 Found 243 hits with Last Name = 'o''shea' and Initial = 'tj'
Found 243 hits with Last Name = 'o''shea' and Initial = 'tj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50312527
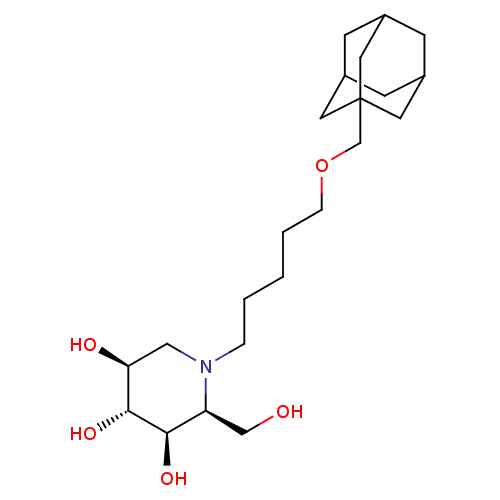
(CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:20.25.19:26,THB:21:20:22.23.27:26,23:22:19:25.24.26,23:24:21.22.27:19| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19-,20+,21+,22?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50312529
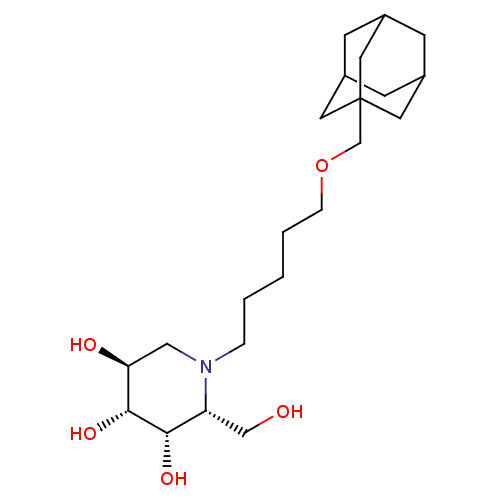
(CHEMBL1088158 | N-[5-(Adamantan-1-yl-methoxy)-pent...)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:20.25.19:26,THB:21:20:22.23.27:26,23:22:19:25.24.26,23:24:21.22.27:19| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20+,21-,22?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18355

((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of lysosomal alpha-glucosidase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301852
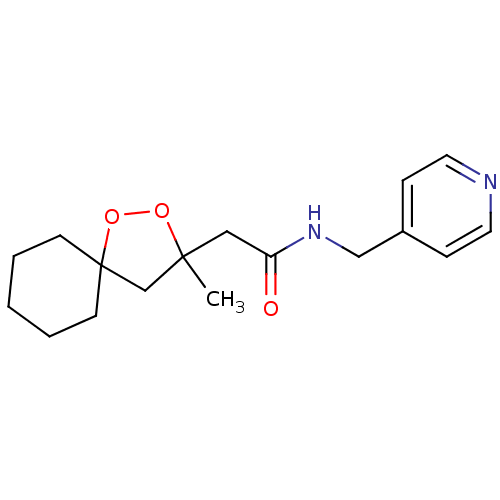
(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(pyri...)Show InChI InChI=1S/C17H24N2O3/c1-16(13-17(22-21-16)7-3-2-4-8-17)11-15(20)19-12-14-5-9-18-10-6-14/h5-6,9-10H,2-4,7-8,11-13H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GCS by cell-based assay |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50312527

(CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:20.25.19:26,THB:21:20:22.23.27:26,23:22:19:25.24.26,23:24:21.22.27:19| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19-,20+,21+,22?/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GCS by cell-based assay |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM18355

((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50312526

(CHEMBL1076754 | N-Butyl-L-ido-1-deoxynojirimycin)Show SMILES CCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO |r| Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8-,9+,10+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50163440
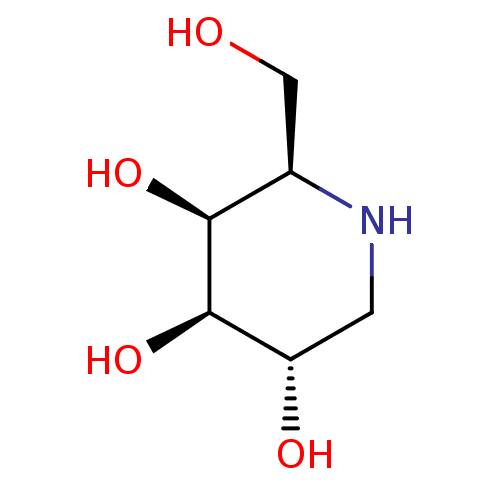
((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5+,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of sucrase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50312528
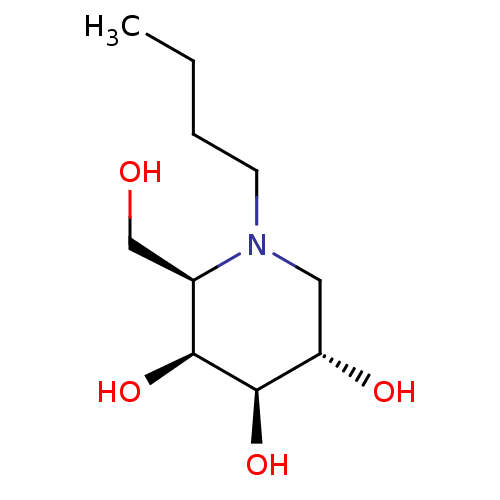
(CHEMBL1086997 | N-Butyl-D-galacto-1-deoxynojirimyc...)Show SMILES CCCCN1C[C@H](O)[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9+,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of lysosomal alpha-glucosidase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50312528

(CHEMBL1086997 | N-Butyl-D-galacto-1-deoxynojirimyc...)Show SMILES CCCCN1C[C@H](O)[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of sucrase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50312529

(CHEMBL1088158 | N-[5-(Adamantan-1-yl-methoxy)-pent...)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:20.25.19:26,THB:21:20:22.23.27:26,23:22:19:25.24.26,23:24:21.22.27:19| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20+,21-,22?/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GCS by cell-based assay |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50312531

(CHEMBL1088292 | N-[5-(Adamantan-1-yl-methoxy)-pent...)Show SMILES OC[C@H]1[C@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:20.25.19:26,THB:21:20:22.23.27:26,23:22:19:25.24.26,23:24:21.22.27:19| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19-,20-,21+,22?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50242271
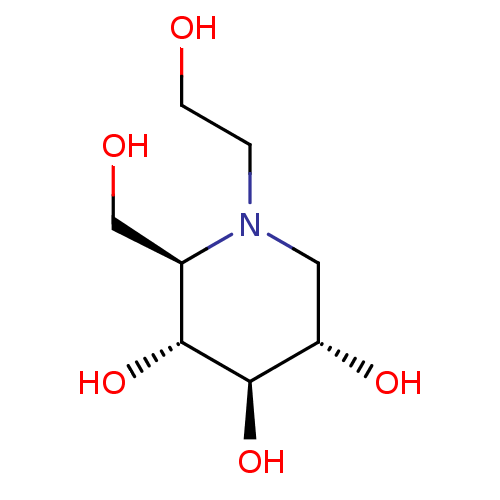
((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...)Show SMILES OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C8H17NO5/c10-2-1-9-3-6(12)8(14)7(13)5(9)4-11/h5-8,10-14H,1-4H2/t5-,6+,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of sucrase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM18355

((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of sucrase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of sucrase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50312529

(CHEMBL1088158 | N-[5-(Adamantan-1-yl-methoxy)-pent...)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:20.25.19:26,THB:21:20:22.23.27:26,23:22:19:25.24.26,23:24:21.22.27:19| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20+,21-,22?/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of lysosomal alpha-glucosidase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50245848
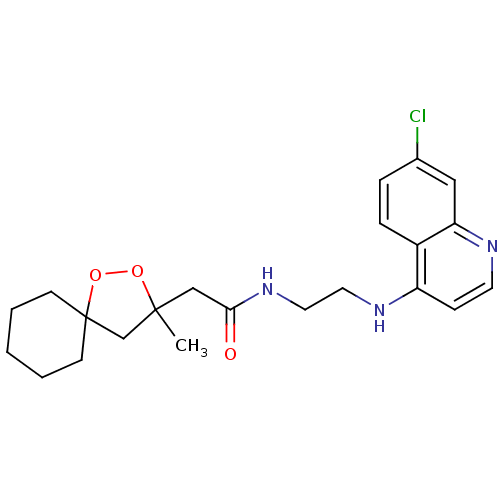
(CHEMBL462360 | N-(2-(7-chloroquinolin-4-ylamino)et...)Show SMILES CC1(CC(=O)NCCNc2ccnc3cc(Cl)ccc23)CC2(CCCCC2)OO1 Show InChI InChI=1S/C22H28ClN3O3/c1-21(15-22(29-28-21)8-3-2-4-9-22)14-20(27)26-12-11-25-18-7-10-24-19-13-16(23)5-6-17(18)19/h5-7,10,13H,2-4,8-9,11-12,14-15H2,1H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301837
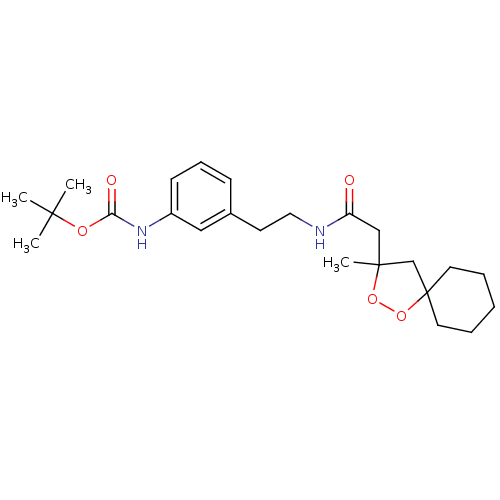
(CHEMBL567374 | tert-butyl 3-(2-(2-(3-methyl-1,2-di...)Show SMILES CC(C)(C)OC(=O)Nc1cccc(CCNC(=O)CC2(C)CC3(CCCCC3)OO2)c1 Show InChI InChI=1S/C24H36N2O5/c1-22(2,3)29-21(28)26-19-10-8-9-18(15-19)11-14-25-20(27)16-23(4)17-24(31-30-23)12-6-5-7-13-24/h8-10,15H,5-7,11-14,16-17H2,1-4H3,(H,25,27)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301847
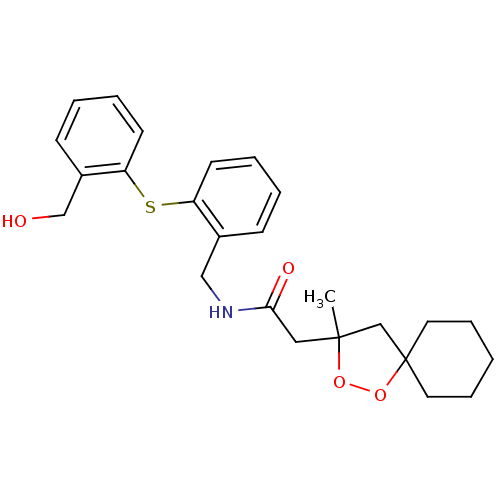
(CHEMBL565360 | N-(2-(2-(hydroxymethyl)phenylthio)b...)Show SMILES CC1(CC(=O)NCc2ccccc2Sc2ccccc2CO)CC2(CCCCC2)OO1 Show InChI InChI=1S/C25H31NO4S/c1-24(18-25(30-29-24)13-7-2-8-14-25)15-23(28)26-16-19-9-3-5-11-21(19)31-22-12-6-4-10-20(22)17-27/h3-6,9-12,27H,2,7-8,13-18H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of lysosomal alpha-glucosidase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301830
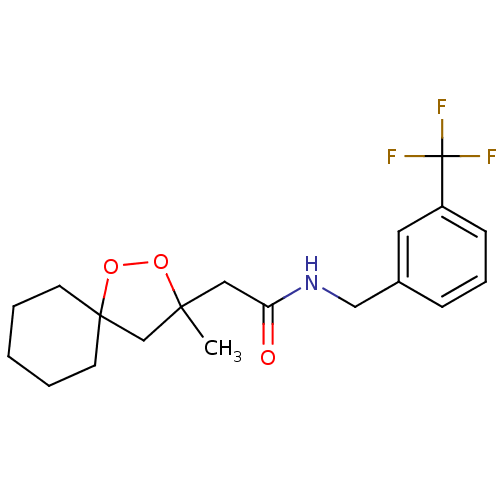
(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(3-(t...)Show SMILES CC1(CC(=O)NCc2cccc(c2)C(F)(F)F)CC2(CCCCC2)OO1 Show InChI InChI=1S/C19H24F3NO3/c1-17(13-18(26-25-17)8-3-2-4-9-18)11-16(24)23-12-14-6-5-7-15(10-14)19(20,21)22/h5-7,10H,2-4,8-9,11-13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50163440

((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5+,6-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of maltase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50312528

(CHEMBL1086997 | N-Butyl-D-galacto-1-deoxynojirimyc...)Show SMILES CCCCN1C[C@H](O)[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9+,10-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of lysosomal alpha-glucosidase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301845
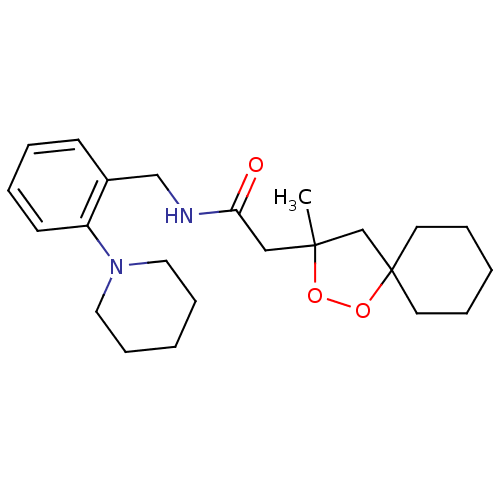
(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(2-(p...)Show SMILES CC1(CC(=O)NCc2ccccc2N2CCCCC2)CC2(CCCCC2)OO1 Show InChI InChI=1S/C23H34N2O3/c1-22(18-23(28-27-22)12-6-2-7-13-23)16-21(26)24-17-19-10-4-5-11-20(19)25-14-8-3-9-15-25/h4-5,10-11H,2-3,6-9,12-18H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301828
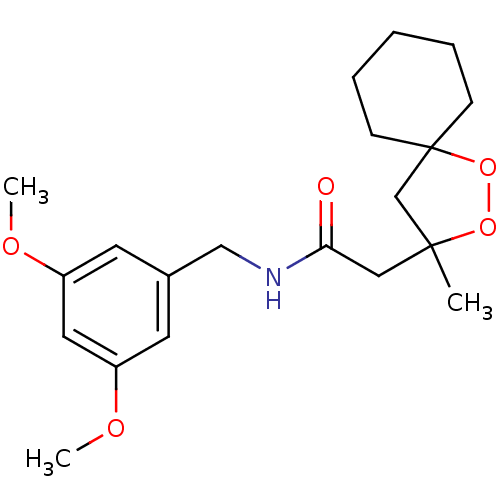
(CHEMBL579199 | N-(3,5-dimethoxybenzyl)-2-(3-methyl...)Show SMILES COc1cc(CNC(=O)CC2(C)CC3(CCCCC3)OO2)cc(OC)c1 Show InChI InChI=1S/C20H29NO5/c1-19(14-20(26-25-19)7-5-4-6-8-20)12-18(22)21-13-15-9-16(23-2)11-17(10-15)24-3/h9-11H,4-8,12-14H2,1-3H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301835
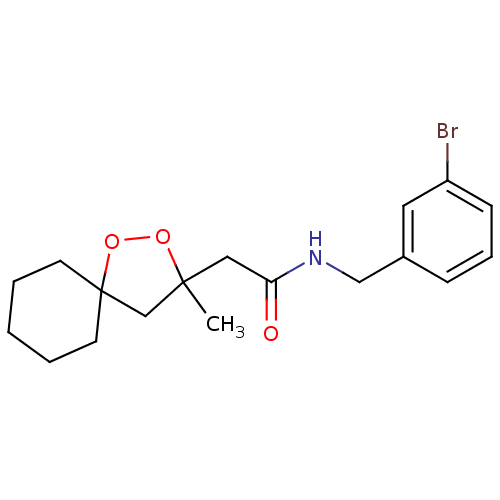
(CHEMBL572184 | N-(3-bromobenzyl)-2-(3-methyl-1,2-d...)Show InChI InChI=1S/C18H24BrNO3/c1-17(13-18(23-22-17)8-3-2-4-9-18)11-16(21)20-12-14-6-5-7-15(19)10-14/h5-7,10H,2-4,8-9,11-13H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301828

(CHEMBL579199 | N-(3,5-dimethoxybenzyl)-2-(3-methyl...)Show SMILES COc1cc(CNC(=O)CC2(C)CC3(CCCCC3)OO2)cc(OC)c1 Show InChI InChI=1S/C20H29NO5/c1-19(14-20(26-25-19)7-5-4-6-8-20)12-18(22)21-13-15-9-16(23-2)11-17(10-15)24-3/h9-11H,4-8,12-14H2,1-3H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301840
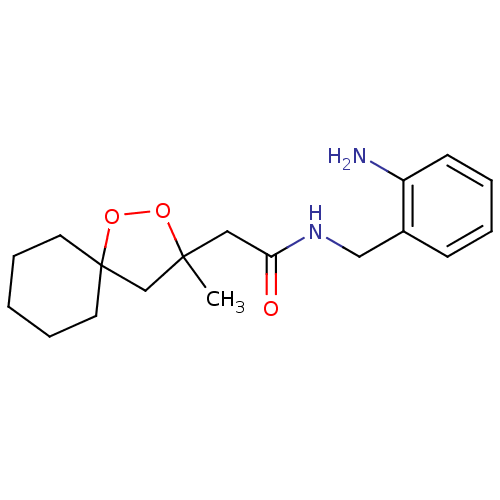
(CHEMBL567376 | N-(2-aminobenzyl)-2-(3-methyl-1,2-d...)Show InChI InChI=1S/C18H26N2O3/c1-17(13-18(23-22-17)9-5-2-6-10-18)11-16(21)20-12-14-7-3-4-8-15(14)19/h3-4,7-8H,2,5-6,9-13,19H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301829
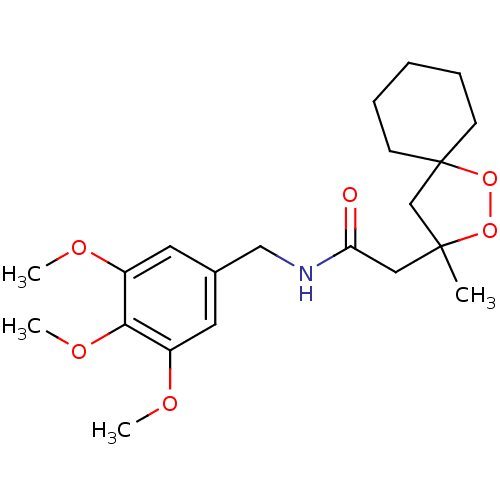
(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(3,4,...)Show SMILES COc1cc(CNC(=O)CC2(C)CC3(CCCCC3)OO2)cc(OC)c1OC Show InChI InChI=1S/C21H31NO6/c1-20(14-21(28-27-20)8-6-5-7-9-21)12-18(23)22-13-15-10-16(24-2)19(26-4)17(11-15)25-3/h10-11H,5-9,12-14H2,1-4H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50242271

((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...)Show SMILES OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C8H17NO5/c10-2-1-9-3-6(12)8(14)7(13)5(9)4-11/h5-8,10-14H,1-4H2/t5-,6+,7-,8-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of lysosomal alpha-glucosidase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of sucrase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of maltase by HPLC |
J Med Chem 53: 689-98 (2010)
Article DOI: 10.1021/jm901281m
BindingDB Entry DOI: 10.7270/Q2M908TC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301834
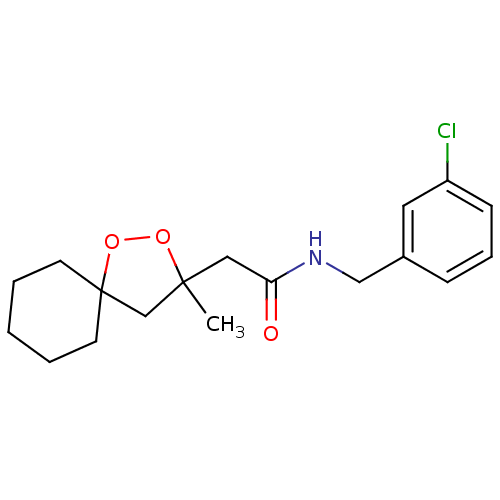
(CHEMBL577694 | N-(3-chlorobenzyl)-2-(3-methyl-1,2-...)Show InChI InChI=1S/C18H24ClNO3/c1-17(13-18(23-22-17)8-3-2-4-9-18)11-16(21)20-12-14-6-5-7-15(19)10-14/h5-7,10H,2-4,8-9,11-13H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50301847

(CHEMBL565360 | N-(2-(2-(hydroxymethyl)phenylthio)b...)Show SMILES CC1(CC(=O)NCc2ccccc2Sc2ccccc2CO)CC2(CCCCC2)OO1 Show InChI InChI=1S/C25H31NO4S/c1-24(18-25(30-29-24)13-7-2-8-14-25)15-23(28)26-16-19-9-3-5-11-21(19)31-22-12-6-4-10-20(22)17-27/h3-6,9-12,27H,2,7-8,13-18H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301845

(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(2-(p...)Show SMILES CC1(CC(=O)NCc2ccccc2N2CCCCC2)CC2(CCCCC2)OO1 Show InChI InChI=1S/C23H34N2O3/c1-22(18-23(28-27-22)12-6-2-7-13-23)16-21(26)24-17-19-10-4-5-11-20(19)25-14-8-3-9-15-25/h4-5,10-11H,2-3,6-9,12-18H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301841
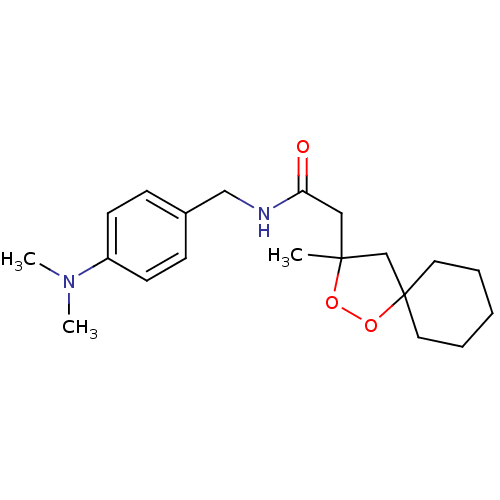
(CHEMBL578588 | N-(4-(dimethylamino)benzyl)-2-(3-me...)Show InChI InChI=1S/C20H30N2O3/c1-19(15-20(25-24-19)11-5-4-6-12-20)13-18(23)21-14-16-7-9-17(10-8-16)22(2)3/h7-10H,4-6,11-15H2,1-3H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301827
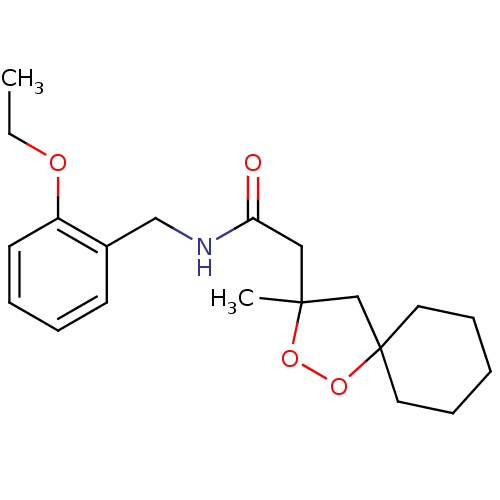
(CHEMBL578968 | N-(2-ethoxybenzyl)-2-(3-methyl-1,2-...)Show InChI InChI=1S/C20H29NO4/c1-3-23-17-10-6-5-9-16(17)14-21-18(22)13-19(2)15-20(25-24-19)11-7-4-8-12-20/h5-6,9-10H,3-4,7-8,11-15H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301848
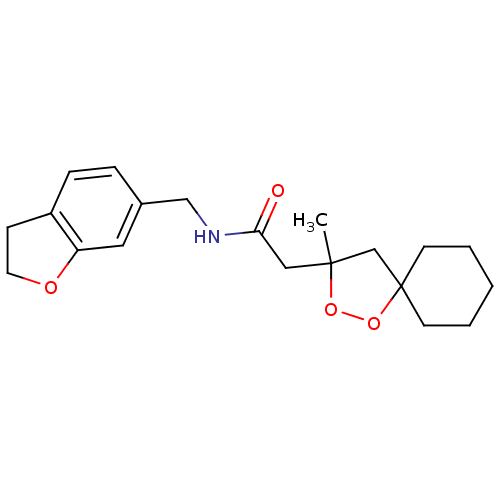
(CHEMBL584886 | N-((2,3-dihydrobenzofuran-6-yl)meth...)Show InChI InChI=1S/C20H27NO4/c1-19(14-20(25-24-19)8-3-2-4-9-20)12-18(22)21-13-15-5-6-16-7-10-23-17(16)11-15/h5-6,11H,2-4,7-10,12-14H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301826
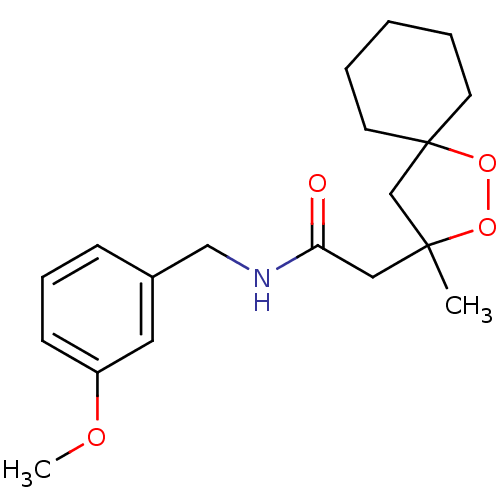
(CHEMBL565361 | N-(3-methoxybenzyl)-2-(3-methyl-1,2...)Show InChI InChI=1S/C19H27NO4/c1-18(14-19(24-23-18)9-4-3-5-10-19)12-17(21)20-13-15-7-6-8-16(11-15)22-2/h6-8,11H,3-5,9-10,12-14H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301836

(CHEMBL565362 | N-(4-bromo-2-fluorobenzyl)-2-(3-met...)Show InChI InChI=1S/C18H23BrFNO3/c1-17(12-18(24-23-17)7-3-2-4-8-18)10-16(22)21-11-13-5-6-14(19)9-15(13)20/h5-6,9H,2-4,7-8,10-12H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301847

(CHEMBL565360 | N-(2-(2-(hydroxymethyl)phenylthio)b...)Show SMILES CC1(CC(=O)NCc2ccccc2Sc2ccccc2CO)CC2(CCCCC2)OO1 Show InChI InChI=1S/C25H31NO4S/c1-24(18-25(30-29-24)13-7-2-8-14-25)15-23(28)26-16-19-9-3-5-11-21(19)31-22-12-6-4-10-20(22)17-27/h3-6,9-12,27H,2,7-8,13-18H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301833
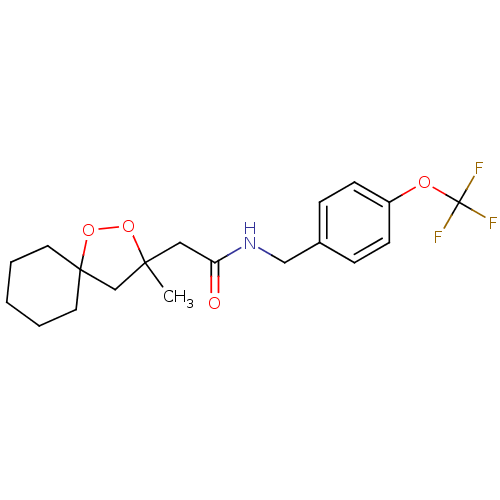
(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(4-(t...)Show SMILES CC1(CC(=O)NCc2ccc(OC(F)(F)F)cc2)CC2(CCCCC2)OO1 Show InChI InChI=1S/C19H24F3NO4/c1-17(13-18(27-26-17)9-3-2-4-10-18)11-16(24)23-12-14-5-7-15(8-6-14)25-19(20,21)22/h5-8H,2-4,9-13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301848

(CHEMBL584886 | N-((2,3-dihydrobenzofuran-6-yl)meth...)Show InChI InChI=1S/C20H27NO4/c1-19(14-20(25-24-19)8-3-2-4-9-20)12-18(22)21-13-15-5-6-16-7-10-23-17(16)11-15/h5-6,11H,2-4,7-10,12-14H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50304834

(CHEMBL578928 | GNF-Pf-2094 | N2-benzyl-N4-cyclohex...)Show InChI InChI=1S/C18H24N4/c1-14-12-17(21-16-10-6-3-7-11-16)22-18(20-14)19-13-15-8-4-2-5-9-15/h2,4-5,8-9,12,16H,3,6-7,10-11,13H2,1H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
Bioorg Med Chem Lett 20: 228-31 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.133
BindingDB Entry DOI: 10.7270/Q2WD40P9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50301830

(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(3-(t...)Show SMILES CC1(CC(=O)NCc2cccc(c2)C(F)(F)F)CC2(CCCCC2)OO1 Show InChI InChI=1S/C19H24F3NO3/c1-17(13-18(26-25-17)8-3-2-4-9-18)11-16(24)23-12-14-6-5-7-15(10-14)19(20,21)22/h5-7,10H,2-4,8-9,11-13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301842
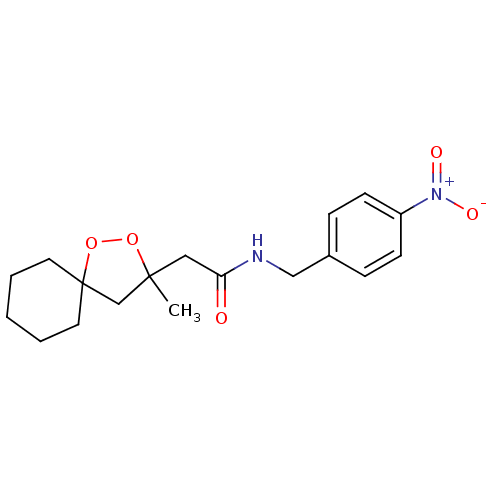
(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(4-ni...)Show SMILES CC1(CC(=O)NCc2ccc(cc2)[N+]([O-])=O)CC2(CCCCC2)OO1 Show InChI InChI=1S/C18H24N2O5/c1-17(13-18(25-24-17)9-3-2-4-10-18)11-16(21)19-12-14-5-7-15(8-6-14)20(22)23/h5-8H,2-4,9-13H2,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50301830

(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(3-(t...)Show SMILES CC1(CC(=O)NCc2cccc(c2)C(F)(F)F)CC2(CCCCC2)OO1 Show InChI InChI=1S/C19H24F3NO3/c1-17(13-18(26-25-17)8-3-2-4-9-18)11-16(24)23-12-14-6-5-7-15(10-14)19(20,21)22/h5-7,10H,2-4,8-9,11-13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data