 Found 388 hits with Last Name = 'rashid' and Initial = 'u'
Found 388 hits with Last Name = 'rashid' and Initial = 'u' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50398388
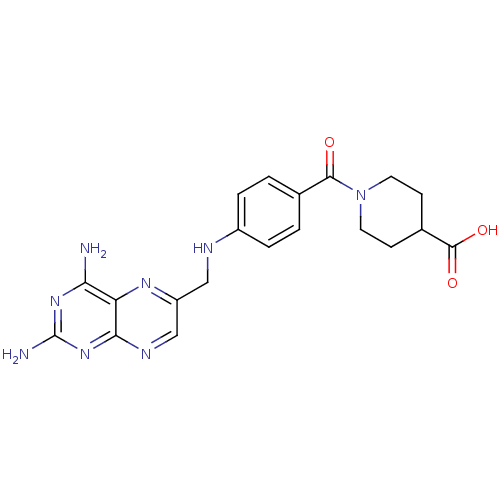
(CHEMBL2178604)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H22N8O3/c21-16-15-17(27-20(22)26-16)24-10-14(25-15)9-23-13-3-1-11(2-4-13)18(29)28-7-5-12(6-8-28)19(30)31/h1-4,10,12,23H,5-9H2,(H,30,31)(H4,21,22,24,26,27) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398388

(CHEMBL2178604)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H22N8O3/c21-16-15-17(27-20(22)26-16)24-10-14(25-15)9-23-13-3-1-11(2-4-13)18(29)28-7-5-12(6-8-28)19(30)31/h1-4,10,12,23H,5-9H2,(H,30,31)(H4,21,22,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562452

(CHEMBL4776197)Show SMILES Nc1cc(cc2nc(c(Nc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)nc12)-c1ccccc1)C(F)(F)F | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562453
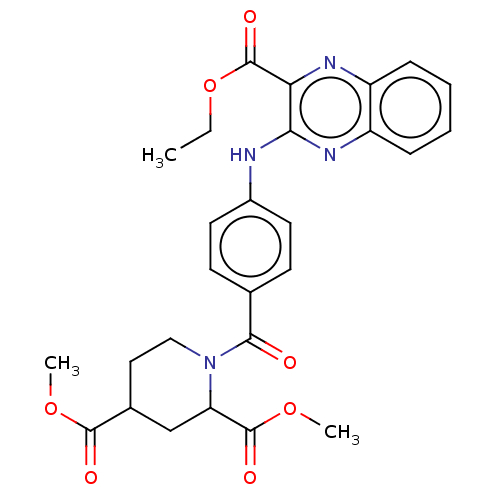
(CHEMBL4783402)Show SMILES CCOC(=O)c1nc2ccccc2nc1Nc1ccc(cc1)C(=O)N1CCC(CC1C(=O)OC)C(=O)OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562451
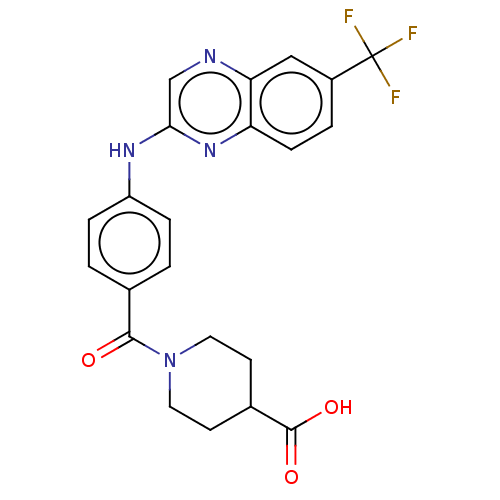
(CHEMBL4763597)Show SMILES OC(=O)C1CCN(CC1)C(=O)c1ccc(Nc2cnc3cc(ccc3n2)C(F)(F)F)cc1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562452

(CHEMBL4776197)Show SMILES Nc1cc(cc2nc(c(Nc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)nc12)-c1ccccc1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562451

(CHEMBL4763597)Show SMILES OC(=O)C1CCN(CC1)C(=O)c1ccc(Nc2cnc3cc(ccc3n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562453

(CHEMBL4783402)Show SMILES CCOC(=O)c1nc2ccccc2nc1Nc1ccc(cc1)C(=O)N1CCC(CC1C(=O)OC)C(=O)OC | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593572
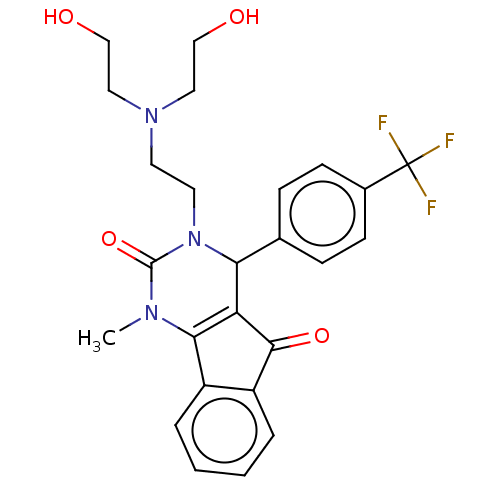
(CHEMBL5198631)Show SMILES CN1C2=C(C(N(CCN(CCO)CCO)C1=O)c1ccc(cc1)C(F)(F)F)C(=O)c1ccccc21 |c:2| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128668
BindingDB Entry DOI: 10.7270/Q2W95F6R |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128668
BindingDB Entry DOI: 10.7270/Q2W95F6R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593577
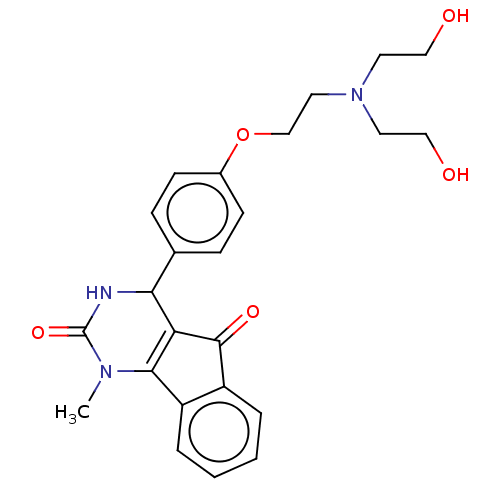
(CHEMBL5176445)Show SMILES CN1C2=C(C(NC1=O)c1ccc(OCCN(CCO)CCO)cc1)C(=O)c1ccccc21 |c:2| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128668
BindingDB Entry DOI: 10.7270/Q2W95F6R |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50606999
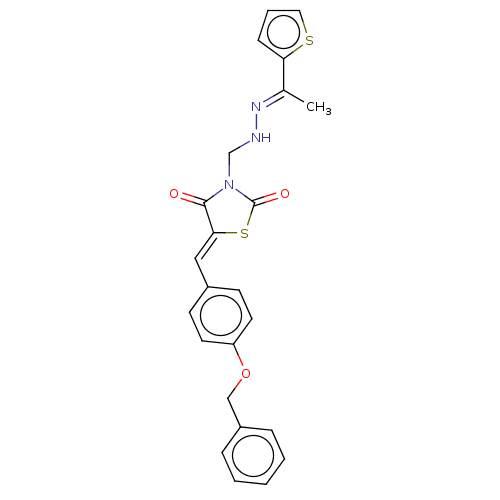
(CHEMBL5219078)Show SMILES C\C(=N/NCN1C(=O)S\C(=C/c2ccc(OCc3ccccc3)cc2)C1=O)c1cccs1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50606995
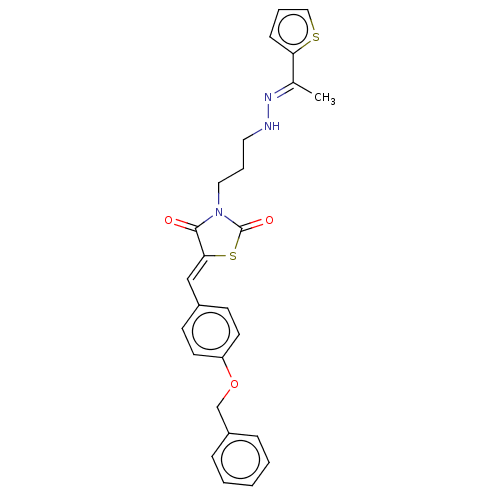
(CHEMBL5220624)Show SMILES C\C(=N/NCCCN1C(=O)S\C(=C/c2ccc(OCc3ccccc3)cc2)C1=O)c1cccs1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562445

(CHEMBL4748139)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50606998
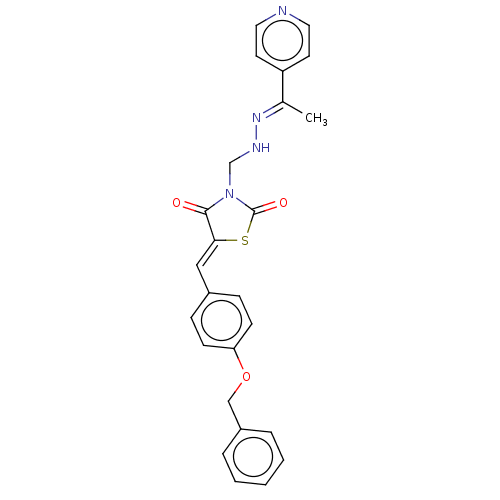
(CHEMBL5219886)Show SMILES C\C(=N/NCN1C(=O)S\C(=C/c2ccc(OCc3ccccc3)cc2)C1=O)c1ccncc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50274904
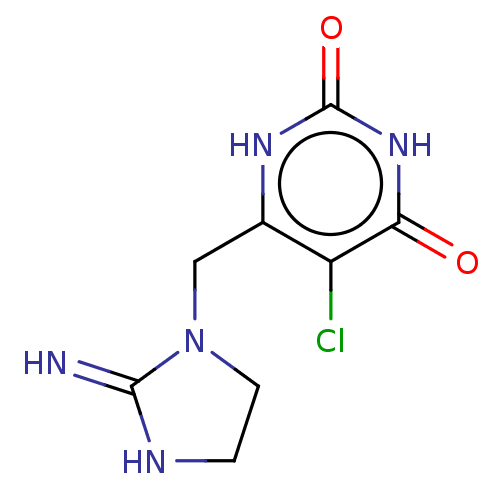
(CHEMBL4129617)Show InChI InChI=1S/C8H10ClN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-3H2,(H2,10,11)(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Imam Abdulrahman Bin Faisal University
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) |
Bioorg Med Chem 26: 3654-3663 (2018)
Article DOI: 10.1016/j.bmc.2018.05.046
BindingDB Entry DOI: 10.7270/Q22V2JKV |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593569
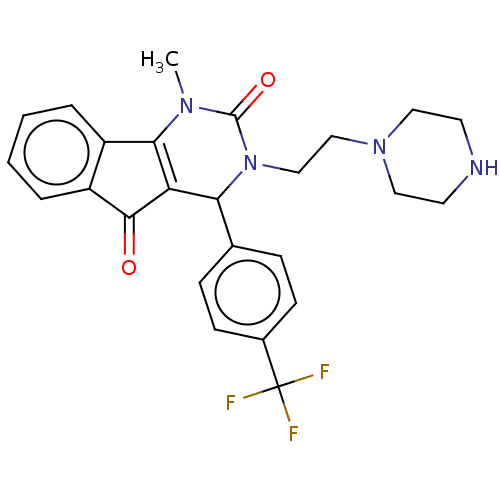
(CHEMBL5199635)Show SMILES CN1C2=C(C(N(CCN3CCNCC3)C1=O)c1ccc(cc1)C(F)(F)F)C(=O)c1ccccc21 |c:2| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128668
BindingDB Entry DOI: 10.7270/Q2W95F6R |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562454

(CHEMBL533684 | TCMDC-141974) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Hazara University
| Assay Description
The synthesized compounds (each separately) were dissolved in a mixture of DMSO (1 ml) and methanol (9 ml) and then diluted in KH2PO4/K2HPO4 buffer (... |
Bioorg Chem 69: 91-101 (2016)
Article DOI: 10.1016/j.bioorg.2016.10.002
BindingDB Entry DOI: 10.7270/Q2X34W8W |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562450
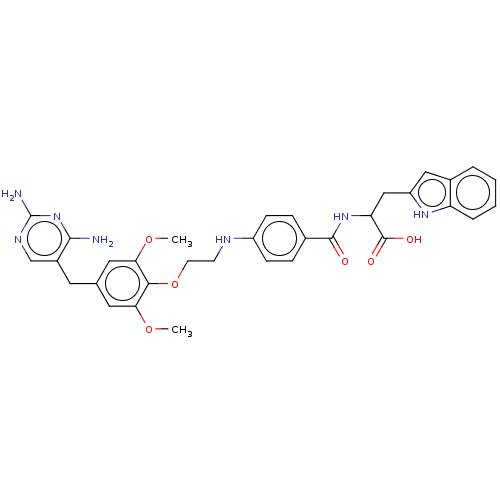
(CHEMBL4757974)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(Cc1cc2ccccc2[nH]1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM19187
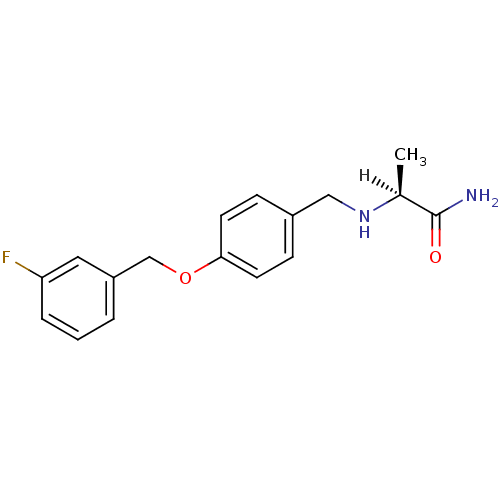
((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...)Show SMILES [H][C@@](C)(NCc1ccc(OCc2cccc(F)c2)cc1)C(N)=O |r| Show InChI InChI=1S/C17H19FN2O2/c1-12(17(19)21)20-10-13-5-7-16(8-6-13)22-11-14-3-2-4-15(18)9-14/h2-9,12,20H,10-11H2,1H3,(H2,19,21)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.0 | 30 |
University of Sargodha
| Assay Description
AChE and BChE inhibitory assay was carried out by following Ellman's methodology [Ellman et al., Biochem. Pharmacol., 7:88-95] using AChE (Electric e... |
Bioorg Chem 72: 256-267 (2017)
BindingDB Entry DOI: 10.7270/Q23B5Z1X |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079
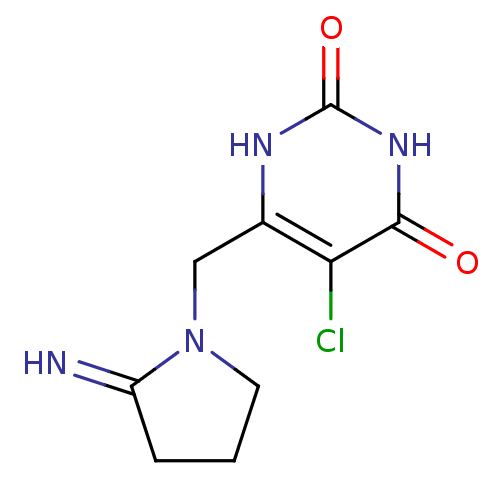
(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Imam Abdulrahman Bin Faisal University
Curated by ChEMBL
| Assay Description
Inhibition of human placenta thymidine phosphorylase using [6-3H]dThd as substrate after 5 mins by scintillation counting method |
Bioorg Med Chem 26: 3654-3663 (2018)
Article DOI: 10.1016/j.bmc.2018.05.046
BindingDB Entry DOI: 10.7270/Q22V2JKV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562443

(CHEMBL4759800)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(C(C)C)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50606996
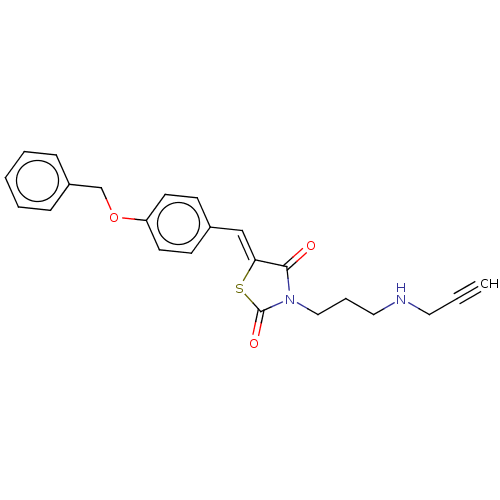
(CHEMBL5219890)Show SMILES O=C1S\C(=C/c2ccc(OCc3ccccc3)cc2)C(=O)N1CCCNCC#C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128668
BindingDB Entry DOI: 10.7270/Q2W95F6R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50606994
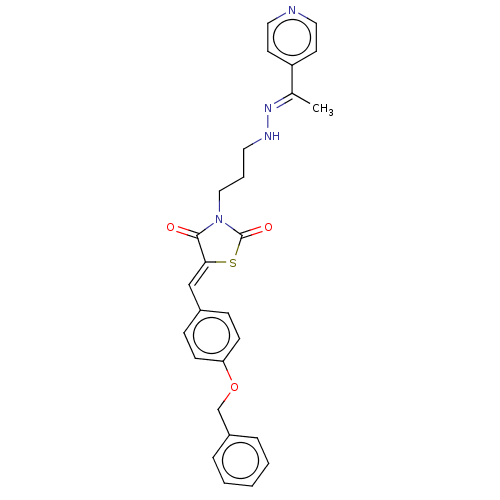
(CHEMBL5220446)Show SMILES C\C(=N/NCCCN1C(=O)S\C(=C/c2ccc(OCc3ccccc3)cc2)C1=O)c1ccncc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf21 cells using arachidonic acid as substrate preincubated for 5 mins followe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111863
BindingDB Entry DOI: 10.7270/Q27S7SFQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593570
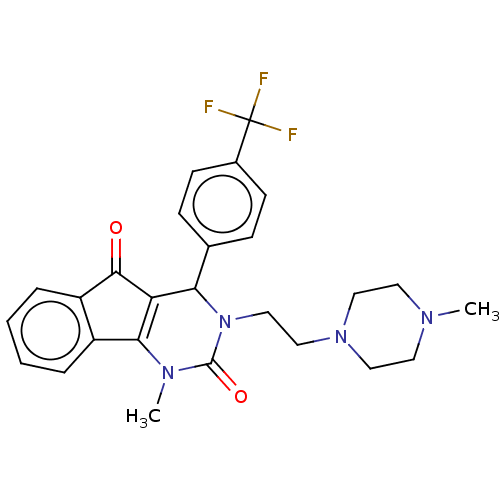
(CHEMBL5175759)Show SMILES CN1CCN(CCN2C(C3=C(N(C)C2=O)c2ccccc2C3=O)c2ccc(cc2)C(F)(F)F)CC1 |c:9| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128668
BindingDB Entry DOI: 10.7270/Q2W95F6R |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50169955
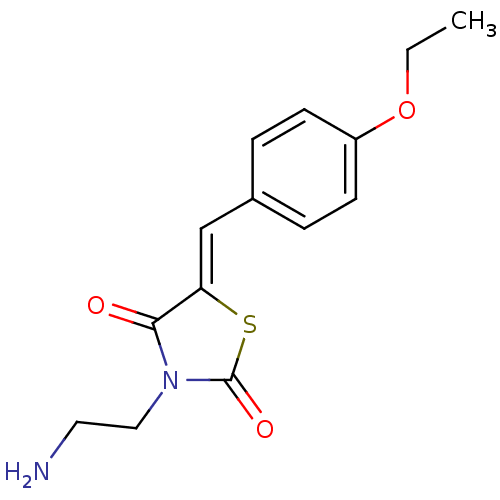
(3-(2-Amino-ethyl)-5-[1-(4-ethoxy-phenyl)-meth-(Z)-...)Show InChI InChI=1S/C14H16N2O3S/c1-2-19-11-5-3-10(4-6-11)9-12-13(17)16(8-7-15)14(18)20-12/h3-6,9H,2,7-8,15H2,1H3/b12-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562444

(CHEMBL4783671)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(Cc1ccccc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50606997

(CHEMBL5219309)Show SMILES [O-][N+](=O)c1ccc(\C=C2\SC(=O)N(CCCNCC#C)C2=O)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562449
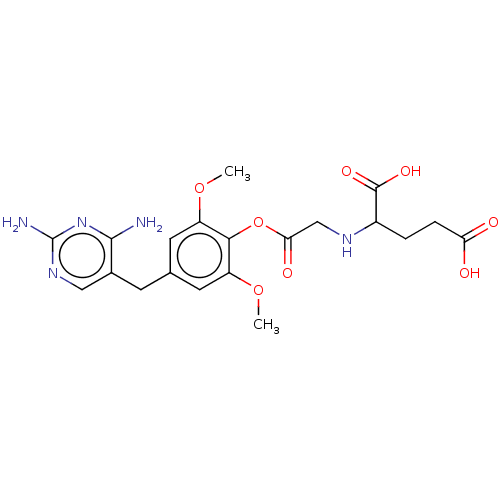
(CHEMBL4745475)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(CCC(O)=O)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50607001
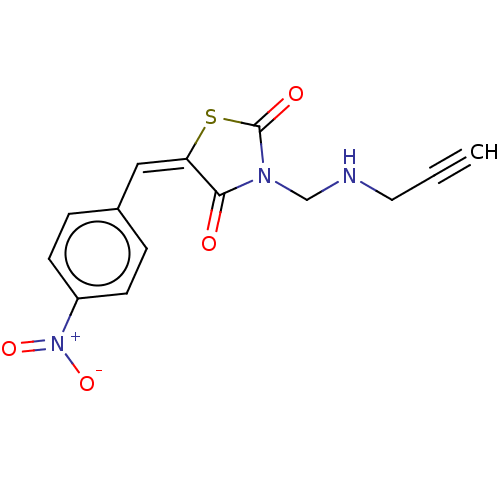
(CHEMBL5219355)Show SMILES [O-][N+](=O)c1ccc(\C=C2\SC(=O)N(CNCC#C)C2=O)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50607000
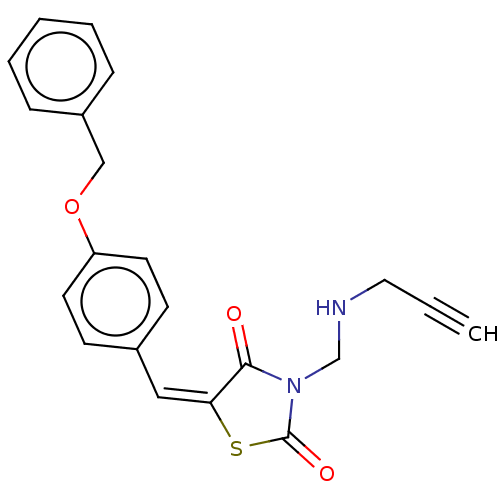
(CHEMBL5219373)Show SMILES O=C1S\C(=C\c2ccc(OCc3ccccc3)cc2)C(=O)N1CNCC#C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562446
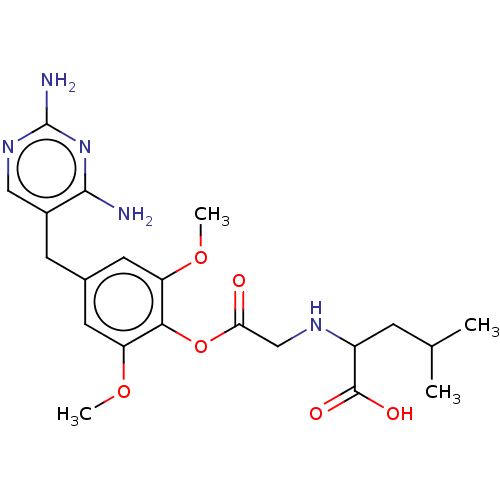
(CHEMBL4752301)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(CC(C)C)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50607003
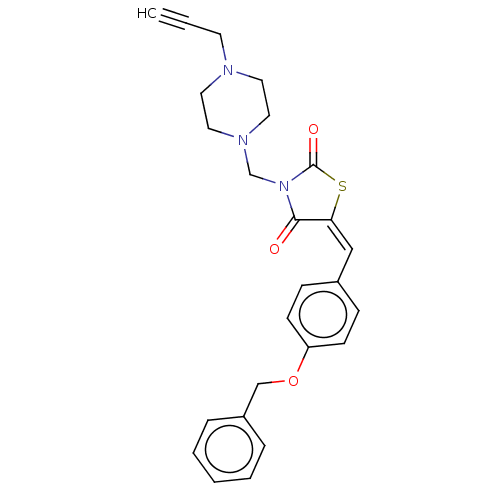
(CHEMBL5220045)Show SMILES O=C1S\C(=C\c2ccc(OCc3ccccc3)cc2)C(=O)N1CN1CCN(CC#C)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562448

(CHEMBL4779765)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(Cc1ccccc1)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM198155

(2-(5-Acetyl-6-methyl-4-phenyl-1,4-dihydropyrimidin...)Show SMILES CC(=O)C1=C(C)NC(SCC(=O)Nc2ccccc2)=NC1c1ccccc1 |c:3,19| Show InChI InChI=1S/C21H21N3O2S/c1-14-19(15(2)25)20(16-9-5-3-6-10-16)24-21(22-14)27-13-18(26)23-17-11-7-4-8-12-17/h3-12,20H,13H2,1-2H3,(H,22,24)(H,23,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Hazara University
| Assay Description
The synthesized compounds (each separately) were dissolved in a mixture of DMSO (1 ml) and methanol (9 ml) and then diluted in KH2PO4/K2HPO4 buffer (... |
Bioorg Chem 69: 91-101 (2016)
Article DOI: 10.1016/j.bioorg.2016.10.002
BindingDB Entry DOI: 10.7270/Q2X34W8W |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562447
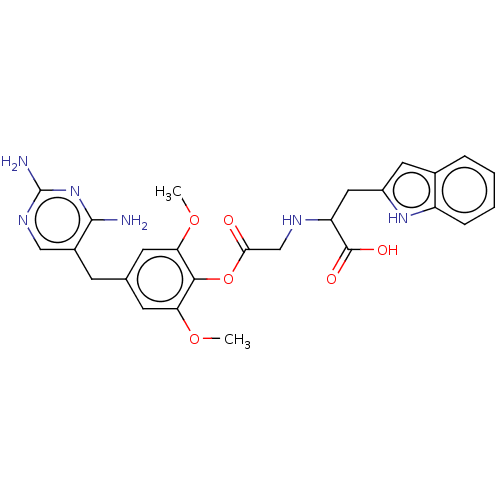
(CHEMBL4761589)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(Cc1cc2ccccc2[nH]1)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562442
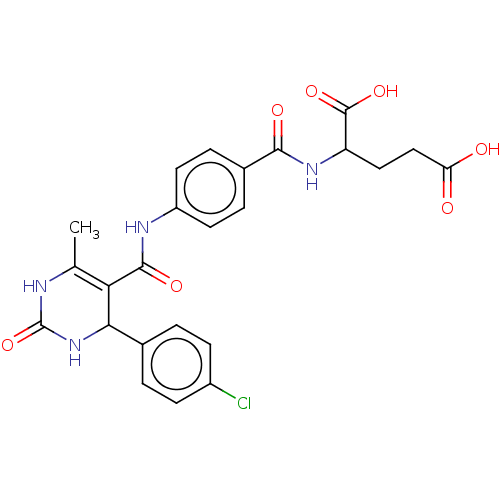
(CHEMBL4748158)Show SMILES CC1=C(C(NC(=O)N1)c1ccc(Cl)cc1)C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50324737

(3-(2-aminoethyl)-5-(4-(dimethylamino)benzylidene)t...)Show InChI InChI=1S/C14H17N3O2S/c1-16(2)11-5-3-10(4-6-11)9-12-13(18)17(8-7-15)14(19)20-12/h3-6,9H,7-8,15H2,1-2H3/b12-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562445

(CHEMBL4748139)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50103636
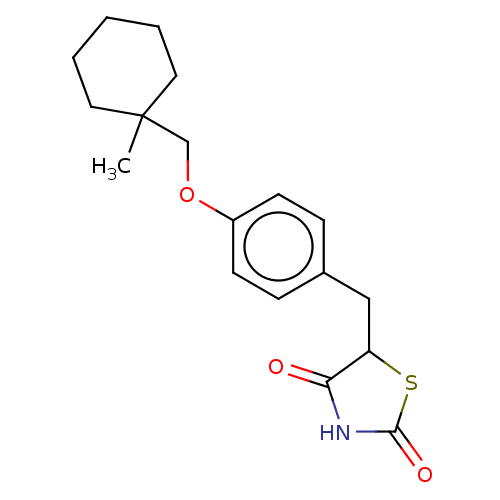
(ADD-3878 | CHEBI:64227 | Ciglitazone | U-63287)Show InChI InChI=1S/C18H23NO3S/c1-18(9-3-2-4-10-18)12-22-14-7-5-13(6-8-14)11-15-16(20)19-17(21)23-15/h5-8,15H,2-4,9-12H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50607002
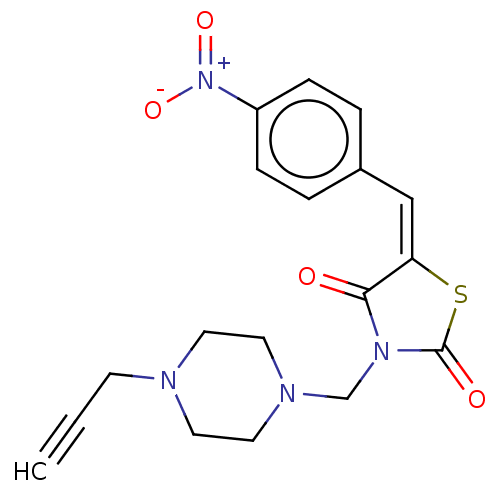
(CHEMBL5219976)Show SMILES [O-][N+](=O)c1ccc(\C=C2\SC(=O)N(CN3CCN(CC#C)CC3)C2=O)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128994
BindingDB Entry DOI: 10.7270/Q2N3021T |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of sheep COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 5 mins by UV-v... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111863
BindingDB Entry DOI: 10.7270/Q27S7SFQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data