1-2H3,(H,21,25)(H3,20,23,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363646

(CHEMBL1947250)Show SMILES NC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:7.8,t:1| Show InChI InChI=1S/C17H15N5O2/c18-17-21-13(16(24)22-17)10-6-7-19-15(23)14-11(10)8-12(20-14)9-4-2-1-3-5-9/h1-5,8,20H,6-7H2,(H,19,23)(H3,18,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363649

(CHEMBL1947253)Show SMILES O=C1N=C(NCc2ccccc2)NC1=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:14.16,t:2| Show InChI InChI=1S/C24H21N5O2/c30-22-21-18(13-19(27-21)16-9-5-2-6-10-16)17(11-12-25-22)20-23(31)29-24(28-20)26-14-15-7-3-1-4-8-15/h1-10,13,27H,11-12,14H2,(H,25,30)(H2,26,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363653

(CHEMBL1944822)Show SMILES CCCNC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:10.21,t:4| Show InChI InChI=1S/C20H21N5O2/c1-2-9-22-20-24-16(19(27)25-20)13-8-10-21-18(26)17-14(13)11-15(23-17)12-6-4-3-5-7-12/h3-7,11,23H,2,8-10H2,1H3,(H,21,26)(H2,22,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363654
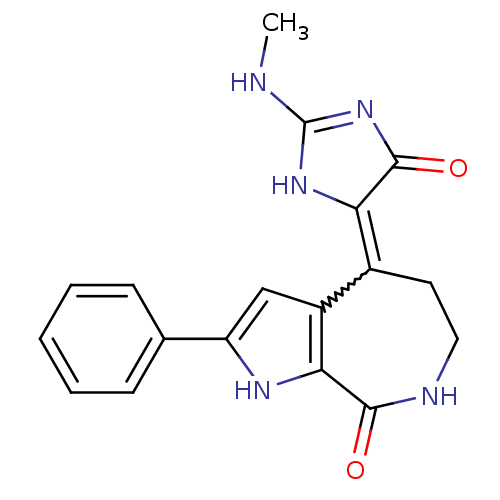
(CHEMBL1944823)Show SMILES CNC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:8.19,t:2| Show InChI InChI=1S/C18H17N5O2/c1-19-18-22-14(17(25)23-18)11-7-8-20-16(24)15-12(11)9-13(21-15)10-5-3-2-4-6-10/h2-6,9,21H,7-8H2,1H3,(H,20,24)(H2,19,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363645
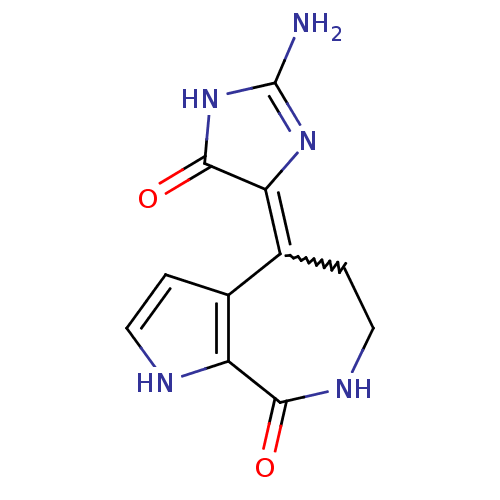
(DEBROMOHYMENIALDISINE)Show SMILES NC1=NC(C(=O)N1)=C1CCNC(=O)c2[nH]ccc12 |w:7.8,t:1| Show InChI InChI=1S/C11H11N5O2/c12-11-15-8(10(18)16-11)6-2-4-14-9(17)7-5(6)1-3-13-7/h1,3,13H,2,4H2,(H,14,17)(H3,12,15,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50025098

(CHEMBL332551)Show SMILES Nc1nc(c(O)[nH]1)C1=C2C(=Nc3ccccc23)C(=O)NCC1 |c:10,t:8| Show InChI InChI=1S/C15H13N5O2/c16-15-19-11(14(22)20-15)8-5-6-17-13(21)12-10(8)7-3-1-2-4-9(7)18-12/h1-4,22H,5-6H2,(H,17,21)(H3,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363652
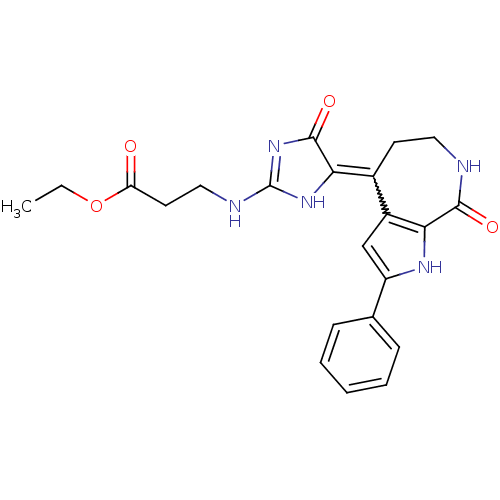
(CHEMBL1944821)Show SMILES CCOC(=O)CCNC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:14.25,t:8| Show InChI InChI=1S/C22H23N5O4/c1-2-31-17(28)9-11-24-22-26-18(21(30)27-22)14-8-10-23-20(29)19-15(14)12-16(25-19)13-6-4-3-5-7-13/h3-7,12,25H,2,8-11H2,1H3,(H,23,29)(H2,24,26,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363650

(CHEMBL1947254)Show SMILES COc1ccc(CNC2=NC(=O)C(N2)=C2CCNC(=O)c3[nH]c(cc23)-c2ccccc2)cc1 |w:14.15,t:8| Show InChI InChI=1S/C25H23N5O3/c1-33-17-9-7-15(8-10-17)14-27-25-29-21(24(32)30-25)18-11-12-26-23(31)22-19(18)13-20(28-22)16-5-3-2-4-6-16/h2-10,13,28H,11-12,14H2,1H3,(H,26,31)(H2,27,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 588 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM32142

(5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...)Show InChI InChI=1S/C9H8N2O/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate expressed in human KMH11 cells assessed as free 7-amino... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM32142

(5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...)Show InChI InChI=1S/C9H8N2O/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate expressed in human UTMC2 cells assessed as free 7-amino... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM32142

(5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...)Show InChI InChI=1S/C9H8N2O/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate expressed in human KMS18 cells assessed as free 7-amino... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM32142

(5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...)Show InChI InChI=1S/C9H8N2O/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate expressed in human OCI-AML2 cells assessed as free 7-am... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM32142

(5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...)Show InChI InChI=1S/C9H8N2O/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate expressed in human K562 cells assessed as free 7-amino-... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM32142

(5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...)Show InChI InChI=1S/C9H8N2O/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate expressed in human MDAY-D2 cells assessed as free 7-ami... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM32142

(5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...)Show InChI InChI=1S/C9H8N2O/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate expressed in human KG1A cells assessed as free 7-amino-... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM32142

(5-amino-8-quinolinol | 5-aminoquinolin-8-ol | 5-az...)Show InChI InChI=1S/C9H8N2O/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate expressed in human NB4 cells assessed as free 7-amino-4... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363645

(DEBROMOHYMENIALDISINE)Show SMILES NC1=NC(C(=O)N1)=C1CCNC(=O)c2[nH]ccc12 |w:7.8,t:1| Show InChI InChI=1S/C11H11N5O2/c12-11-15-8(10(18)16-11)6-2-4-14-9(17)7-5(6)1-3-13-7/h1,3,13H,2,4H2,(H,14,17)(H3,12,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 725 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363647

(CHEMBL1947251)Show SMILES COc1ccc(cc1)-c1cc2c([nH]1)C(=O)NCCC2=C1NC(N)=NC1=O |w:18.19,c:25| Show InChI InChI=1S/C18H17N5O3/c1-26-10-4-2-9(3-5-10)13-8-12-11(14-17(25)23-18(19)22-14)6-7-20-16(24)15(12)21-13/h2-5,8,21H,6-7H2,1H3,(H,20,24)(H3,19,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 867 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363649

(CHEMBL1947253)Show SMILES O=C1N=C(NCc2ccccc2)NC1=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:14.16,t:2| Show InChI InChI=1S/C24H21N5O2/c30-22-21-18(13-19(27-21)16-9-5-2-6-10-16)17(11-12-25-22)20-23(31)29-24(28-20)26-14-15-7-3-1-4-8-15/h1-10,13,27H,11-12,14H2,(H,25,30)(H2,26,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 905 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363646

(CHEMBL1947250)Show SMILES NC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:7.8,t:1| Show InChI InChI=1S/C17H15N5O2/c18-17-21-13(16(24)22-17)10-6-7-19-15(23)14-11(10)8-12(20-14)9-4-2-1-3-5-9/h1-5,8,20H,6-7H2,(H,19,23)(H3,18,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363651

(CHEMBL1947255)Show SMILES O=C1N=C(NC2CCCCC2)NC1=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:13.25,t:2| Show InChI InChI=1S/C23H25N5O2/c29-21-20-17(13-18(26-20)14-7-3-1-4-8-14)16(11-12-24-21)19-22(30)28-23(27-19)25-15-9-5-2-6-10-15/h1,3-4,7-8,13,15,26H,2,5-6,9-12H2,(H,24,29)(H2,25,27,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human Chk2 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363648

(CHEMBL1947252)Show SMILES COc1ccc(cc1OC)-c1cc2c([nH]1)C(=O)NCCC2=C1NC(N)=NC1=O |w:20.21,c:27| Show InChI InChI=1S/C19H19N5O4/c1-27-13-4-3-9(7-14(13)28-2)12-8-11-10(15-18(26)24-19(20)23-15)5-6-21-17(25)16(11)22-12/h3-4,7-8,22H,5-6H2,1-2H3,(H,21,25)(H3,20,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171081
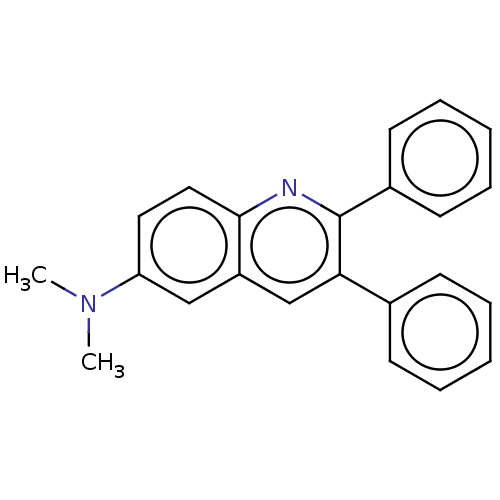
(CHEMBL3805036)Show InChI InChI=1S/C23H20N2/c1-25(2)20-13-14-22-19(15-20)16-21(17-9-5-3-6-10-17)23(24-22)18-11-7-4-8-12-18/h3-16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate assessed as fluorescence quinching control by fluoresce... |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50170997
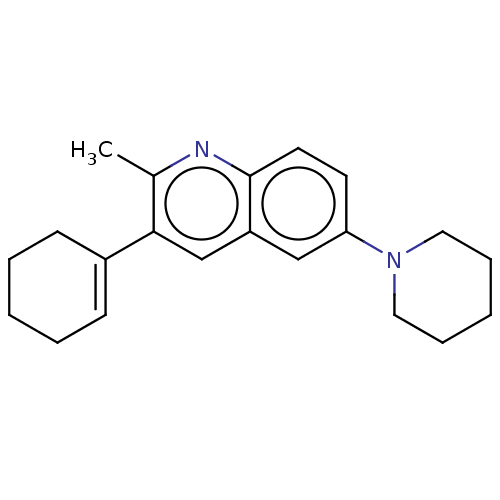
(CHEMBL3805801)Show InChI InChI=1S/C21H26N2/c1-16-20(17-8-4-2-5-9-17)15-18-14-19(10-11-21(18)22-16)23-12-6-3-7-13-23/h8,10-11,14-15H,2-7,9,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171045
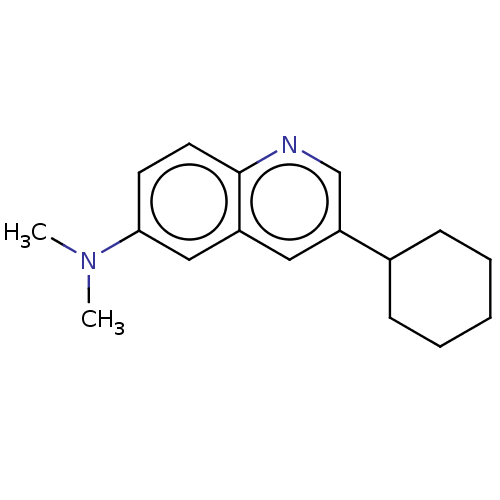
(CHEMBL3805324)Show InChI InChI=1S/C17H22N2/c1-19(2)16-8-9-17-14(11-16)10-15(12-18-17)13-6-4-3-5-7-13/h8-13H,3-7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50170999
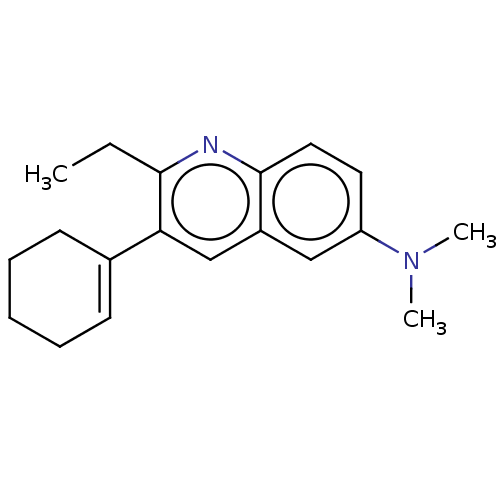
(CHEMBL3805496)Show InChI InChI=1S/C19H24N2/c1-4-18-17(14-8-6-5-7-9-14)13-15-12-16(21(2)3)10-11-19(15)20-18/h8,10-13H,4-7,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171000
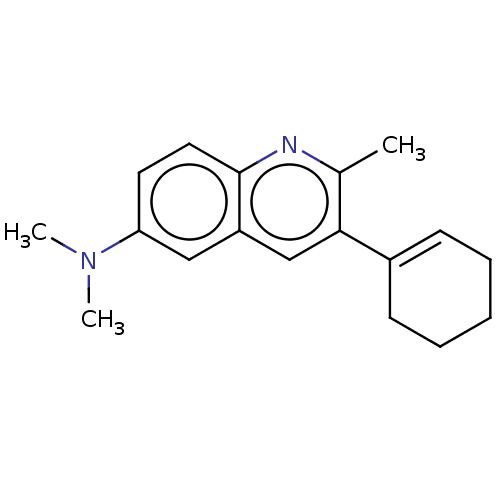
(CHEMBL3805486)Show InChI InChI=1S/C18H22N2/c1-13-17(14-7-5-4-6-8-14)12-15-11-16(20(2)3)9-10-18(15)19-13/h7,9-12H,4-6,8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171001
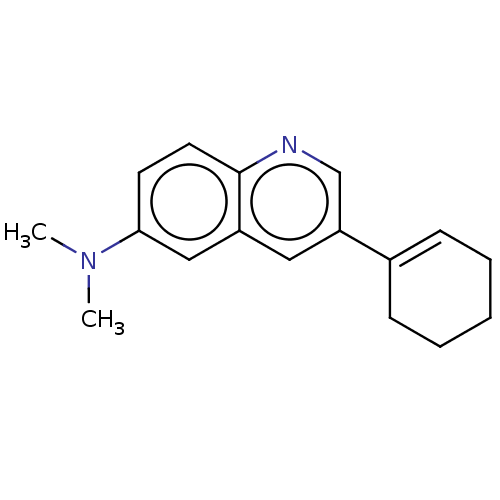
(CHEMBL3805988)Show InChI InChI=1S/C17H20N2/c1-19(2)16-8-9-17-14(11-16)10-15(12-18-17)13-6-4-3-5-7-13/h6,8-12H,3-5,7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363654

(CHEMBL1944823)Show SMILES CNC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:8.19,t:2| Show InChI InChI=1S/C18H17N5O2/c1-19-18-22-14(17(25)23-18)11-7-8-20-16(24)15-12(11)9-13(21-15)10-5-3-2-4-6-10/h2-6,9,21H,7-8H2,1H3,(H,20,24)(H2,19,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171046
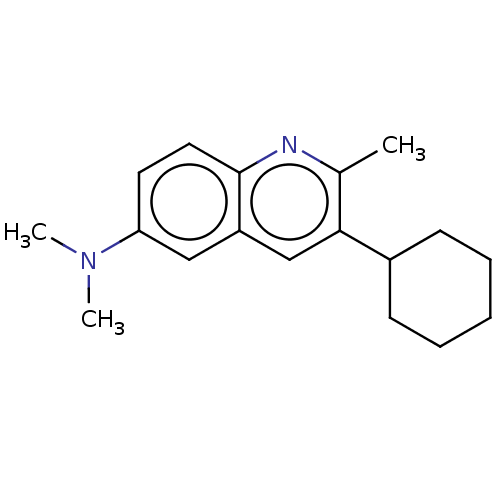
(CHEMBL3805440)Show InChI InChI=1S/C18H24N2/c1-13-17(14-7-5-4-6-8-14)12-15-11-16(20(2)3)9-10-18(15)19-13/h9-12,14H,4-8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171002

(CHEMBL3806320)Show InChI InChI=1S/C20H25N/c1-3-4-8-16-11-12-20-18(13-16)14-19(15(2)21-20)17-9-6-5-7-10-17/h9,11-14H,3-8,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171047
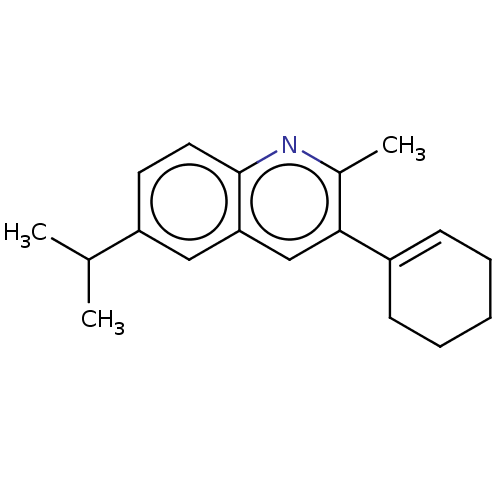
(CHEMBL3805100)Show InChI InChI=1S/C19H23N/c1-13(2)16-9-10-19-17(11-16)12-18(14(3)20-19)15-7-5-4-6-8-15/h7,9-13H,4-6,8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171034
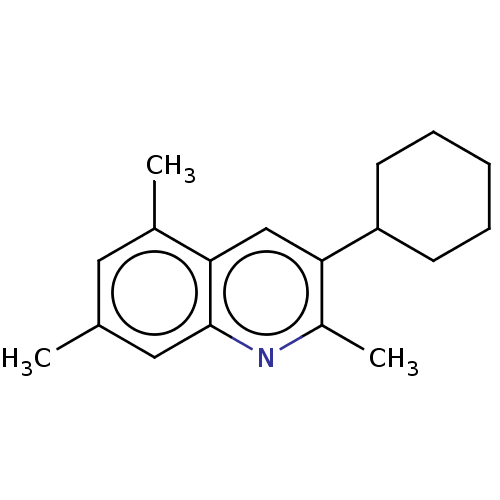
(CHEMBL3805013)Show InChI InChI=1S/C18H23N/c1-12-9-13(2)16-11-17(14(3)19-18(16)10-12)15-7-5-4-6-8-15/h9-11,15H,4-8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171003
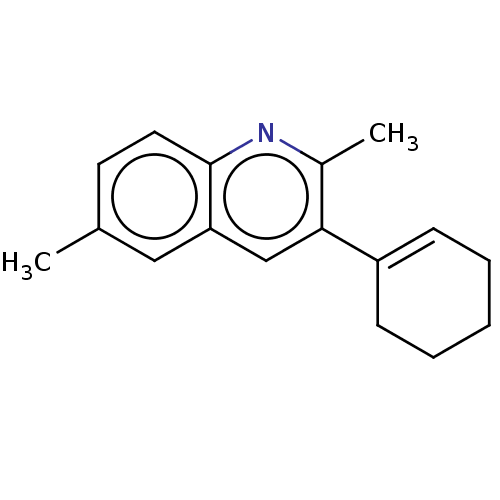
(CHEMBL3805932)Show InChI InChI=1S/C17H19N/c1-12-8-9-17-15(10-12)11-16(13(2)18-17)14-6-4-3-5-7-14/h6,8-11H,3-5,7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50170998
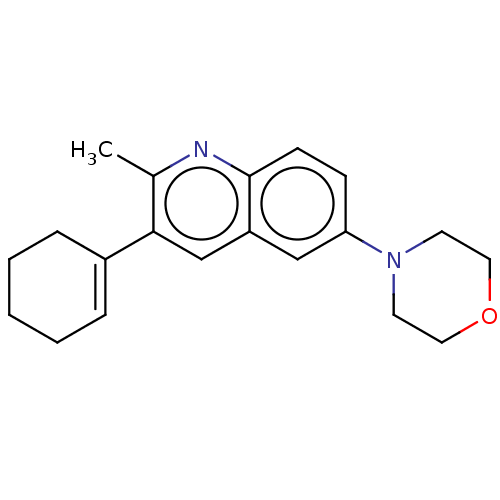
(CHEMBL3805812)Show InChI InChI=1S/C20H24N2O/c1-15-19(16-5-3-2-4-6-16)14-17-13-18(7-8-20(17)21-15)22-9-11-23-12-10-22/h5,7-8,13-14H,2-4,6,9-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171006
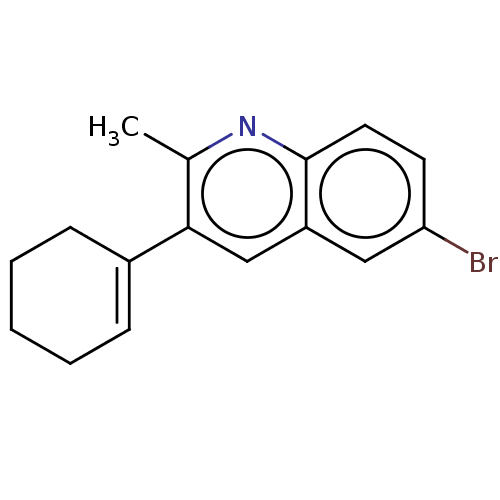
(CHEMBL3805160)Show InChI InChI=1S/C16H16BrN/c1-11-15(12-5-3-2-4-6-12)10-13-9-14(17)7-8-16(13)18-11/h5,7-10H,2-4,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363650

(CHEMBL1947254)Show SMILES COc1ccc(CNC2=NC(=O)C(N2)=C2CCNC(=O)c3[nH]c(cc23)-c2ccccc2)cc1 |w:14.15,t:8| Show InChI InChI=1S/C25H23N5O3/c1-33-17-9-7-15(8-10-17)14-27-25-29-21(24(32)30-25)18-11-12-26-23(31)22-19(18)13-20(28-22)16-5-3-2-4-6-16/h2-10,13,28H,11-12,14H2,1H3,(H,26,31)(H2,27,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363651

(CHEMBL1947255)Show SMILES O=C1N=C(NC2CCCCC2)NC1=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:13.25,t:2| Show InChI InChI=1S/C23H25N5O2/c29-21-20-17(13-18(26-20)14-7-3-1-4-8-14)16(11-12-24-21)19-22(30)28-23(27-19)25-15-9-5-2-6-10-15/h1,3-4,7-8,13,15,26H,2,5-6,9-12H2,(H,24,29)(H2,25,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363653

(CHEMBL1944822)Show SMILES CCCNC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:10.21,t:4| Show InChI InChI=1S/C20H21N5O2/c1-2-9-22-20-24-16(19(27)25-20)13-8-10-21-18(26)17-14(13)11-15(23-17)12-6-4-3-5-7-12/h3-7,11,23H,2,8-10H2,1H3,(H,21,26)(H2,22,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50363652

(CHEMBL1944821)Show SMILES CCOC(=O)CCNC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:14.25,t:8| Show InChI InChI=1S/C22H23N5O4/c1-2-31-17(28)9-11-24-22-26-18(21(30)27-22)14-8-10-23-20(29)19-15(14)12-16(25-19)13-6-4-3-5-7-13/h3-7,12,25H,2,8-11H2,1H3,(H,23,29)(H2,24,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using STK1 as substrate after 10 mins by HTRF assay |
Bioorg Med Chem 20: 1475-81 (2012)
Article DOI: 10.1016/j.bmc.2011.12.054
BindingDB Entry DOI: 10.7270/Q20865RK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171080
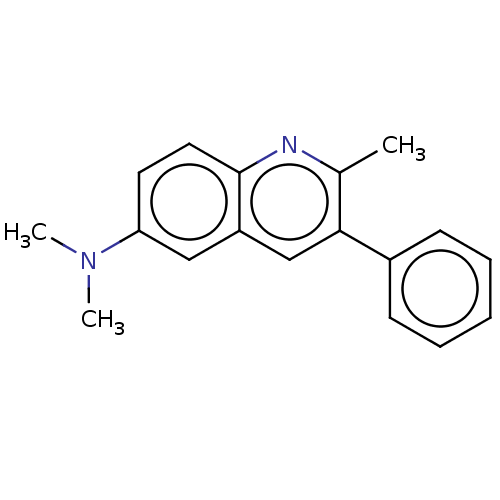
(CHEMBL3806316)Show InChI InChI=1S/C18H18N2/c1-13-17(14-7-5-4-6-8-14)12-15-11-16(20(2)3)9-10-18(15)19-13/h4-12H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50170997

(CHEMBL3805801)Show InChI InChI=1S/C21H26N2/c1-16-20(17-8-4-2-5-9-17)15-18-14-19(10-11-21(18)22-16)23-12-6-3-7-13-23/h8,10-11,14-15H,2-7,9,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome caspase beta1-like activity using Z-LLE-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171036
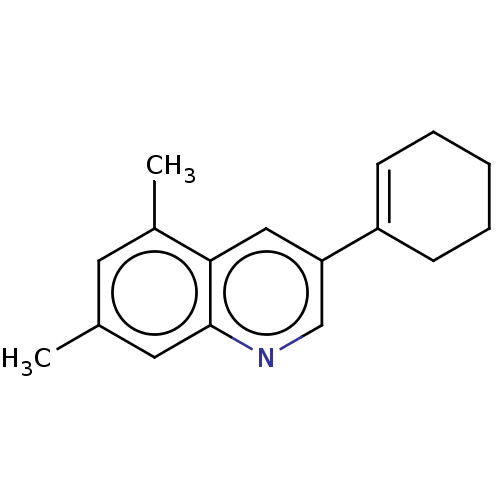
(CHEMBL3805729)Show InChI InChI=1S/C17H19N/c1-12-8-13(2)16-10-15(11-18-17(16)9-12)14-6-4-3-5-7-14/h6,8-11H,3-5,7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50171038
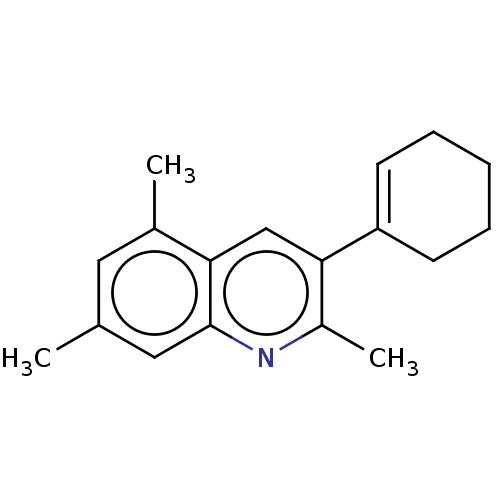
(CHEMBL3805213)Show InChI InChI=1S/C18H21N/c1-12-9-13(2)16-11-17(14(3)19-18(16)10-12)15-7-5-4-6-8-15/h7,9-11H,4-6,8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
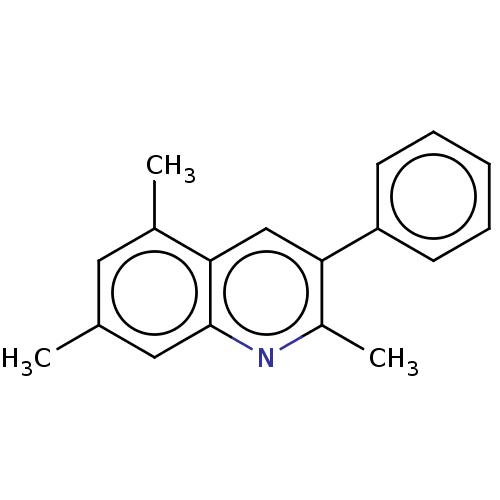
(Homo sapiens (Human)) | BDBM50171039
(CHEMBL3806263)Show InChI InChI=1S/C18H17N/c1-12-9-13(2)16-11-17(14(3)19-18(16)10-12)15-7-5-4-6-8-15/h4-11H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
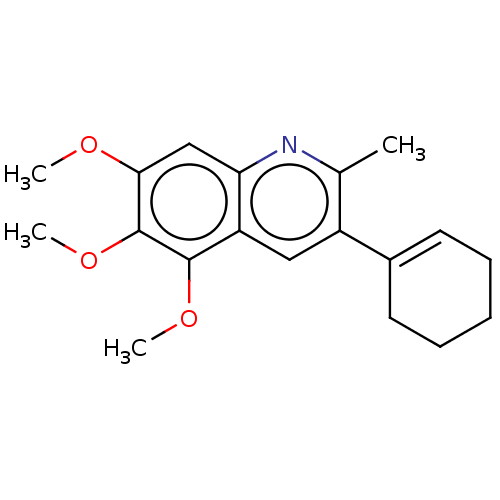
(Homo sapiens (Human)) | BDBM50171044
(CHEMBL3805167)Show SMILES COc1cc2nc(C)c(cc2c(OC)c1OC)C1=CCCCC1 |t:19| Show InChI InChI=1S/C19H23NO3/c1-12-14(13-8-6-5-7-9-13)10-15-16(20-12)11-17(21-2)19(23-4)18(15)22-3/h8,10-11H,5-7,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome chymotryptic-like activity using Suc-LLVY-AMC as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem 24: 2441-50 (2016)
Article DOI: 10.1016/j.bmc.2016.04.005
BindingDB Entry DOI: 10.7270/Q2PK0J26 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data