

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50426561 (CHEMBL2323965) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en... | US Patent US8975276 (2015) BindingDB Entry DOI: 10.7270/Q25M64F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50426559 (CHEMBL2323964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en... | US Patent US8975276 (2015) BindingDB Entry DOI: 10.7270/Q25M64F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50426560 (CHEMBL2323963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en... | US Patent US8975276 (2015) BindingDB Entry DOI: 10.7270/Q25M64F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM297163 (2-{8-Fluoro-3-[4-(2H3)methyl-1-methyl-1H-1,2,3-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00219 BindingDB Entry DOI: 10.7270/Q20R9TDX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300145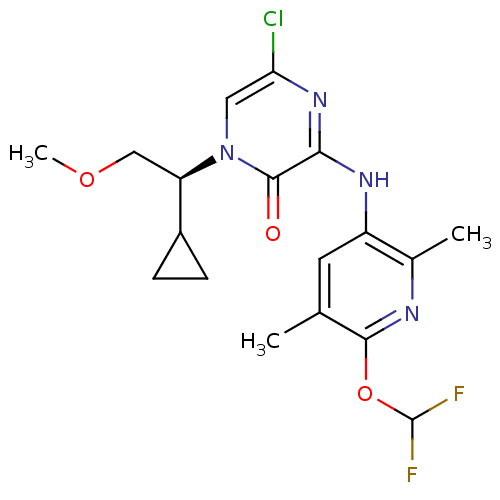 ((S)-5-Chloro-1-(cyclopropyl-2-methoxyethyl)-3-[6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | Bioorg Med Chem Lett 20: 1890-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.129 BindingDB Entry DOI: 10.7270/Q29G5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293912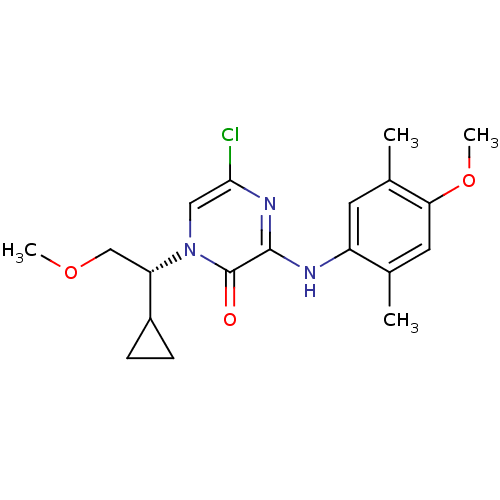 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293912 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293974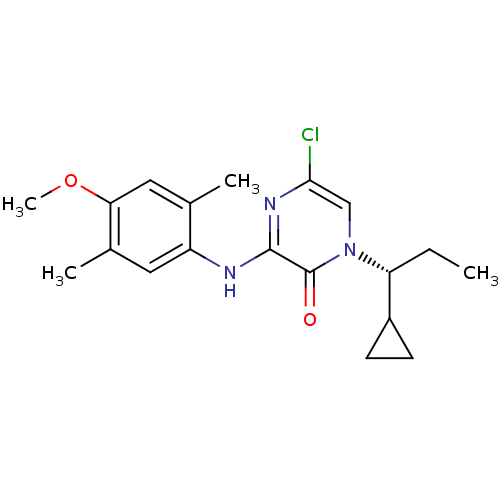 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(4-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293964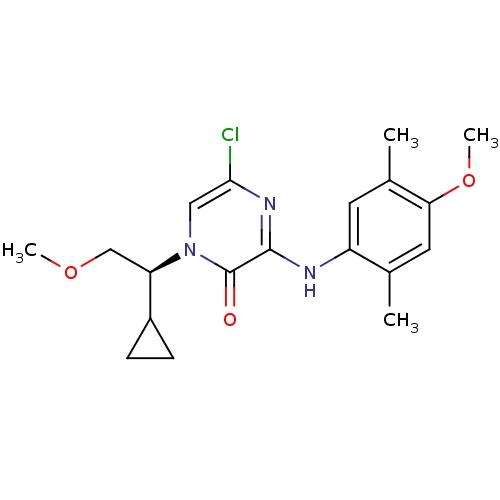 ((S)-5-Chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300142 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-[6-(difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | Bioorg Med Chem Lett 20: 1890-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.129 BindingDB Entry DOI: 10.7270/Q29G5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149699 (US8975276, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en... | US Patent US8975276 (2015) BindingDB Entry DOI: 10.7270/Q25M64F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293941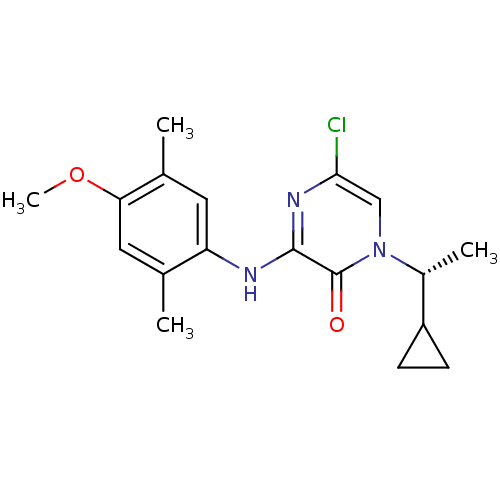 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(4-methoxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293941 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(4-methoxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293916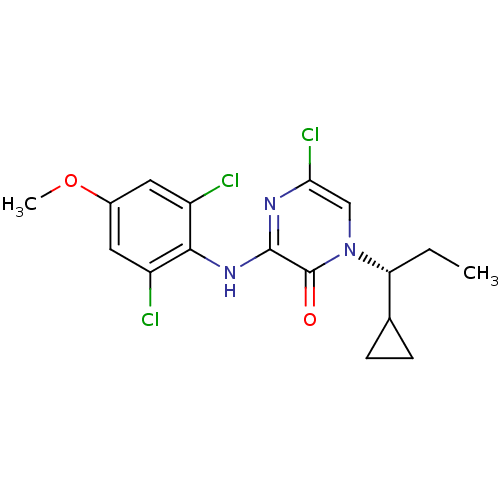 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,6-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293935 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-[2,6-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293973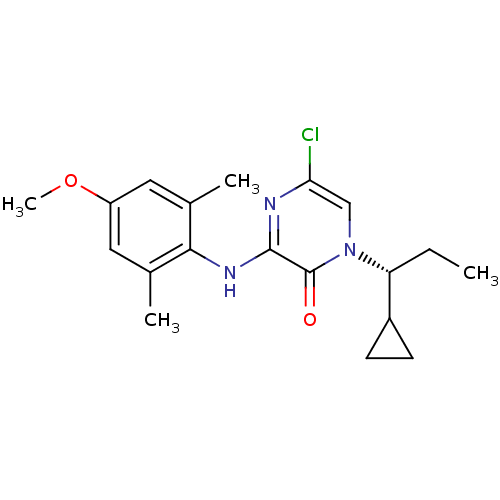 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(4-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293914 ((R)-5-chloro-1-(1-cyclopropylpropyl)-3-(2,4-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293914 ((R)-5-chloro-1-(1-cyclopropylpropyl)-3-(2,4-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing hormone receptor 2 (Sus scrofa) | BDBM50158983 (CHEMBL439883 | E G P P I S I D L S L E L L R K M I...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF2 receptor in pig choroid plexus by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293953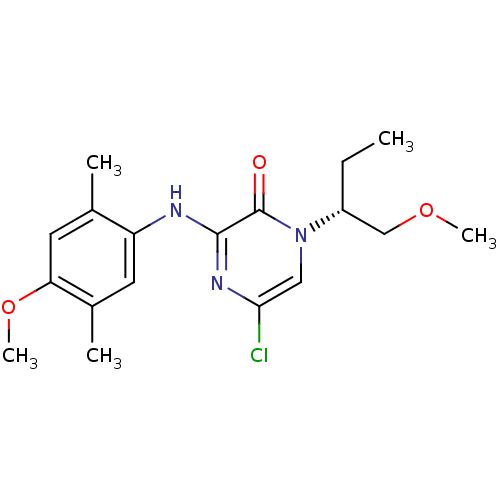 ((R)-5-Chloro-3-(4-methoxy-2,5-dimethylphenylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293952 (5-Chloro-3-(4-methoxy-2,5-dimethylphenylamino)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293981 ((R)-5-Chloro-3-(7-chloro-5-methoxyindolin-1-yl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293913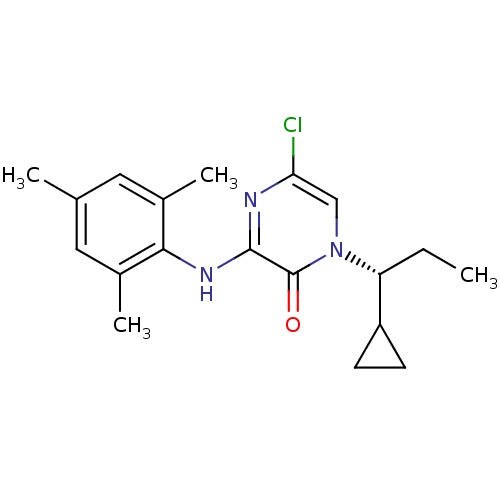 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,4,6-trim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293913 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,4,6-trim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50313860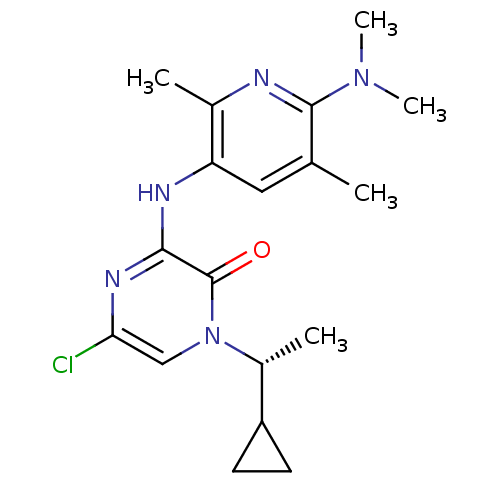 ((R)-5-chloro-1-(1-cyclopropylethyl)-3-(6-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | Bioorg Med Chem Lett 20: 1890-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.129 BindingDB Entry DOI: 10.7270/Q29G5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293951 (5-Chloro-1-(1-ethylpropyl)-3-(4-methoxy-2,5-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM297236 (2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-methoxy-5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00219 BindingDB Entry DOI: 10.7270/Q20R9TDX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50313869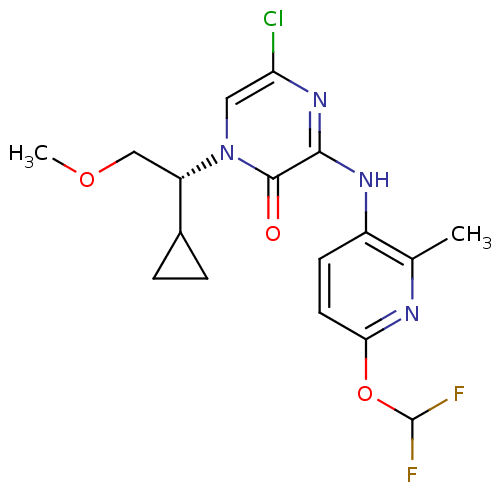 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | Bioorg Med Chem Lett 20: 1890-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.129 BindingDB Entry DOI: 10.7270/Q29G5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50320241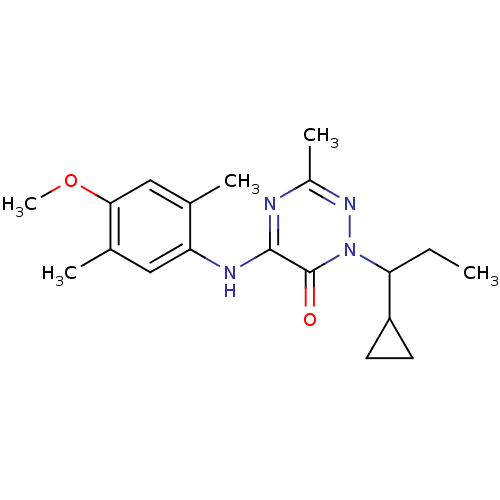 (1-(1-cyclopropylpropyl)-5-(4-methoxy-2,5-dimethylp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine CRF from rat CRF1 receptor | Bioorg Med Chem Lett 20: 3579-83 (2010) Article DOI: 10.1016/j.bmcl.2010.04.121 BindingDB Entry DOI: 10.7270/Q2SX6DDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM297070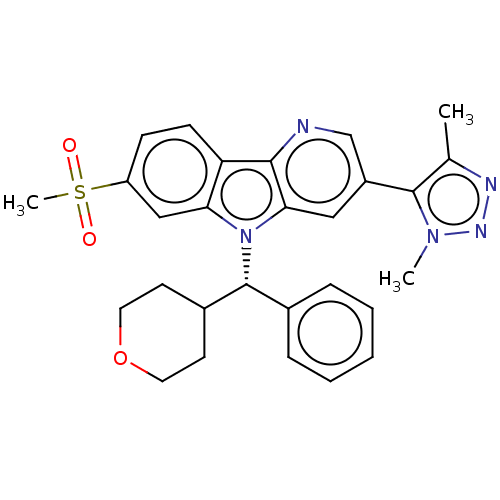 (5-{7-Methanesulfonyl-5-[(S)-oxan-4-yl(phenyl)methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00219 BindingDB Entry DOI: 10.7270/Q20R9TDX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM297163 (2-{8-Fluoro-3-[4-(2H3)methyl-1-methyl-1H-1,2,3-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00219 BindingDB Entry DOI: 10.7270/Q20R9TDX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293930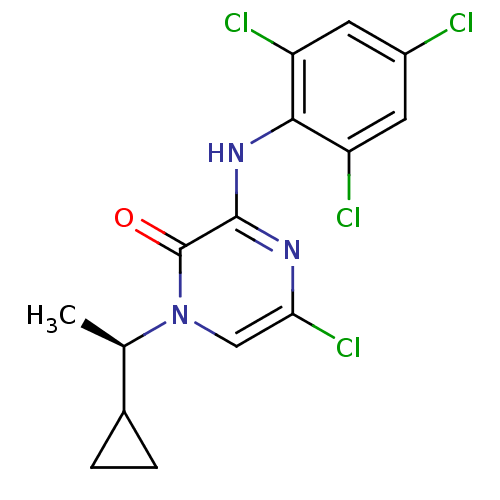 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(2,4,6-trich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293982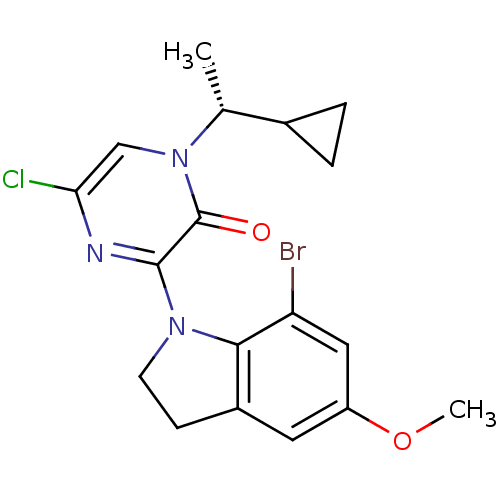 ((R)-3-(7-Bromo-5-methoxyindolin-1-yl)-5-chloro-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293929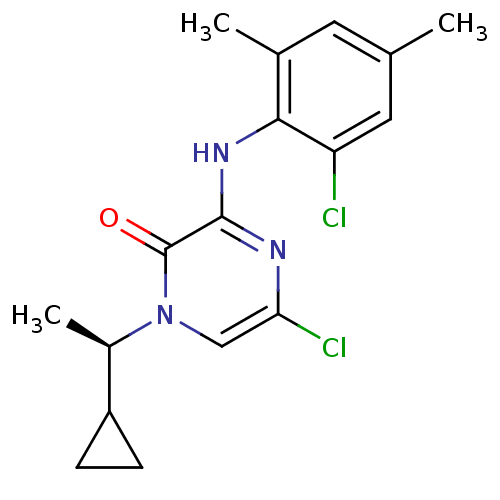 ((R)-5-Chloro-3-(2-chloro-4,6-dimethylphenylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331306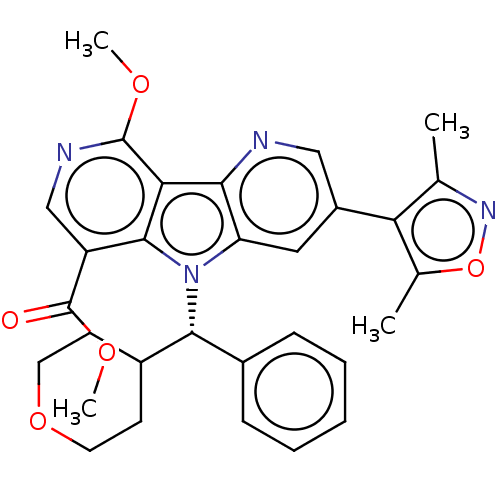 (Methyl 5-(dimethyl-1,2-oxazol-4-yl)-13-methoxy-8-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331316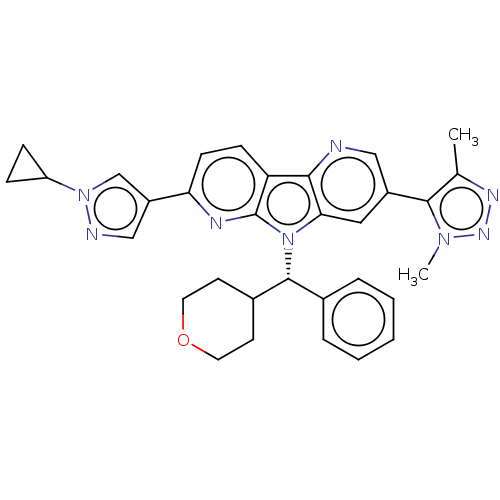 (11-(1-Cyclopropyl-1H-pyrazol-4-yl)-5-(dimethyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331355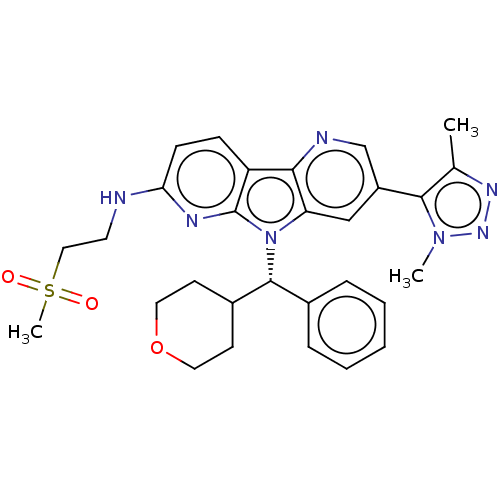 (N-(2-Methanesulfonylethyl)-5-[4-(2H3)methyl-1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331356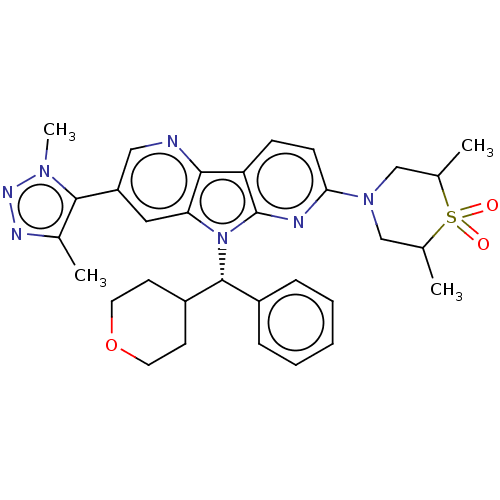 (4-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331380 (5-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331379 (4-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331385 ((2-{[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331389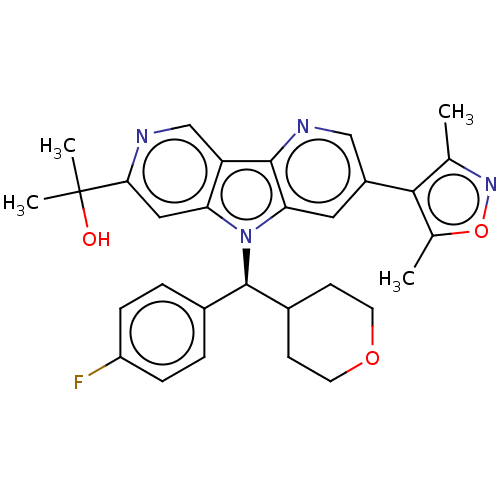 (2-[5-(Dimethyl-1,2-oxazol-4-yl)-8-[(S)-(4-fluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331412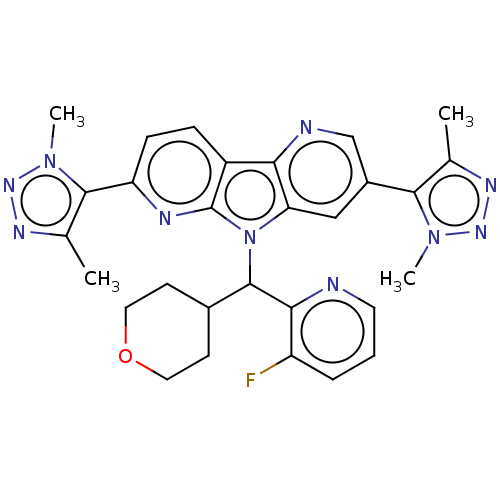 (8-[(3-Fluoropyridin-2-yl)(oxan-4-yl)methyl]-5,11-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331417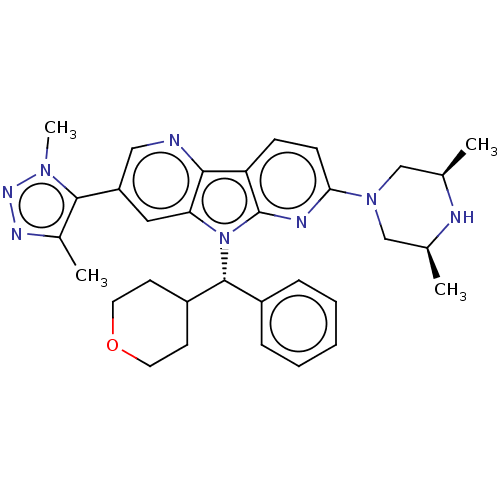 (11-[(3R,5S)-3,5-Dimethylpiperazin-1-yl]-5-[4-(2H3)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331441 (2-{10-Fluoro-5-[4-(2H3)methyl-1-methyl-1H-1,2,3-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331446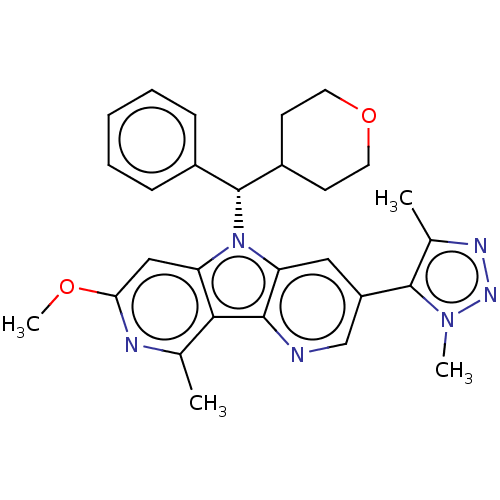 (5-(Dimethyl-1H-1,2,3-triazol-5-yl)-11-methoxy-13-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331447 (2-{5-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331451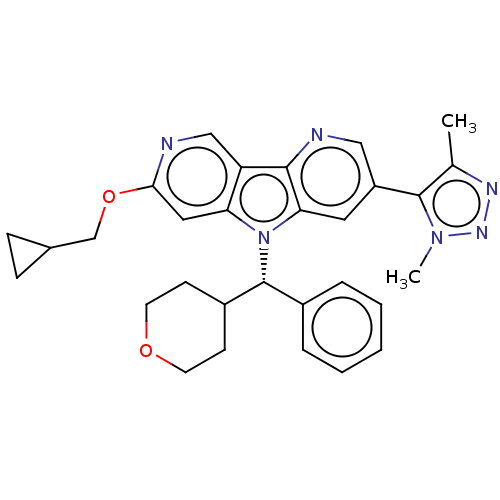 (11-(Cyclopropylmethoxy)-5-[4-(2H3)methyl-1-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331505 (2-{8-[(4,4-Difluorocyclohexyl)(phenyl)methyl]-13-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM297144 (2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-9-methoxy-5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00219 BindingDB Entry DOI: 10.7270/Q20R9TDX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 662 total ) | Next | Last >> |