 Found 103 hits with Last Name = 'welstead' and Initial = 'wj'
Found 103 hits with Last Name = 'welstead' and Initial = 'wj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50007538
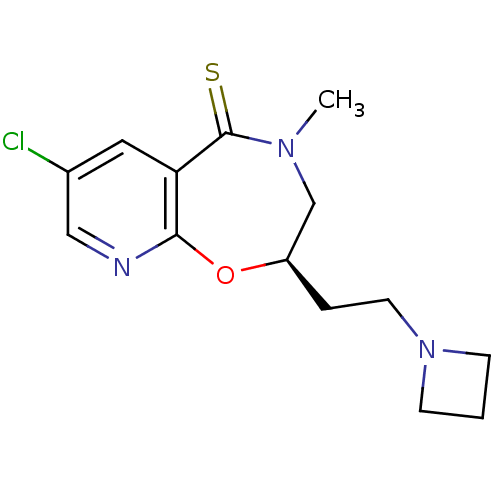
(8-(2-Azetidin-1-yl-ethyl)-3-chloro-6-methyl-7,8-di...)Show InChI InChI=1S/C14H18ClN3OS/c1-17-9-11(3-6-18-4-2-5-18)19-13-12(14(17)20)7-10(15)8-16-13/h7-8,11H,2-6,9H2,1H3/t11-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
A. H. Robins Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-mepyramine binding to the histamine receptor in guinea pig cortex |
J Med Chem 34: 1314-28 (1991)
BindingDB Entry DOI: 10.7270/Q23F4NMG |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50007542
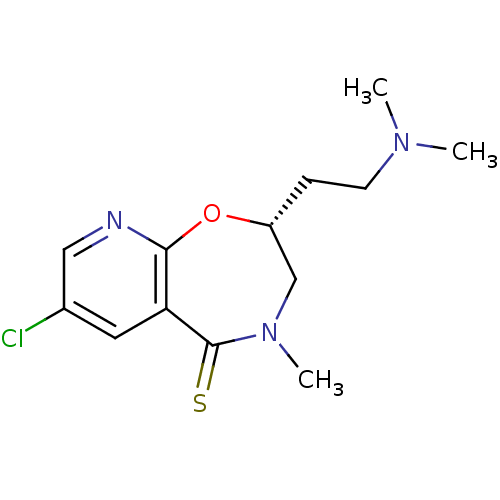
(3-Chloro-8-(2-dimethylamino-ethyl)-6-methyl-7,8-di...)Show InChI InChI=1S/C13H18ClN3OS/c1-16(2)5-4-10-8-17(3)13(19)11-6-9(14)7-15-12(11)18-10/h6-7,10H,4-5,8H2,1-3H3/t10-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
A. H. Robins Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-mepyramine binding to the histamine receptor in guinea pig cortex |
J Med Chem 34: 1314-28 (1991)
BindingDB Entry DOI: 10.7270/Q23F4NMG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225193

(CHEMBL432463)Show SMILES [Na+].CSc1ccc(cc1)C(=O)c1cc(F)cc(CC([O-])=O)c1N Show InChI InChI=1S/C16H14FNO3S/c1-22-12-4-2-9(3-5-12)16(21)13-8-11(17)6-10(15(13)18)7-14(19)20/h2-6,8H,7,18H2,1H3,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM35938
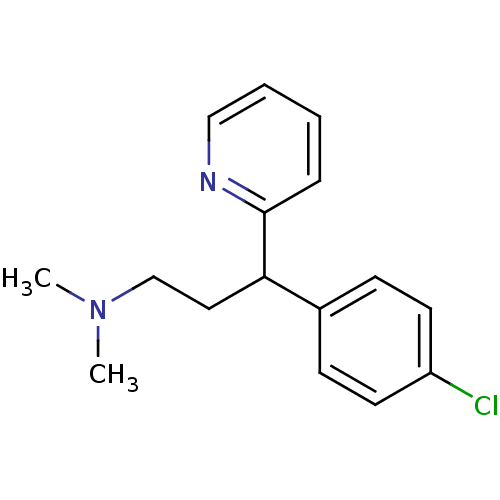
(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
A. H. Robins Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-mepyramine binding to the Histamine H1 receptor in guinea pig cortex |
J Med Chem 34: 1314-28 (1991)
BindingDB Entry DOI: 10.7270/Q23F4NMG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225184
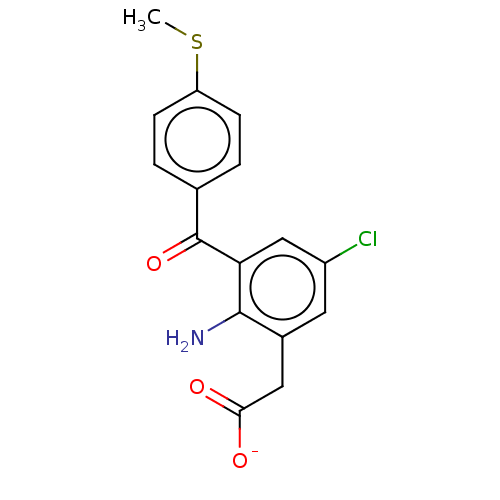
(CHEMBL28976)Show SMILES [Na+].CSc1ccc(cc1)C(=O)c1cc(Cl)cc(CC([O-])=O)c1N Show InChI InChI=1S/C16H14ClNO3S/c1-22-12-4-2-9(3-5-12)16(21)13-8-11(17)6-10(15(13)18)7-14(19)20/h2-6,8H,7,18H2,1H3,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225190
(CHEMBL28508)Show SMILES [Na+].Cc1ccc(cc1)C(=O)c1cc(Cl)cc(CC([O-])=O)c1N Show InChI InChI=1S/C16H14ClNO3/c1-9-2-4-10(5-3-9)16(21)13-8-12(17)6-11(15(13)18)7-14(19)20/h2-6,8H,7,18H2,1H3,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225114

(CHEMBL28507)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(Cl)cc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C15H11BrClNO3/c16-10-3-1-8(2-4-10)15(21)12-7-11(17)5-9(14(12)18)6-13(19)20/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50004172
(CHEMBL544763 | Sulfamic acid 2-(4-imidazol-1-yl-ph...)Show InChI InChI=1S/C11H13N3O4S/c12-19(15,16)18-8-7-17-11-3-1-10(2-4-11)14-6-5-13-9-14/h1-6,9H,7-8H2,(H2,12,15,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50007536

(CHEMBL24517 | [3-(5-Chloro-pyridin-2-yl)-3-pyridin...)Show InChI InChI=1S/C15H18ClN3/c1-19(2)10-8-13(14-5-3-4-9-17-14)15-7-6-12(16)11-18-15/h3-7,9,11,13H,8,10H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
A. H. Robins Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-mepyramine binding to the Histamine H1 receptor in guinea pig cortex |
J Med Chem 34: 1314-28 (1991)
BindingDB Entry DOI: 10.7270/Q23F4NMG |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50007539
(8-(2-Dimethylamino-ethyl)-6-methyl-7,8-dihydro-6H-...)Show InChI InChI=1S/C13H19N3OS/c1-15(2)8-6-10-9-16(3)13(18)11-5-4-7-14-12(11)17-10/h4-5,7,10H,6,8-9H2,1-3H3/t10-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
A. H. Robins Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-mepyramine binding to the histamine receptor in guinea pig cortex |
J Med Chem 34: 1314-28 (1991)
BindingDB Entry DOI: 10.7270/Q23F4NMG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225186
(CHEMBL281012)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(Cl)cc1C(=O)c1ccc(I)cc1 Show InChI InChI=1S/C15H11ClINO3/c16-10-5-9(6-13(19)20)14(18)12(7-10)15(21)8-1-3-11(17)4-2-8/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225122
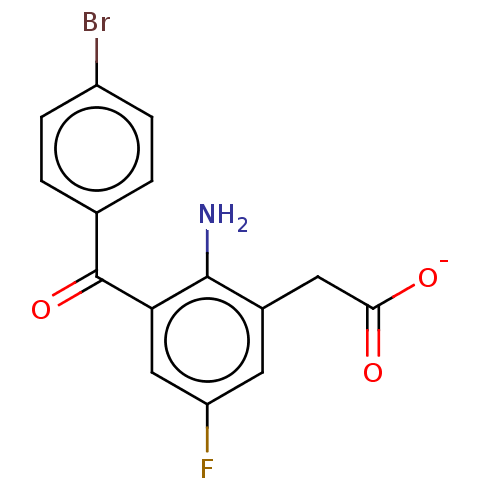
(CHEMBL29047)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(F)cc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C15H11BrFNO3/c16-10-3-1-8(2-4-10)15(21)12-7-11(17)5-9(14(12)18)6-13(19)20/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50230218
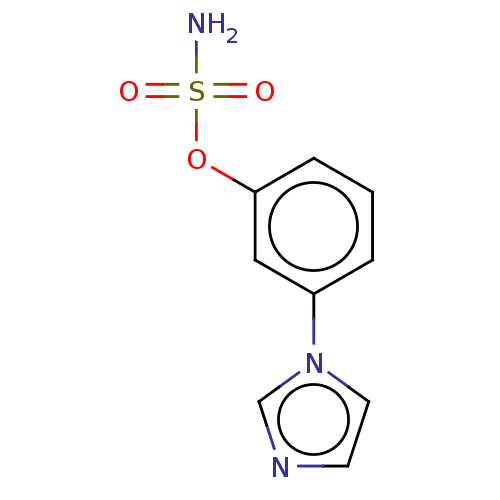
(CHEMBL545555)Show InChI InChI=1S/C9H9N3O3S/c10-16(13,14)15-9-3-1-2-8(6-9)12-5-4-11-7-12/h1-7H,(H2,10,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225141
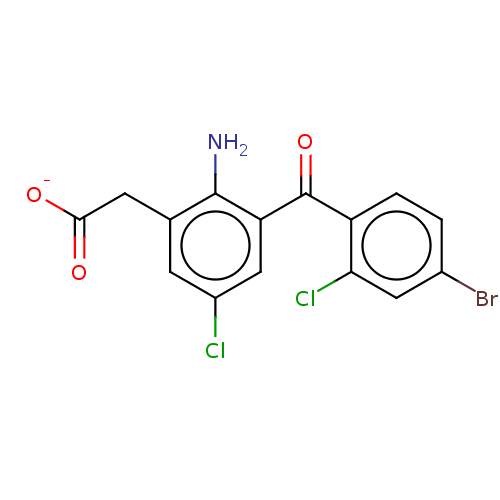
(CHEMBL418035)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(Cl)cc1C(=O)c1ccc(Br)cc1Cl Show InChI InChI=1S/C15H10BrCl2NO3/c16-8-1-2-10(12(18)5-8)15(22)11-6-9(17)3-7(14(11)19)4-13(20)21/h1-3,5-6H,4,19H2,(H,20,21)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50230217
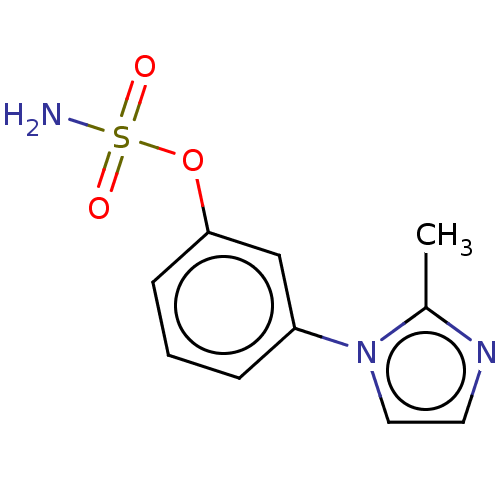
(CHEMBL555867)Show InChI InChI=1S/C10H11N3O3S/c1-8-12-5-6-13(8)9-3-2-4-10(7-9)16-17(11,14)15/h2-7H,1H3,(H2,11,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225136

(CHEMBL538408)Show SMILES [Na+].Nc1c(CC([O-])=O)cccc1C(=O)c1ccc(Br)cc1Cl Show InChI InChI=1S/C15H11BrClNO3/c16-9-4-5-10(12(17)7-9)15(21)11-3-1-2-8(14(11)18)6-13(19)20/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50230221

(CHEMBL541599)Show InChI InChI=1S/C11H13N3O4S/c12-19(15,16)18-7-6-17-11-3-1-2-10(8-11)14-5-4-13-9-14/h1-5,8-9H,6-7H2,(H2,12,15,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225195

(CHEMBL31313)Show SMILES [Na+].Nc1c(CC([O-])=O)cccc1C(=O)c1ccc(I)cc1 Show InChI InChI=1S/C15H12INO3/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19/h1-7H,8,17H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225123
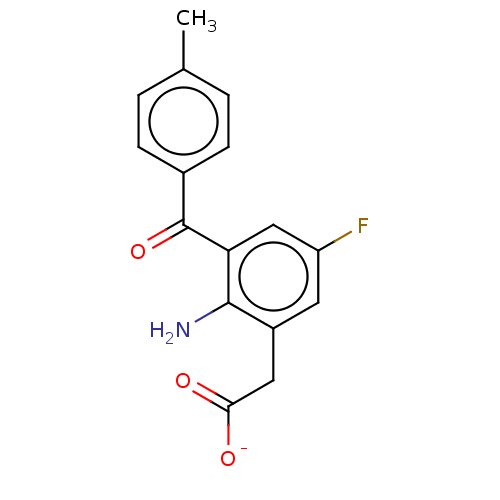
(CHEMBL28543)Show SMILES [Na+].Cc1ccc(cc1)C(=O)c1cc(F)cc(CC([O-])=O)c1N Show InChI InChI=1S/C16H14FNO3/c1-9-2-4-10(5-3-9)16(21)13-8-12(17)6-11(15(13)18)7-14(19)20/h2-6,8H,7,18H2,1H3,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50230214

(CHEMBL555738)Show InChI InChI=1S/C12H15N3O4S/c1-10-14-5-6-15(10)11-3-2-4-12(9-11)18-7-8-19-20(13,16)17/h2-6,9H,7-8H2,1H3,(H2,13,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225126

(CHEMBL283267)Show SMILES [Na+].Nc1c(CC([O-])=O)cccc1C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H17NO3/c22-20-17(13-19(23)24)7-4-8-18(20)21(25)16-11-9-15(10-12-16)14-5-2-1-3-6-14/h1-12H,13,22H2,(H,23,24)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225143
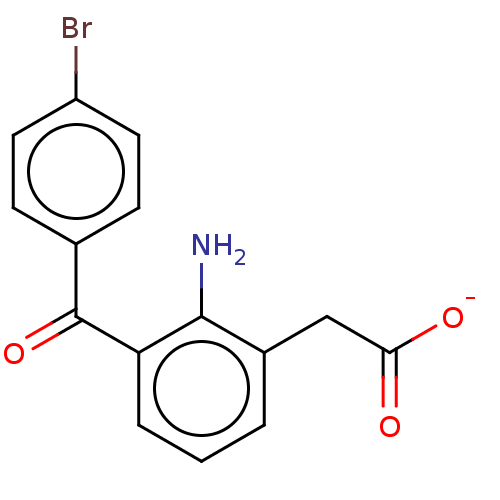
(AHR-10282B | Bromday | Bromfenac Sodium | Bromsite...)Show SMILES [Na+].Nc1c(CC([O-])=O)cccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C15H12BrNO3/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19/h1-7H,8,17H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50230219
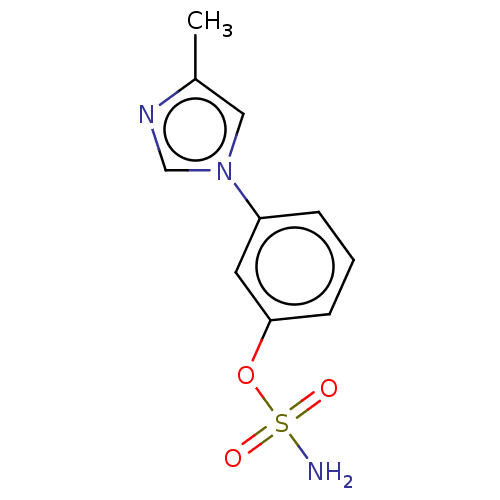
(CHEMBL545088)Show InChI InChI=1S/C10H11N3O3S/c1-8-6-13(7-12-8)9-3-2-4-10(5-9)16-17(11,14)15/h2-7H,1H3,(H2,11,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225176
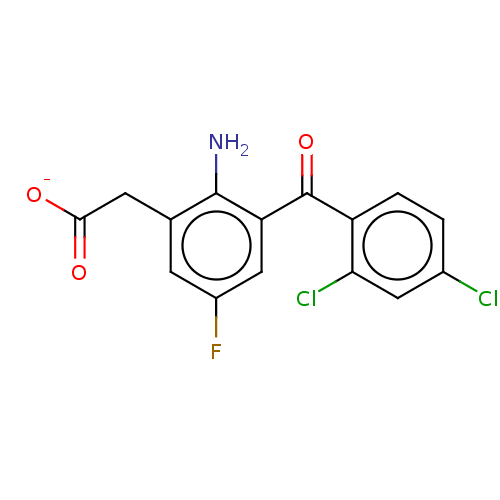
(CHEMBL29291)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(F)cc1C(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C15H10Cl2FNO3/c16-8-1-2-10(12(17)5-8)15(22)11-6-9(18)3-7(14(11)19)4-13(20)21/h1-3,5-6H,4,19H2,(H,20,21)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225134

(CHEMBL32037)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(F)cc1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H11ClFNO3/c16-10-3-1-8(2-4-10)15(21)12-7-11(17)5-9(14(12)18)6-13(19)20/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225121
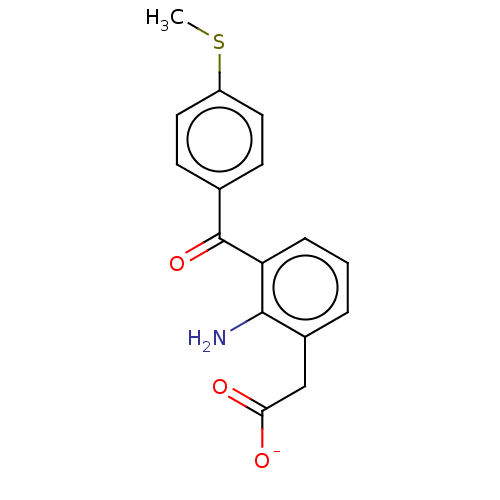
(CHEMBL286716)Show SMILES [Na+].CSc1ccc(cc1)C(=O)c1cccc(CC([O-])=O)c1N Show InChI InChI=1S/C16H15NO3S/c1-21-12-7-5-10(6-8-12)16(20)13-4-2-3-11(15(13)17)9-14(18)19/h2-8H,9,17H2,1H3,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225118
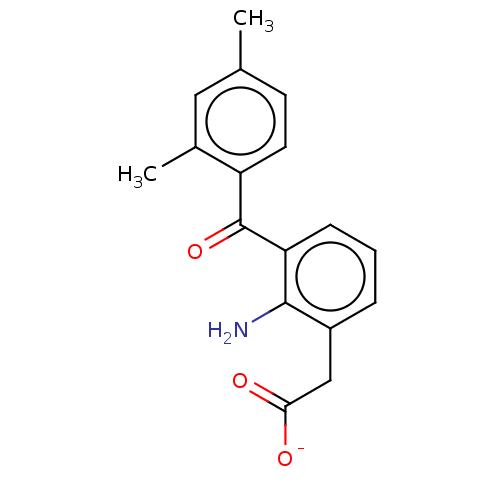
(CHEMBL436147)Show SMILES [Na+].Cc1ccc(C(=O)c2cccc(CC([O-])=O)c2N)c(C)c1 Show InChI InChI=1S/C17H17NO3/c1-10-6-7-13(11(2)8-10)17(21)14-5-3-4-12(16(14)18)9-15(19)20/h3-8H,9,18H2,1-2H3,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50225187
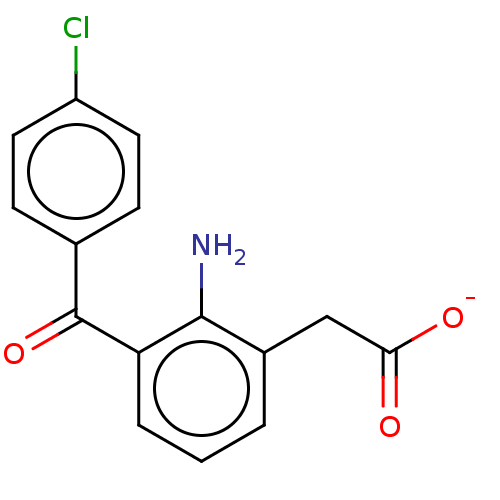
(CHEMBL28700)Show SMILES [Na+].Nc1c(CC([O-])=O)cccc1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H12ClNO3/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19/h1-7H,8,17H2,(H,18,19)/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase |
J Med Chem 33: 2296-304 (1990)
BindingDB Entry DOI: 10.7270/Q2MS3RR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50224285

(AHR-5850D | Amfenac Sodium | CHEBI:75918)Show InChI InChI=1S/C15H13NO3/c16-14-11(9-13(17)18)7-4-8-12(14)15(19)10-5-2-1-3-6-10/h1-8H,9,16H2,(H,17,18)/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase |
J Med Chem 33: 2296-304 (1990)
BindingDB Entry DOI: 10.7270/Q2MS3RR7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50230216
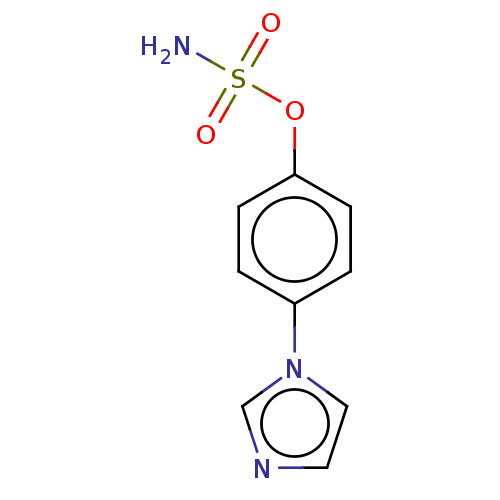
(CHEMBL553542)Show InChI InChI=1S/C9H9N3O3S/c10-16(13,14)15-9-3-1-8(2-4-9)12-6-5-11-7-12/h1-7H,(H2,10,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225181

(CHEMBL282828)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(F)cc1C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C15H11F2NO3/c16-10-3-1-8(2-4-10)15(21)12-7-11(17)5-9(14(12)18)6-13(19)20/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase |
J Med Chem 33: 2296-304 (1990)
BindingDB Entry DOI: 10.7270/Q2MS3RR7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225140

(CHEMBL28955)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(F)cc1C(=O)c1ccccc1 Show InChI InChI=1S/C15H12FNO3/c16-11-6-10(7-13(18)19)14(17)12(8-11)15(20)9-4-2-1-3-5-9/h1-6,8H,7,17H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50224285

(AHR-5850D | Amfenac Sodium | CHEBI:75918)Show InChI InChI=1S/C15H13NO3/c16-14-11(9-13(17)18)7-4-8-12(14)15(19)10-5-2-1-3-6-10/h1-8H,9,16H2,(H,17,18)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50224285

(AHR-5850D | Amfenac Sodium | CHEBI:75918)Show InChI InChI=1S/C15H13NO3/c16-14-11(9-13(17)18)7-4-8-12(14)15(19)10-5-2-1-3-6-10/h1-8H,9,16H2,(H,17,18)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of H-Ras-mediated farnesylation expressed in mouse NIH3T3 cells |
J Med Chem 25: 446-51 (1982)
BindingDB Entry DOI: 10.7270/Q28P62QH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50230220

(CHEMBL555771)Show InChI InChI=1S/C12H15N3O4S/c1-10-8-15(9-14-10)11-3-2-4-12(7-11)18-5-6-19-20(13,16)17/h2-4,7-9H,5-6H2,1H3,(H2,13,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Bos taurus) | BDBM50230215
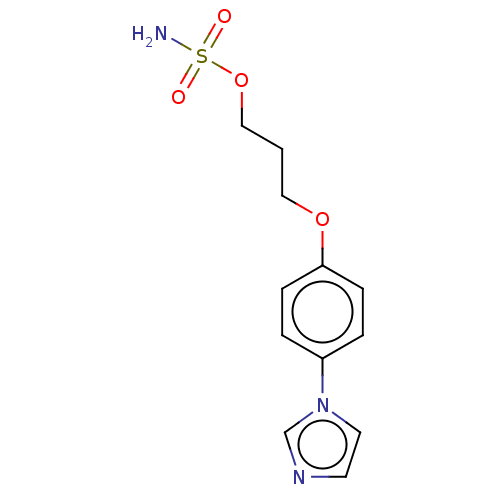
(CHEMBL543683)Show InChI InChI=1S/C12H15N3O4S/c13-20(16,17)19-9-1-8-18-12-4-2-11(3-5-12)15-7-6-14-10-15/h2-7,10H,1,8-9H2,(H2,13,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Bovine erythrocyte carbonic anhydrase inhibitory activity of the compound. |
J Med Chem 35: 4790-4 (1992)
BindingDB Entry DOI: 10.7270/Q2MS3W1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225117
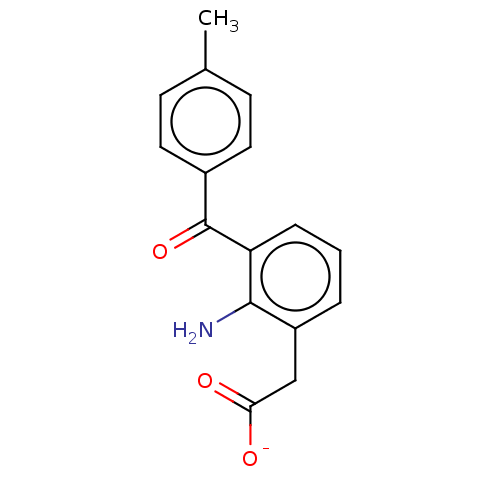
(CHEMBL28703)Show SMILES [Na+].Cc1ccc(cc1)C(=O)c1cccc(CC([O-])=O)c1N Show InChI InChI=1S/C16H15NO3/c1-10-5-7-11(8-6-10)16(20)13-4-2-3-12(15(13)17)9-14(18)19/h2-8H,9,17H2,1H3,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225135

(CHEMBL284647)Show InChI InChI=1S/C15H12ClNO3/c16-12-7-2-1-5-10(12)15(20)11-6-3-4-9(14(11)17)8-13(18)19/h1-7H,8,17H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225187

(CHEMBL28700)Show SMILES [Na+].Nc1c(CC([O-])=O)cccc1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H12ClNO3/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19/h1-7H,8,17H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225130
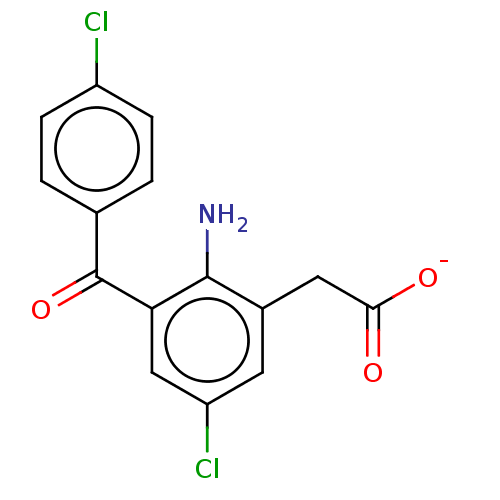
(CHEMBL27620)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(Cl)cc1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H11Cl2NO3/c16-10-3-1-8(2-4-10)15(21)12-7-11(17)5-9(14(12)18)6-13(19)20/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50228722

(CHEMBL75896)Show InChI InChI=1S/C15H19NO3/c16-14-11(9-13(17)18)7-4-8-12(14)15(19)10-5-2-1-3-6-10/h4,7-8,10H,1-3,5-6,9,16H2,(H,17,18)/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase |
J Med Chem 33: 2296-304 (1990)
BindingDB Entry DOI: 10.7270/Q2MS3RR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225185
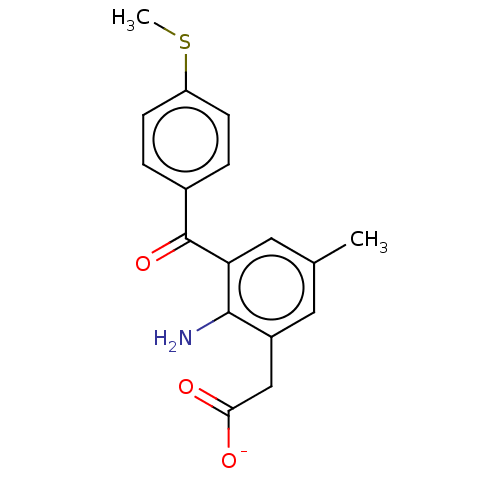
(CHEMBL28511)Show SMILES [Na+].CSc1ccc(cc1)C(=O)c1cc(C)cc(CC([O-])=O)c1N Show InChI InChI=1S/C17H17NO3S/c1-10-7-12(9-15(19)20)16(18)14(8-10)17(21)11-3-5-13(22-2)6-4-11/h3-8H,9,18H2,1-2H3,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50007540

(CHEMBL279907 | [3-(4-Chloro-phenyl)-3-(5-chloro-py...)Show InChI InChI=1S/C16H18Cl2N2/c1-20(2)10-9-15(12-3-5-13(17)6-4-12)16-8-7-14(18)11-19-16/h3-8,11,15H,9-10H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
A. H. Robins Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-mepyramine binding to the Histamine H1 receptor in guinea pig cortex |
J Med Chem 34: 1314-28 (1991)
BindingDB Entry DOI: 10.7270/Q23F4NMG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225120

(CHEMBL28469)Show SMILES [Na+].Nc1c(CC([O-])=O)cccc1C(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C15H11Cl2NO3/c16-9-4-5-10(12(17)7-9)15(21)11-3-1-2-8(14(11)18)6-13(19)20/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225178

(CHEMBL282824)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(Br)cc1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H11BrClNO3/c16-10-5-9(6-13(19)20)14(18)12(7-10)15(21)8-1-3-11(17)4-2-8/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225191

(CHEMBL284879)Show SMILES [Na+].Cc1cc(CC([O-])=O)c(N)c(c1)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C16H14ClNO3/c1-9-6-11(8-14(19)20)15(18)13(7-9)16(21)10-2-4-12(17)5-3-10/h2-7H,8,18H2,1H3,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225113

(CHEMBL441960)Show SMILES [Na+].Cc1cc(CC([O-])=O)c(N)c(c1)C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C16H14BrNO3/c1-9-6-11(8-14(19)20)15(18)13(7-9)16(21)10-2-4-12(17)5-3-10/h2-7H,8,18H2,1H3,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Bos taurus) | BDBM50225139

(CHEMBL29112)Show SMILES [Na+].Nc1c(CC([O-])=O)cc(Br)cc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C15H11Br2NO3/c16-10-3-1-8(2-4-10)15(21)12-7-11(17)5-9(14(12)18)6-13(19)20/h1-5,7H,6,18H2,(H,19,20)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. |
J Med Chem 27: 1379-88 (1984)
BindingDB Entry DOI: 10.7270/Q2222X0V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data