 Found 634 hits with Last Name = 'cody' and Initial = 'v'
Found 634 hits with Last Name = 'cody' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040861
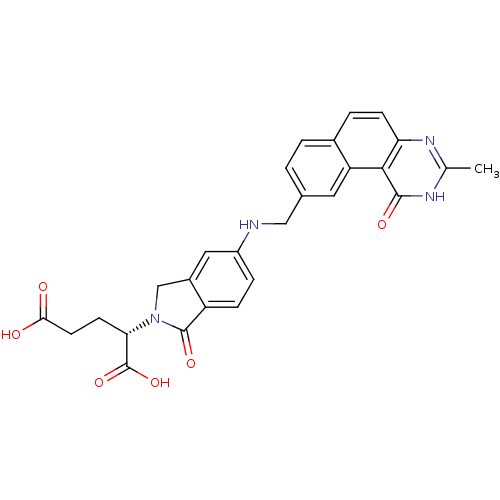
((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...)Show SMILES Cc1nc2ccc3ccc(CNc4ccc5C(=O)N(Cc5c4)[C@@H](CCC(O)=O)C(O)=O)cc3c2c(=O)[nH]1 Show InChI InChI=1S/C27H24N4O6/c1-14-29-21-7-4-16-3-2-15(10-20(16)24(21)25(34)30-14)12-28-18-5-6-19-17(11-18)13-31(26(19)35)22(27(36)37)8-9-23(32)33/h2-7,10-11,22,28H,8-9,12-13H2,1H3,(H,32,33)(H,36,37)(H,29,30,34)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of TS by spectrophotometry |
Bioorg Med Chem 19: 3585-94 (2011)
Article DOI: 10.1016/j.bmc.2011.03.067
BindingDB Entry DOI: 10.7270/Q22V2GGP |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM85093
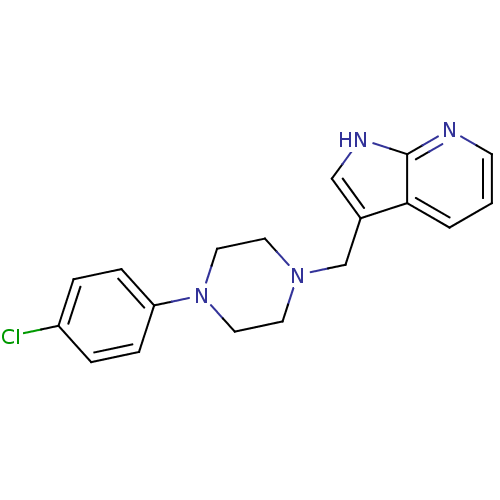
(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50155515
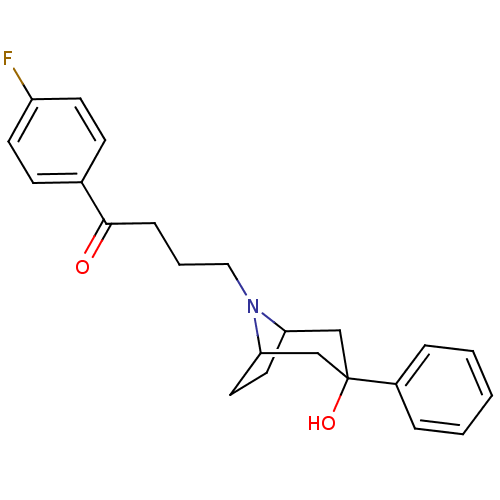
(1-(4-Fluoro-phenyl)-4-(3-hydroxy-3-phenyl-8-aza-bi...)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccccc1 |TLB:0:1:8:4.5,9:8:1.7.2:4.5| Show InChI InChI=1S/C23H26FNO2/c24-19-10-8-17(9-11-19)22(26)7-4-14-25-20-12-13-21(25)16-23(27,15-20)18-5-2-1-3-6-18/h1-3,5-6,8-11,20-21,27H,4,7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D2 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50028549
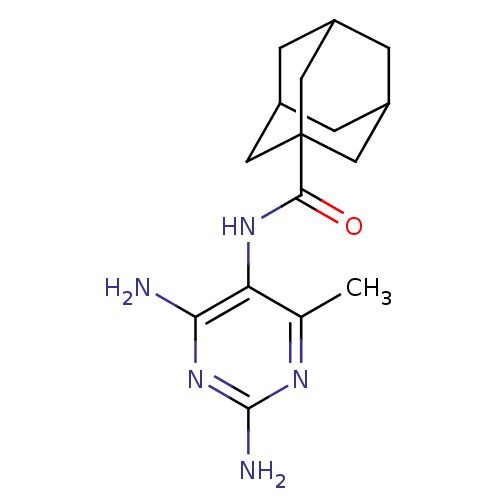
(Adamantane-1-carboxylic acid (2,4-diamino-6-methyl...)Show SMILES Cc1nc(N)nc(N)c1NC(=O)C12CC3CC(CC(C3)C1)C2 |TLB:15:16:20:13.14.19,19:14:21:20.18.17,19:18:21:13.14.15,THB:15:14:20:21.16.17| Show InChI InChI=1S/C16H23N5O/c1-8-12(13(17)21-15(18)19-8)20-14(22)16-5-9-2-10(6-16)4-11(3-9)7-16/h9-11H,2-7H2,1H3,(H,20,22)(H4,17,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of mammalian dihydrofolate reductase |
J Med Chem 25: 427-30 (1982)
BindingDB Entry DOI: 10.7270/Q20P10KH |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50155520
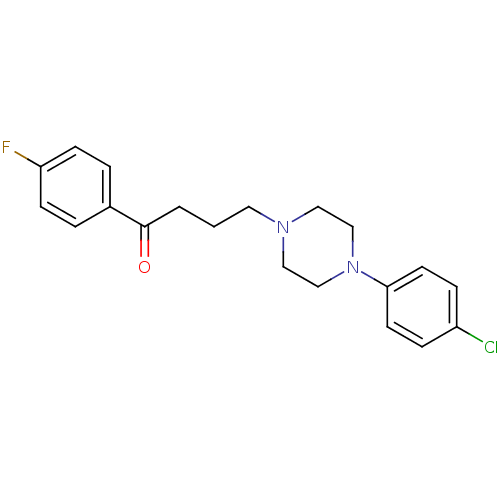
(4-[4-(4-Chloro-phenyl)-piperazin-1-yl]-1-(4-fluoro...)Show InChI InChI=1S/C20H22ClFN2O/c21-17-5-9-19(10-6-17)24-14-12-23(13-15-24)11-1-2-20(25)16-3-7-18(22)8-4-16/h3-10H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50155515

(1-(4-Fluoro-phenyl)-4-(3-hydroxy-3-phenyl-8-aza-bi...)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccccc1 |TLB:0:1:8:4.5,9:8:1.7.2:4.5| Show InChI InChI=1S/C23H26FNO2/c24-19-10-8-17(9-11-19)22(26)7-4-14-25-20-12-13-21(25)16-23(27,15-20)18-5-2-1-3-6-18/h1-3,5-6,8-11,20-21,27H,4,7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50061255

(1-(4-Fluoro-phenyl)-4-(3-phenyl-8-aza-bicyclo[3.2....)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1CC(C2)c1ccccc1 |TLB:11:12:18.19.17:14.15| Show InChI InChI=1S/C23H26FNO/c24-20-10-8-18(9-11-20)23(26)7-4-14-25-21-12-13-22(25)16-19(15-21)17-5-2-1-3-6-17/h1-3,5-6,8-11,19,21-22H,4,7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D2 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50061255

(1-(4-Fluoro-phenyl)-4-(3-phenyl-8-aza-bicyclo[3.2....)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1CC(C2)c1ccccc1 |TLB:11:12:18.19.17:14.15| Show InChI InChI=1S/C23H26FNO/c24-20-10-8-18(9-11-20)23(26)7-4-14-25-21-12-13-22(25)16-19(15-21)17-5-2-1-3-6-17/h1-3,5-6,8-11,19,21-22H,4,7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50155523
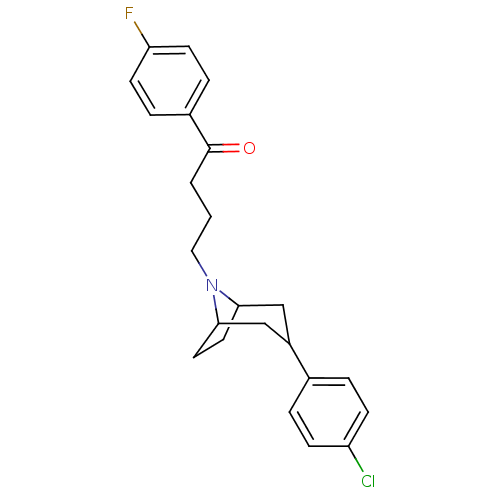
(4-[3-(4-Chloro-phenyl)-8-aza-bicyclo[3.2.1]oct-8-y...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1CC(C2)c1ccc(Cl)cc1 |TLB:11:12:18.19.17:14.15| Show InChI InChI=1S/C23H25ClFNO/c24-19-7-3-16(4-8-19)18-14-21-11-12-22(15-18)26(21)13-1-2-23(27)17-5-9-20(25)10-6-17/h3-10,18,21-22H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D2 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50155520

(4-[4-(4-Chloro-phenyl)-piperazin-1-yl]-1-(4-fluoro...)Show InChI InChI=1S/C20H22ClFN2O/c21-17-5-9-19(10-6-17)24-14-12-23(13-15-24)11-1-2-20(25)16-3-7-18(22)8-4-16/h3-10H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D2 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50155523

(4-[3-(4-Chloro-phenyl)-8-aza-bicyclo[3.2.1]oct-8-y...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1CC(C2)c1ccc(Cl)cc1 |TLB:11:12:18.19.17:14.15| Show InChI InChI=1S/C23H25ClFNO/c24-19-7-3-16(4-8-19)18-14-21-11-12-22(15-18)26(21)13-1-2-23(27)17-5-9-20(25)10-6-17/h3-10,18,21-22H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50155517
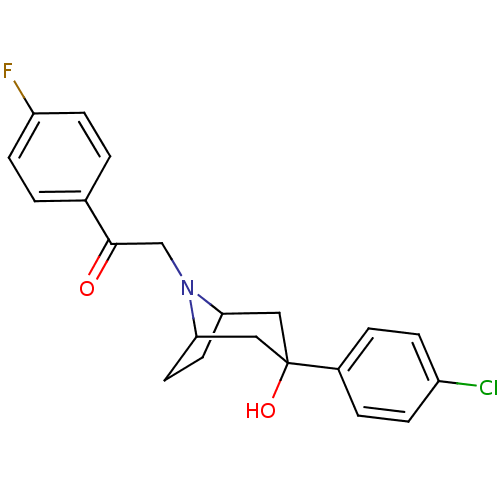
(2-[3-(4-Chloro-phenyl)-3-hydroxy-8-aza-bicyclo[3.2...)Show SMILES OC1(CC2CCC(C1)N2CC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:0:1:8:4.5,9:8:1.7.2:4.5| Show InChI InChI=1S/C21H21ClFNO2/c22-16-5-3-15(4-6-16)21(26)11-18-9-10-19(12-21)24(18)13-20(25)14-1-7-17(23)8-2-14/h1-8,18-19,26H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 789 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM85093

(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D2 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50155516
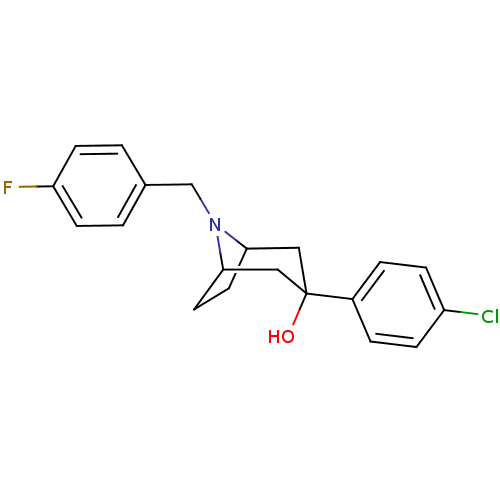
(3-(4-Chloro-phenyl)-8-(4-fluoro-benzyl)-8-aza-bicy...)Show SMILES OC1(CC2CCC(C1)N2Cc1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.7.2:4.5,0:1:8:4.5| Show InChI InChI=1S/C20H21ClFNO/c21-16-5-3-15(4-6-16)20(24)11-18-9-10-19(12-20)23(18)13-14-1-7-17(22)8-2-14/h1-8,18-19,24H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50155516

(3-(4-Chloro-phenyl)-8-(4-fluoro-benzyl)-8-aza-bicy...)Show SMILES OC1(CC2CCC(C1)N2Cc1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.7.2:4.5,0:1:8:4.5| Show InChI InChI=1S/C20H21ClFNO/c21-16-5-3-15(4-6-16)20(24)11-18-9-10-19(12-20)23(18)13-14-1-7-17(22)8-2-14/h1-8,18-19,24H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D2 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50155517

(2-[3-(4-Chloro-phenyl)-3-hydroxy-8-aza-bicyclo[3.2...)Show SMILES OC1(CC2CCC(C1)N2CC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:0:1:8:4.5,9:8:1.7.2:4.5| Show InChI InChI=1S/C21H21ClFNO2/c22-16-5-3-15(4-6-16)21(26)11-18-9-10-19(12-21)24(18)13-20(25)14-1-7-17(23)8-2-14/h1-8,18-19,26H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D2 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50028550
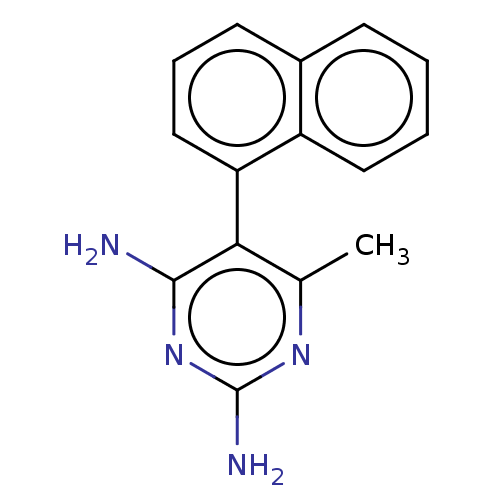
(6-Methyl-5-naphthalen-1-yl-pyrimidine-2,4-diamine;...)Show SMILES Cc1nc(N)nc(N)c1-c1cccc2ccccc12 |(6.16,-5.11,;4.82,-4.34,;3.47,-5.11,;2.16,-4.34,;.81,-5.11,;2.16,-2.8,;3.47,-2.03,;3.47,-.49,;4.82,-2.8,;6.13,-2,;7.16,-3.15,;8.66,-2.83,;9.14,-1.39,;8.12,-.24,;8.6,1.24,;7.61,2.36,;6.1,2.04,;5.59,.6,;6.61,-.56,)| Show InChI InChI=1S/C15H14N4/c1-9-13(14(16)19-15(17)18-9)12-8-4-6-10-5-2-3-7-11(10)12/h2-8H,1H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of mammalian Dihydrofolate reductase |
J Med Chem 25: 427-30 (1982)
BindingDB Entry DOI: 10.7270/Q20P10KH |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHFR |
J Med Chem 56: 4422-41 (2013)
Article DOI: 10.1021/jm400086g
BindingDB Entry DOI: 10.7270/Q2F47QH3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and NADPH |
Bioorg Med Chem 26: 2640-2650 (2018)
Article DOI: 10.1016/j.bmc.2018.04.032
BindingDB Entry DOI: 10.7270/Q2QJ7KXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in consumption of NADPH using 9 uM DHFA as substrate |
Bioorg Med Chem Lett 29: 1874-1880 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.004
BindingDB Entry DOI: 10.7270/Q2P84GBC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM31777
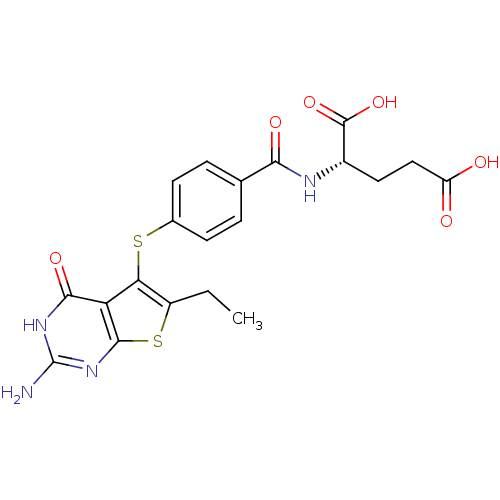
(thieno[2,3-d]pyrimidine deriv., 2)Show SMILES CCc1sc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H20N4O6S2/c1-2-12-15(14-17(28)23-20(21)24-18(14)32-12)31-10-5-3-9(4-6-10)16(27)22-11(19(29)30)7-8-13(25)26/h3-6,11H,2,7-8H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H3,21,23,24,28)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
| Assay Description
DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... |
J Med Chem 52: 4892-902 (2009)
Article DOI: 10.1021/jm900490a
BindingDB Entry DOI: 10.7270/Q2CR5RPK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in consumption of NADPH using 18 uM DHFA as substrate |
Bioorg Med Chem Lett 29: 1874-1880 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.004
BindingDB Entry DOI: 10.7270/Q2P84GBC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHFR |
J Med Chem 56: 4422-41 (2013)
Article DOI: 10.1021/jm400086g
BindingDB Entry DOI: 10.7270/Q2F47QH3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50066495
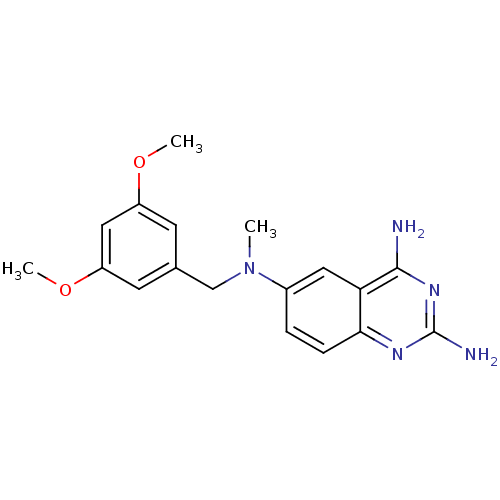
(CHEMBL113816 | N*6*-(3,5-Dimethoxy-benzyl)-N*6*-me...)Show InChI InChI=1S/C18H21N5O2/c1-23(10-11-6-13(24-2)9-14(7-11)25-3)12-4-5-16-15(8-12)17(19)22-18(20)21-16/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant Dihydrofolate reductase from Escherichia coli (ecDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Rat liver |
J Med Chem 42: 4853-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S46R5R |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase from rat liver |
J Med Chem 41: 1263-71 (1998)
Article DOI: 10.1021/jm970537w
BindingDB Entry DOI: 10.7270/Q2J965J6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase from rat liver (rlDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHFR |
J Med Chem 56: 4422-41 (2013)
Article DOI: 10.1021/jm400086g
BindingDB Entry DOI: 10.7270/Q2F47QH3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in consumption of NADPH using 18 uM DHFA as substrate |
Bioorg Med Chem Lett 29: 1874-1880 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.004
BindingDB Entry DOI: 10.7270/Q2P84GBC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50066490
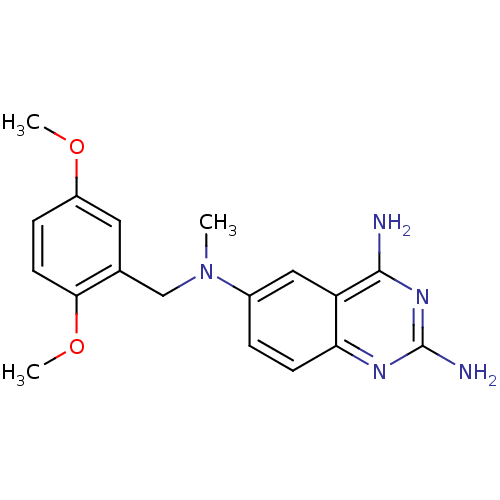
(2,4-DIAMINO-6-[N-(2',5'-DIMETHOXYBENZYL)-N-METHYLA...)Show InChI InChI=1S/C18H21N5O2/c1-23(10-11-8-13(24-2)5-7-16(11)25-3)12-4-6-15-14(9-12)17(19)22-18(20)21-15/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50066490

(2,4-DIAMINO-6-[N-(2',5'-DIMETHOXYBENZYL)-N-METHYLA...)Show InChI InChI=1S/C18H21N5O2/c1-23(10-11-8-13(24-2)5-7-16(11)25-3)12-4-6-15-14(9-12)17(19)22-18(20)21-15/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50066496
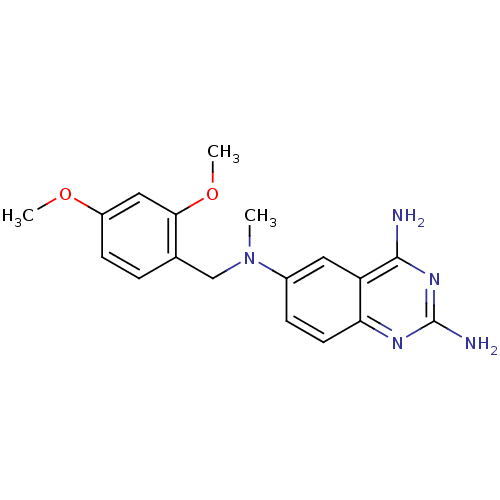
(CHEMBL115804 | N*6*-(2,4-Dimethoxy-benzyl)-N*6*-me...)Show InChI InChI=1S/C18H21N5O2/c1-23(10-11-4-6-13(24-2)9-16(11)25-3)12-5-7-15-14(8-12)17(19)22-18(20)21-15/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50066497
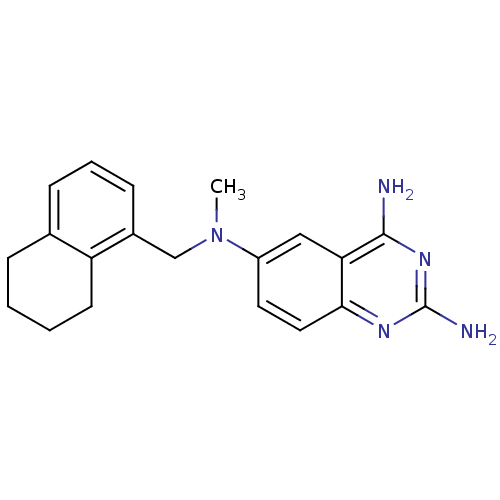
(CHEMBL114354 | N*6*-Methyl-N*6*-(5,6,7,8-tetrahydr...)Show InChI InChI=1S/C20H23N5/c1-25(12-14-7-4-6-13-5-2-3-8-16(13)14)15-9-10-18-17(11-15)19(21)24-20(22)23-18/h4,6-7,9-11H,2-3,5,8,12H2,1H3,(H4,21,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50433813
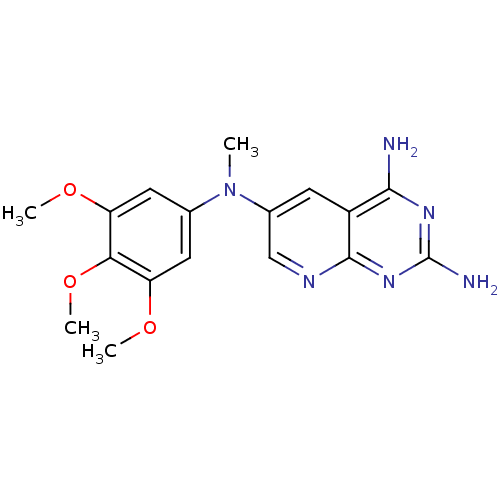
(CHEMBL2382330)Show InChI InChI=1S/C17H20N6O3/c1-23(9-6-12(24-2)14(26-4)13(7-9)25-3)10-5-11-15(18)21-17(19)22-16(11)20-8-10/h5-8H,1-4H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHFR |
J Med Chem 56: 4422-41 (2013)
Article DOI: 10.1021/jm400086g
BindingDB Entry DOI: 10.7270/Q2F47QH3 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50066495

(CHEMBL113816 | N*6*-(3,5-Dimethoxy-benzyl)-N*6*-me...)Show InChI InChI=1S/C18H21N5O2/c1-23(10-11-6-13(24-2)9-14(7-11)25-3)12-4-5-16-15(8-12)17(19)22-18(20)21-16/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50066495

(CHEMBL113816 | N*6*-(3,5-Dimethoxy-benzyl)-N*6*-me...)Show InChI InChI=1S/C18H21N5O2/c1-23(10-11-6-13(24-2)9-14(7-11)25-3)12-4-5-16-15(8-12)17(19)22-18(20)21-16/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50433814
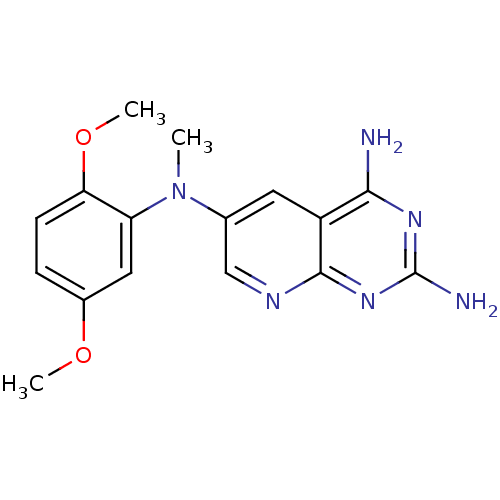
(CHEMBL2382329)Show InChI InChI=1S/C16H18N6O2/c1-22(12-7-10(23-2)4-5-13(12)24-3)9-6-11-14(17)20-16(18)21-15(11)19-8-9/h4-8H,1-3H3,(H4,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHFR |
J Med Chem 56: 4422-41 (2013)
Article DOI: 10.1021/jm400086g
BindingDB Entry DOI: 10.7270/Q2F47QH3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50066497

(CHEMBL114354 | N*6*-Methyl-N*6*-(5,6,7,8-tetrahydr...)Show InChI InChI=1S/C20H23N5/c1-25(12-14-7-4-6-13-5-2-3-8-16(13)14)15-9-10-18-17(11-15)19(21)24-20(22)23-18/h4,6-7,9-11H,2-3,5,8,12H2,1H3,(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant Dihydrofolate reductase from Escherichia coli (ecDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50066485

(CHEMBL117750 | N*6*-Methyl-N*6*-(2,3,4-trimethoxy-...)Show SMILES COc1ccc(CN(C)c2ccc3nc(N)nc(N)c3c2)c(OC)c1OC Show InChI InChI=1S/C19H23N5O3/c1-24(10-11-5-8-15(25-2)17(27-4)16(11)26-3)12-6-7-14-13(9-12)18(20)23-19(21)22-14/h5-9H,10H2,1-4H3,(H4,20,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50066485

(CHEMBL117750 | N*6*-Methyl-N*6*-(2,3,4-trimethoxy-...)Show SMILES COc1ccc(CN(C)c2ccc3nc(N)nc(N)c3c2)c(OC)c1OC Show InChI InChI=1S/C19H23N5O3/c1-24(10-11-5-8-15(25-2)17(27-4)16(11)26-3)12-6-7-14-13(9-12)18(20)23-19(21)22-14/h5-9H,10H2,1-4H3,(H4,20,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50433809
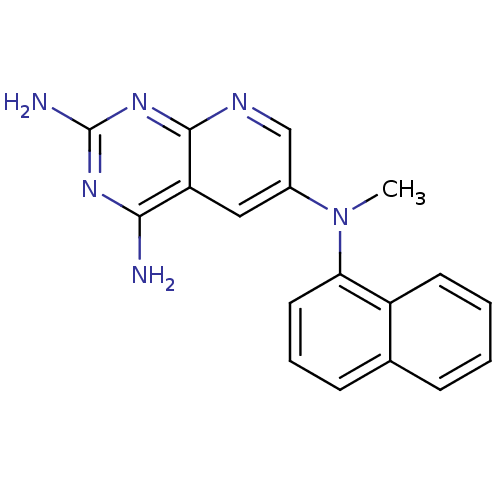
(CHEMBL2382334)Show InChI InChI=1S/C18H16N6/c1-24(15-8-4-6-11-5-2-3-7-13(11)15)12-9-14-16(19)22-18(20)23-17(14)21-10-12/h2-10H,1H3,(H4,19,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHFR |
J Med Chem 56: 4422-41 (2013)
Article DOI: 10.1021/jm400086g
BindingDB Entry DOI: 10.7270/Q2F47QH3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against isolated Escherichia coli Dihydrofolate reductase |
J Med Chem 43: 3837-51 (2000)
BindingDB Entry DOI: 10.7270/Q2S1835M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant Dihydrofolate reductase from Toxoplasma gondii (tgDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50066490

(2,4-DIAMINO-6-[N-(2',5'-DIMETHOXYBENZYL)-N-METHYLA...)Show InChI InChI=1S/C18H21N5O2/c1-23(10-11-8-13(24-2)5-7-16(11)25-3)12-4-6-15-14(9-12)17(19)22-18(20)21-15/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant Dihydrofolate reductase from Escherichia coli (ecDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM31776
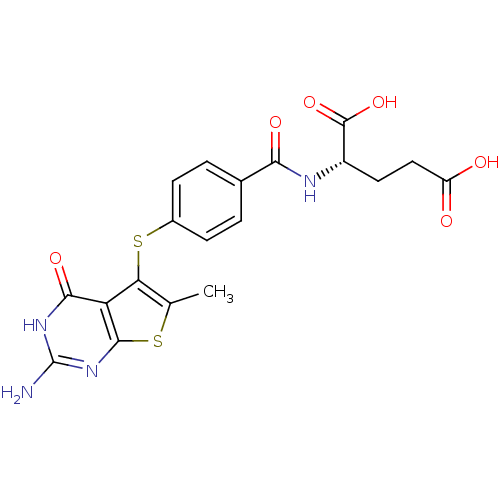
(thieno[2,3-d]pyrimidine deriv., 1)Show SMILES Cc1sc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H18N4O6S2/c1-8-14(13-16(27)22-19(20)23-17(13)30-8)31-10-4-2-9(3-5-10)15(26)21-11(18(28)29)6-7-12(24)25/h2-5,11H,6-7H2,1H3,(H,21,26)(H,24,25)(H,28,29)(H3,20,22,23,27)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
| Assay Description
DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... |
J Med Chem 52: 4892-902 (2009)
Article DOI: 10.1021/jm900490a
BindingDB Entry DOI: 10.7270/Q2CR5RPK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50066485

(CHEMBL117750 | N*6*-Methyl-N*6*-(2,3,4-trimethoxy-...)Show SMILES COc1ccc(CN(C)c2ccc3nc(N)nc(N)c3c2)c(OC)c1OC Show InChI InChI=1S/C19H23N5O3/c1-24(10-11-5-8-15(25-2)17(27-4)16(11)26-3)12-6-7-14-13(9-12)18(20)23-19(21)22-14/h5-9H,10H2,1-4H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant Dihydrofolate reductase from Escherichia coli (ecDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM31782
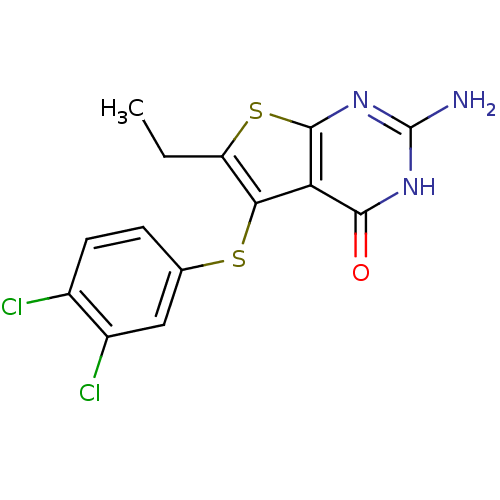
(thieno[2,3-d]pyrimidine deriv., 2e)Show InChI InChI=1S/C14H11Cl2N3OS2/c1-2-9-11(21-6-3-4-7(15)8(16)5-6)10-12(20)18-14(17)19-13(10)22-9/h3-5H,2H2,1H3,(H3,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
| Assay Description
DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... |
J Med Chem 52: 4892-902 (2009)
Article DOI: 10.1021/jm900490a
BindingDB Entry DOI: 10.7270/Q2CR5RPK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50066495

(CHEMBL113816 | N*6*-(3,5-Dimethoxy-benzyl)-N*6*-me...)Show InChI InChI=1S/C18H21N5O2/c1-23(10-11-6-13(24-2)9-14(7-11)25-3)12-4-5-16-15(8-12)17(19)22-18(20)21-16/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase from rat liver (rlDHFR) |
J Med Chem 41: 3426-34 (1998)
Article DOI: 10.1021/jm980081y
BindingDB Entry DOI: 10.7270/Q27H1HQZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM31784
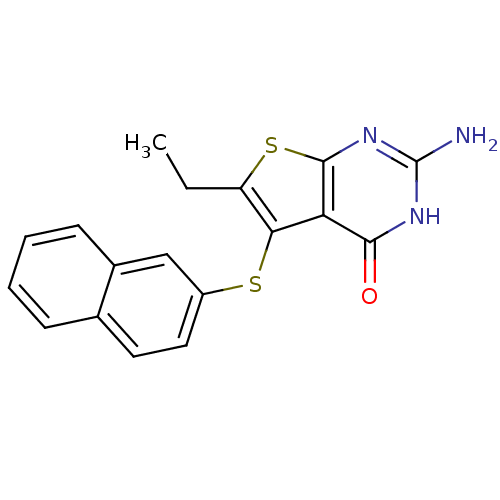
(thieno[2,3-d]pyrimidine deriv., 2g)Show InChI InChI=1S/C18H15N3OS2/c1-2-13-15(14-16(22)20-18(19)21-17(14)24-13)23-12-8-7-10-5-3-4-6-11(10)9-12/h3-9H,2H2,1H3,(H3,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
| Assay Description
DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... |
J Med Chem 52: 4892-902 (2009)
Article DOI: 10.1021/jm900490a
BindingDB Entry DOI: 10.7270/Q2CR5RPK |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM31780
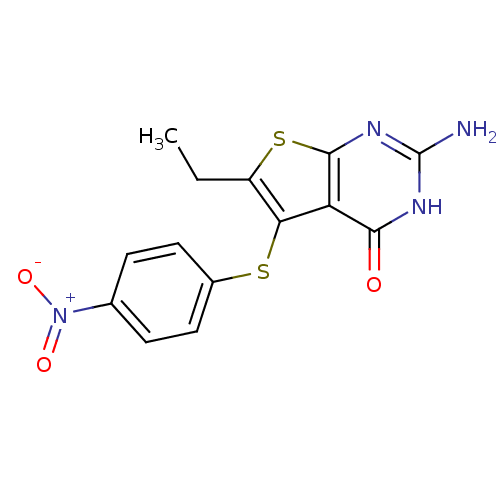
(thieno[2,3-d]pyrimidine deriv., 2c)Show SMILES CCc1sc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C14H12N4O3S2/c1-2-9-11(10-12(19)16-14(15)17-13(10)23-9)22-8-5-3-7(4-6-8)18(20)21/h3-6H,2H2,1H3,(H3,15,16,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
| Assay Description
DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... |
J Med Chem 52: 4892-902 (2009)
Article DOI: 10.1021/jm900490a
BindingDB Entry DOI: 10.7270/Q2CR5RPK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data