 Found 3893 hits with Last Name = 'gao' and Initial = 'x'
Found 3893 hits with Last Name = 'gao' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499195

(CHEMBL3735504)Show SMILES CC(C)(C)C(=O)Nc1ccc2n([C@@H]3CC[C@H](CO)CC3)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r,wD:12.11,15.15,(-7.45,.88,;-6.39,1.5,;-7.46,2.11,;-6.4,2.74,;-5.05,.74,;-5.04,-.49,;-3.72,1.53,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;3.72,-3.12,;4.09,-4.62,;2.98,-5.68,;3.36,-7.18,;4.54,-7.52,;1.5,-5.26,;1.13,-3.76,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,)| Show InChI InChI=1S/C27H31F3N4O3/c1-26(2,3)24(37)31-19-9-12-22-21(14-19)32-25(34(22)20-10-7-16(15-35)8-11-20)33-23(36)17-5-4-6-18(13-17)27(28,29)30/h4-6,9,12-14,16,20,35H,7-8,10-11,15H2,1-3H3,(H,31,37)(H,32,33,36)/t16-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256911

(CHEMBL4095621)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C24H30ClN7O5/c1-13(30-23(37)19(32-24(27)28)11-15-4-8-17(33)9-5-15)22(36)29-12-20(34)31-18(21(26)35)10-14-2-6-16(25)7-3-14/h2-9,13,18-19,33H,10-12H2,1H3,(H2,26,35)(H,29,36)(H,30,37)(H,31,34)(H4,27,28,32)/t13-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256914
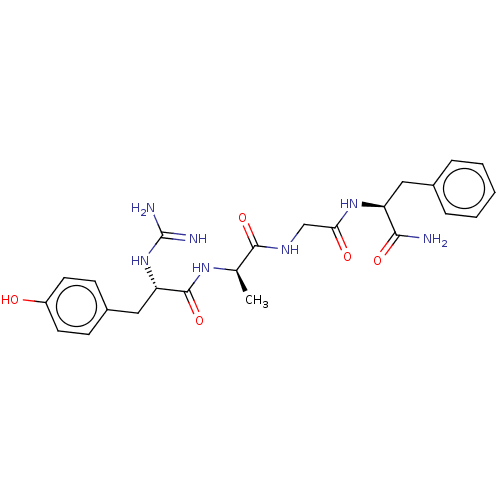
(CHEMBL4061665)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C24H31N7O5/c1-14(29-23(36)19(31-24(26)27)12-16-7-9-17(32)10-8-16)22(35)28-13-20(33)30-18(21(25)34)11-15-5-3-2-4-6-15/h2-10,14,18-19,32H,11-13H2,1H3,(H2,25,34)(H,28,35)(H,29,36)(H,30,33)(H4,26,27,31)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499203
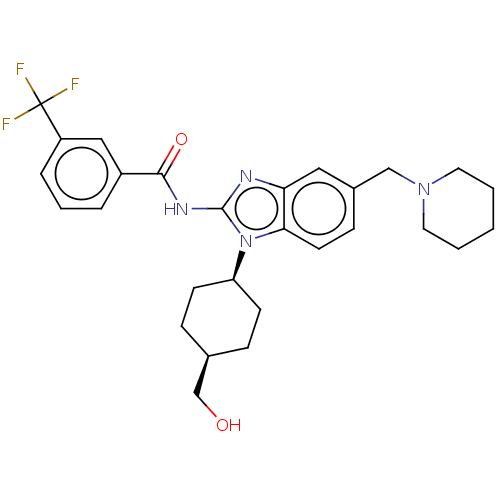
(CHEMBL3736036)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C28H33F3N4O2/c29-28(30,31)22-6-4-5-21(16-22)26(37)33-27-32-24-15-20(17-34-13-2-1-3-14-34)9-12-25(24)35(27)23-10-7-19(18-36)8-11-23/h4-6,9,12,15-16,19,23,36H,1-3,7-8,10-11,13-14,17-18H2,(H,32,33,37)/t19-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499205
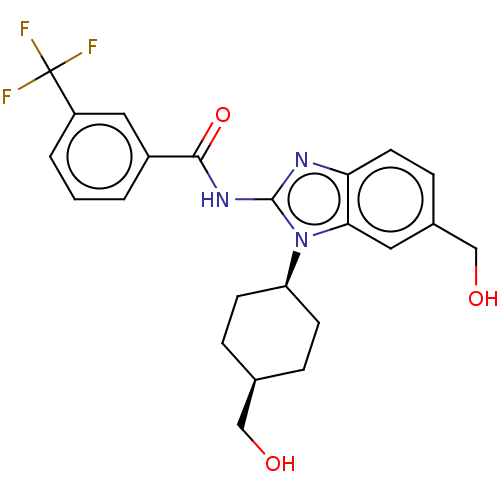
(CHEMBL3734814)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2ccc(CO)cc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.81,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-3.72,-1.53,;-3.72,-2.76,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C23H24F3N3O3/c24-23(25,26)17-3-1-2-16(11-17)21(32)28-22-27-19-9-6-15(13-31)10-20(19)29(22)18-7-4-14(12-30)5-8-18/h1-3,6,9-11,14,18,30-31H,4-5,7-8,12-13H2,(H,27,28,32)/t14-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499208
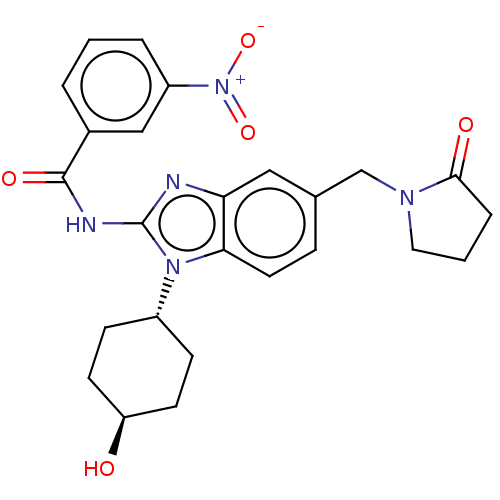
(CHEMBL3734872)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCC3=O)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-5.17,-.78,;-6.68,-1.11,;-7.46,.21,;-6.44,1.37,;-6.7,2.57,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H27N5O5/c31-20-9-7-18(8-10-20)29-22-11-6-16(15-28-12-2-5-23(28)32)13-21(22)26-25(29)27-24(33)17-3-1-4-19(14-17)30(34)35/h1,3-4,6,11,13-14,18,20,31H,2,5,7-10,12,15H2,(H,26,27,33)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499194

(CHEMBL3734854)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33N5O4/c33-18-19-7-10-22(11-8-19)31-25-12-9-20(17-30-13-2-1-3-14-30)15-24(25)28-27(31)29-26(34)21-5-4-6-23(16-21)32(35)36/h4-6,9,12,15-16,19,22,33H,1-3,7-8,10-11,13-14,17-18H2,(H,28,29,34)/t19-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256915

(CHEMBL4068851)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C23H28ClN5O5/c1-13(28-23(34)18(25)10-14-4-8-17(30)9-5-14)22(33)27-12-20(31)29-19(21(26)32)11-15-2-6-16(24)7-3-15/h2-9,13,18-19,30H,10-12,25H2,1H3,(H2,26,32)(H,27,33)(H,28,34)(H,29,31)/t13-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499197
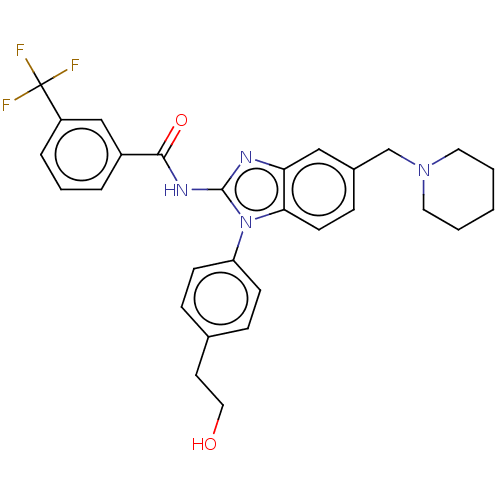
(CHEMBL3736465)Show SMILES OCCc1ccc(cc1)-n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C29H29F3N4O2/c30-29(31,32)23-6-4-5-22(18-23)27(38)34-28-33-25-17-21(19-35-14-2-1-3-15-35)9-12-26(25)36(28)24-10-7-20(8-11-24)13-16-37/h4-12,17-18,37H,1-3,13-16,19H2,(H,33,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499206

(CHEMBL3735949)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.45,1.38,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C21H21FN4O4/c22-15-6-9-19-18(11-15)23-21(25(19)16-7-4-13(12-27)5-8-16)24-20(28)14-2-1-3-17(10-14)26(29)30/h1-3,6,9-11,13,16,27H,4-5,7-8,12H2,(H,23,24,28)/t13-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50453766
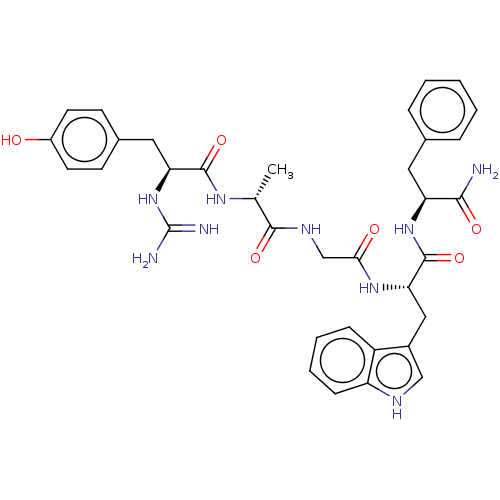
(CHEMBL4210361)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H41N9O6/c1-20(41-33(49)28(44-35(37)38)16-22-11-13-24(45)14-12-22)32(48)40-19-30(46)42-29(17-23-18-39-26-10-6-5-9-25(23)26)34(50)43-27(31(36)47)15-21-7-3-2-4-8-21/h2-14,18,20,27-29,39,45H,15-17,19H2,1H3,(H2,36,47)(H,40,48)(H,41,49)(H,42,46)(H,43,50)(H4,37,38,44)/t20-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor in Wistar rat brain membranes preincubated for 1 hr measured after 1hr by scintillation counting m... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21123
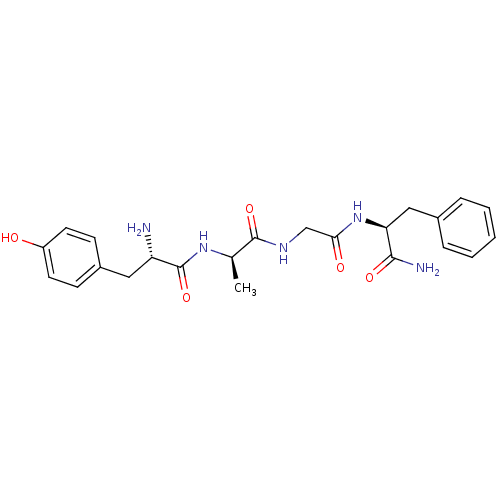
((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C23H29N5O5/c1-14(27-23(33)18(24)11-16-7-9-17(29)10-8-16)22(32)26-13-20(30)28-19(21(25)31)12-15-5-3-2-4-6-15/h2-10,14,18-19,29H,11-13,24H2,1H3,(H2,25,31)(H,26,32)(H,27,33)(H,28,30)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499198
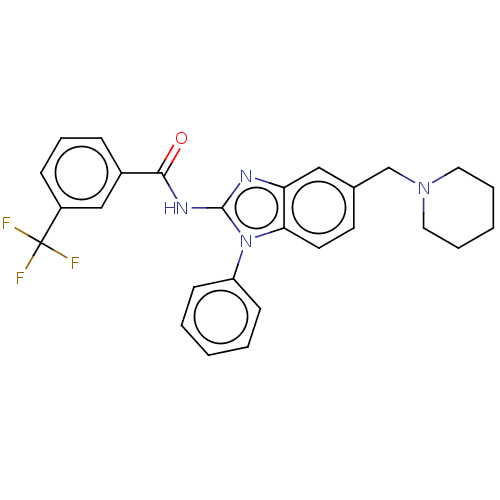
(CHEMBL3736278)Show SMILES FC(F)(F)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C27H25F3N4O/c28-27(29,30)21-9-7-8-20(17-21)25(35)32-26-31-23-16-19(18-33-14-5-2-6-15-33)12-13-24(23)34(26)22-10-3-1-4-11-22/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499199

(CHEMBL3735673)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C26H31N5O4/c32-22-10-8-20(9-11-22)30-24-12-7-18(17-29-13-2-1-3-14-29)15-23(24)27-26(30)28-25(33)19-5-4-6-21(16-19)31(34)35/h4-7,12,15-16,20,22,32H,1-3,8-11,13-14,17H2,(H,27,28,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50453765
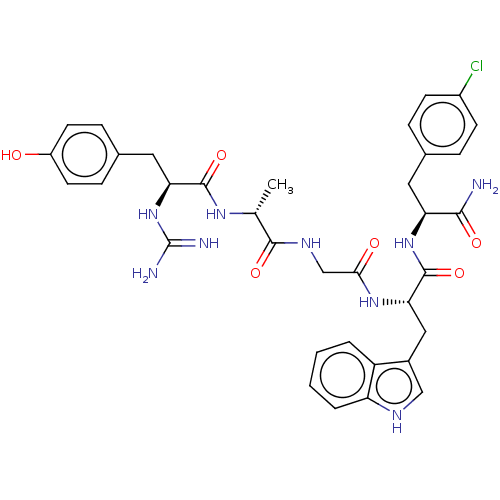
(CHEMBL4215224)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C35H40ClN9O6/c1-19(42-33(50)28(45-35(38)39)15-21-8-12-24(46)13-9-21)32(49)41-18-30(47)43-29(16-22-17-40-26-5-3-2-4-25(22)26)34(51)44-27(31(37)48)14-20-6-10-23(36)11-7-20/h2-13,17,19,27-29,40,46H,14-16,18H2,1H3,(H2,37,48)(H,41,49)(H,42,50)(H,43,47)(H,44,51)(H4,38,39,45)/t19-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor in Wistar rat brain membranes preincubated for 1 hr measured after 1hr by scintillation counting m... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499202

(CHEMBL3735523)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCOCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H29N5O5/c31-21-7-5-19(6-8-21)29-23-9-4-17(16-28-10-12-35-13-11-28)14-22(23)26-25(29)27-24(32)18-2-1-3-20(15-18)30(33)34/h1-4,9,14-15,19,21,31H,5-8,10-13,16H2,(H,26,27,32)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50222015
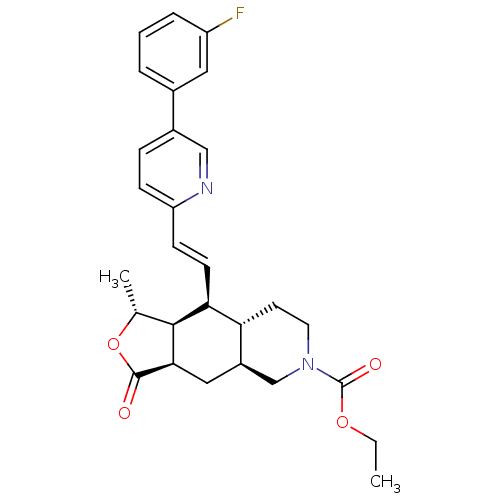
((1R,3aR,4aS,8aS,9S,9aS)-decahydro-1-methyl-3-oxo-9...)Show SMILES CCOC(=O)N1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1 Show InChI InChI=1S/C28H31FN2O4/c1-3-34-28(33)31-12-11-23-20(16-31)14-25-26(17(2)35-27(25)32)24(23)10-9-22-8-7-19(15-30-22)18-5-4-6-21(29)13-18/h4-10,13,15,17,20,23-26H,3,11-12,14,16H2,1-2H3/b10-9+/t17-,20-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
J Med Chem 50: 5147-60 (2007)
Article DOI: 10.1021/jm070704k
BindingDB Entry DOI: 10.7270/Q2SN08PK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor in Wistar rat brain membranes preincubated for 1 hr measured after 1hr by scintillation counting m... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499200
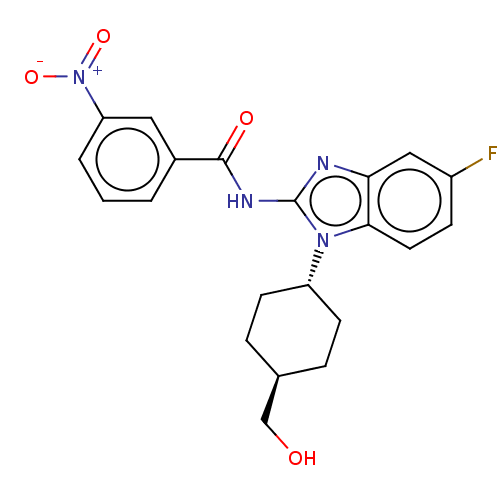
(CHEMBL3735044)Show SMILES OC[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r,wU:5.8,wD:2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.45,1.38,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C21H21FN4O4/c22-15-6-9-19-18(11-15)23-21(25(19)16-7-4-13(12-27)5-8-16)24-20(28)14-2-1-3-17(10-14)26(29)30/h1-3,6,9-11,13,16,27H,4-5,7-8,12H2,(H,23,24,28)/t13-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499210
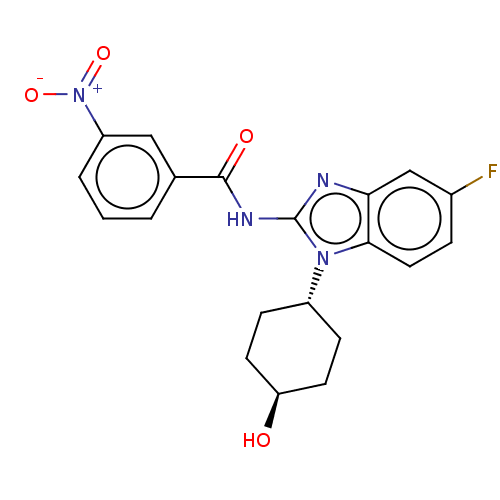
(CHEMBL3734846)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.45,1.38,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C20H19FN4O4/c21-13-4-9-18-17(11-13)22-20(24(18)14-5-7-16(26)8-6-14)23-19(27)12-2-1-3-15(10-12)25(28)29/h1-4,9-11,14,16,26H,5-8H2,(H,22,23,27)/t14-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50453764
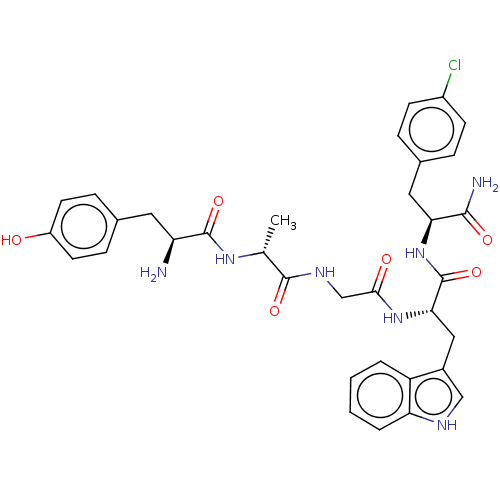
(CHEMBL4211768)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C34H38ClN7O6/c1-19(40-33(47)26(36)14-20-8-12-24(43)13-9-20)32(46)39-18-30(44)41-29(16-22-17-38-27-5-3-2-4-25(22)27)34(48)42-28(31(37)45)15-21-6-10-23(35)11-7-21/h2-13,17,19,26,28-29,38,43H,14-16,18,36H2,1H3,(H2,37,45)(H,39,46)(H,40,47)(H,41,44)(H,42,48)/t19-,26+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor in Wistar rat brain membranes preincubated for 1 hr measured after 1hr by scintillation counting m... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499211
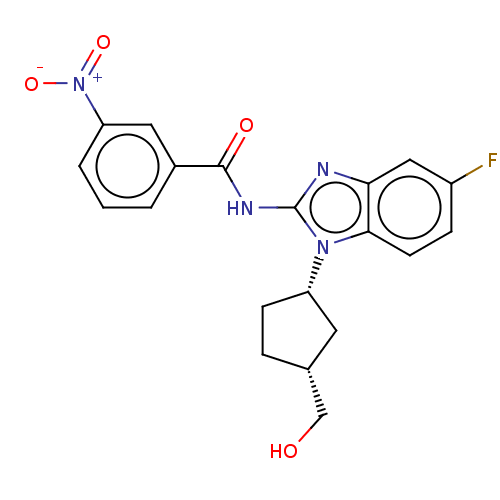
(CHEMBL3735247)Show SMILES OC[C@@H]1CC[C@@H](C1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r| Show InChI InChI=1S/C20H19FN4O4/c21-14-5-7-18-17(10-14)22-20(24(18)15-6-4-12(8-15)11-26)23-19(27)13-2-1-3-16(9-13)25(28)29/h1-3,5,7,9-10,12,15,26H,4,6,8,11H2,(H,22,23,27)/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499204
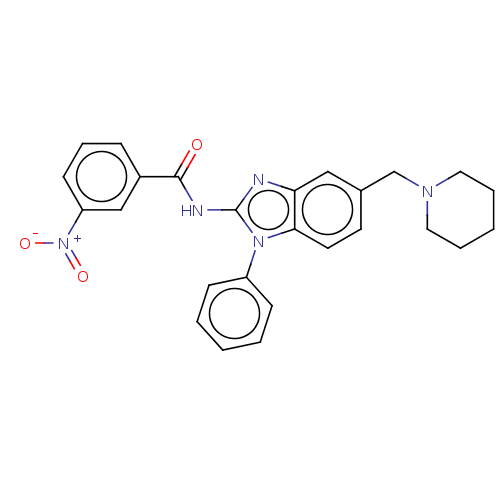
(CHEMBL3735719)Show SMILES [O-][N+](=O)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C26H25N5O3/c32-25(20-8-7-11-22(17-20)31(33)34)28-26-27-23-16-19(18-29-14-5-2-6-15-29)12-13-24(23)30(26)21-9-3-1-4-10-21/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,27,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499201

(CHEMBL3736469)Show SMILES OC[C@H]1CC[C@@H](C1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r| Show InChI InChI=1S/C20H19FN4O4/c21-14-5-7-18-17(10-14)22-20(24(18)15-6-4-12(8-15)11-26)23-19(27)13-2-1-3-16(9-13)25(28)29/h1-3,5,7,9-10,12,15,26H,4,6,8,11H2,(H,22,23,27)/t12-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499207

(CHEMBL3735975)Show SMILES OCc1ccc2n([C@H]3CC[C@H](O)CC3)c(NC(=O)c3cccc(c3)[N+]([O-])=O)nc2c1 |r,wU:7.6,wD:10.10,(-4.78,.9,;-3.72,1.53,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;3.72,-3.12,;4.09,-4.62,;2.98,-5.68,;3.28,-6.88,;1.5,-5.26,;1.13,-3.76,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,)| Show InChI InChI=1S/C21H22N4O5/c26-12-13-4-9-19-18(10-13)22-21(24(19)15-5-7-17(27)8-6-15)23-20(28)14-2-1-3-16(11-14)25(29)30/h1-4,9-11,15,17,26-27H,5-8,12H2,(H,22,23,28)/t15-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50499197

(CHEMBL3736465)Show SMILES OCCc1ccc(cc1)-n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C29H29F3N4O2/c30-29(31,32)23-6-4-5-22(18-23)27(38)34-28-33-25-17-21(19-35-14-2-1-3-15-35)9-12-26(25)36(28)24-10-7-20(8-11-24)13-16-37/h4-12,17-18,37H,1-3,13-16,19H2,(H,33,34,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK-1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50453767
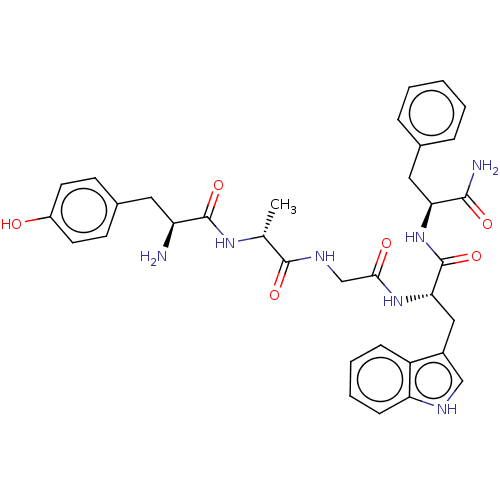
(CHEMBL4202727)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H39N7O6/c1-20(39-33(46)26(35)15-22-11-13-24(42)14-12-22)32(45)38-19-30(43)40-29(17-23-18-37-27-10-6-5-9-25(23)27)34(47)41-28(31(36)44)16-21-7-3-2-4-8-21/h2-14,18,20,26,28-29,37,42H,15-17,19,35H2,1H3,(H2,36,44)(H,38,45)(H,39,46)(H,40,43)(H,41,47)/t20-,26+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor in Wistar rat brain membranes preincubated for 1 hr measured after 1hr by scintillation counting m... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499199

(CHEMBL3735673)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C26H31N5O4/c32-22-10-8-20(9-11-22)30-24-12-7-18(17-29-13-2-1-3-14-29)15-23(24)27-26(30)28-25(33)19-5-4-6-21(16-19)31(34)35/h4-7,12,15-16,20,22,32H,1-3,8-11,13-14,17H2,(H,27,28,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50499199

(CHEMBL3735673)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C26H31N5O4/c32-22-10-8-20(9-11-22)30-24-12-7-18(17-29-13-2-1-3-14-29)15-23(24)27-26(30)28-25(33)19-5-4-6-21(16-19)31(34)35/h4-7,12,15-16,20,22,32H,1-3,8-11,13-14,17H2,(H,27,28,33)/t20-,22- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK-1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499194

(CHEMBL3734854)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33N5O4/c33-18-19-7-10-22(11-8-19)31-25-12-9-20(17-30-13-2-1-3-14-30)15-24(25)28-27(31)29-26(34)21-5-4-6-23(16-21)32(35)36/h4-6,9,12,15-16,19,22,33H,1-3,7-8,10-11,13-14,17-18H2,(H,28,29,34)/t19-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499209
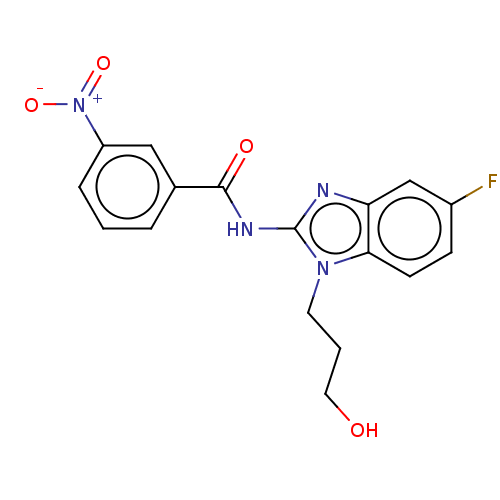
(CHEMBL3735096)Show SMILES OCCCn1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 Show InChI InChI=1S/C17H15FN4O4/c18-12-5-6-15-14(10-12)19-17(21(15)7-2-8-23)20-16(24)11-3-1-4-13(9-11)22(25)26/h1,3-6,9-10,23H,2,7-8H2,(H,19,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50499194

(CHEMBL3734854)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33N5O4/c33-18-19-7-10-22(11-8-19)31-25-12-9-20(17-30-13-2-1-3-14-30)15-24(25)28-27(31)29-26(34)21-5-4-6-23(16-21)32(35)36/h4-6,9,12,15-16,19,22,33H,1-3,7-8,10-11,13-14,17-18H2,(H,28,29,34)/t19-,22+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK-1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50499203

(CHEMBL3736036)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C28H33F3N4O2/c29-28(30,31)22-6-4-5-21(16-22)26(37)33-27-32-24-15-20(17-34-13-2-1-3-14-34)9-12-25(24)35(27)23-10-7-19(18-36)8-11-23/h4-6,9,12,15-16,19,23,36H,1-3,7-8,10-11,13-14,17-18H2,(H,32,33,37)/t19-,23+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK-1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256911

(CHEMBL4095621)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C24H30ClN7O5/c1-13(30-23(37)19(32-24(27)28)11-15-4-8-17(33)9-5-15)22(36)29-12-20(34)31-18(21(26)35)10-14-2-6-16(25)7-3-14/h2-9,13,18-19,33H,10-12H2,1H3,(H2,26,35)(H,29,36)(H,30,37)(H,31,34)(H4,27,28,32)/t13-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta-opioid receptor in Wistar rat brain membrane after 3 hrs by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499203

(CHEMBL3736036)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C28H33F3N4O2/c29-28(30,31)22-6-4-5-21(16-22)26(37)33-27-32-24-15-20(17-34-13-2-1-3-14-34)9-12-25(24)35(27)23-10-7-19(18-36)8-11-23/h4-6,9,12,15-16,19,23,36H,1-3,7-8,10-11,13-14,17-18H2,(H,32,33,37)/t19-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256916

(CHEMBL4096142)Show SMILES NC(=N)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C26H33N7O5/c27-23(36)19(13-16-5-2-1-3-6-16)31-22(35)15-30-24(37)21-7-4-12-33(21)25(38)20(32-26(28)29)14-17-8-10-18(34)11-9-17/h1-3,5-6,8-11,19-21,34H,4,7,12-15H2,(H2,27,36)(H,30,37)(H,31,35)(H4,28,29,32)/t19-,20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50499204

(CHEMBL3735719)Show SMILES [O-][N+](=O)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C26H25N5O3/c32-25(20-8-7-11-22(17-20)31(33)34)28-26-27-23-16-19(18-29-14-5-2-6-15-29)12-13-24(23)30(26)21-9-3-1-4-10-21/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,27,28,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK-1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232245
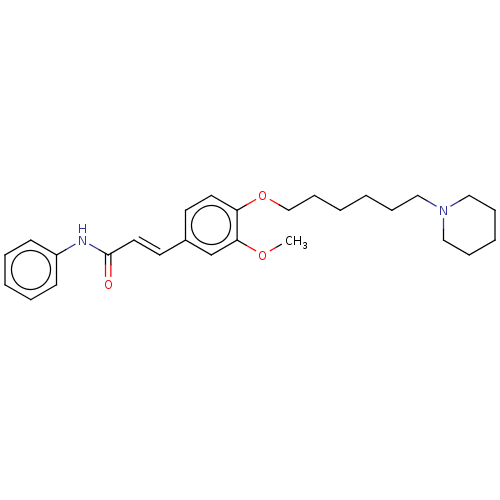
(CHEMBL4080105)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN1CCCCC1 Show InChI InChI=1S/C27H36N2O3/c1-31-26-22-23(15-17-27(30)28-24-12-6-4-7-13-24)14-16-25(26)32-21-11-3-2-8-18-29-19-9-5-10-20-29/h4,6-7,12-17,22H,2-3,5,8-11,18-21H2,1H3,(H,28,30)/b17-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Competitive inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Michaelis-Menten plot analysis |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50499198

(CHEMBL3736278)Show SMILES FC(F)(F)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C27H25F3N4O/c28-27(29,30)21-9-7-8-20(17-21)25(35)32-26-31-23-16-19(18-33-14-5-2-6-15-33)12-13-24(23)34(26)22-10-3-1-4-11-22/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,31,32,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 608 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK-1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499197

(CHEMBL3736465)Show SMILES OCCc1ccc(cc1)-n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C29H29F3N4O2/c30-29(31,32)23-6-4-5-22(18-23)27(38)34-28-33-25-17-21(19-35-14-2-1-3-15-35)9-12-26(25)36(28)24-10-7-20(8-11-24)13-16-37/h4-12,17-18,37H,1-3,13-16,19H2,(H,33,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499204

(CHEMBL3735719)Show SMILES [O-][N+](=O)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C26H25N5O3/c32-25(20-8-7-11-22(17-20)31(33)34)28-26-27-23-16-19(18-29-14-5-2-6-15-29)12-13-24(23)30(26)21-9-3-1-4-10-21/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,27,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256914

(CHEMBL4061665)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C24H31N7O5/c1-14(29-23(36)19(31-24(26)27)12-16-7-9-17(32)10-8-16)22(35)28-13-20(33)30-18(21(25)34)11-15-5-3-2-4-6-15/h2-10,14,18-19,32H,11-13H2,1H3,(H2,25,34)(H,28,35)(H,29,36)(H,30,33)(H4,26,27,31)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta-opioid receptor in Wistar rat brain membrane after 3 hrs by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50401506
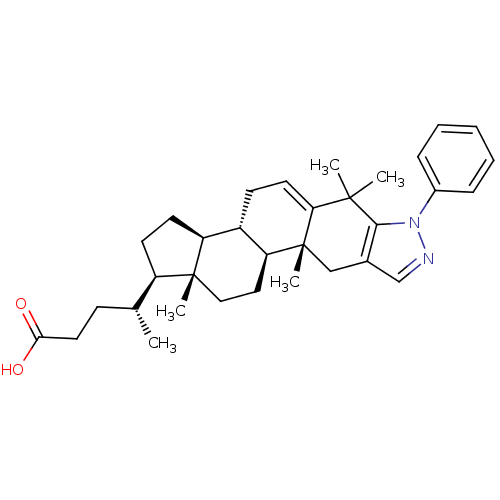
(CHEMBL2205895)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C(C)(C)c5c(C[C@]4(C)[C@H]3CC[C@]12C)cnn5-c1ccccc1 |r,t:13| Show InChI InChI=1S/C33H44N2O2/c1-21(11-16-29(36)37)25-13-14-26-24-12-15-28-31(2,3)30-22(20-34-35(30)23-9-7-6-8-10-23)19-33(28,5)27(24)17-18-32(25,26)4/h6-10,15,20-21,24-27H,11-14,16-19H2,1-5H3,(H,36,37)/t21-,24+,25-,26+,27+,32-,33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Competitive inhibition of PTP1B by para-nitrophenyl phosphate release assay |
Bioorg Med Chem Lett 22: 7237-42 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.040
BindingDB Entry DOI: 10.7270/Q2JH3NCP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232245

(CHEMBL4080105)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN1CCCCC1 Show InChI InChI=1S/C27H36N2O3/c1-31-26-22-23(15-17-27(30)28-24-12-6-4-7-13-24)14-16-25(26)32-21-11-3-2-8-18-29-19-9-5-10-20-29/h4,6-7,12-17,22H,2-3,5,8-11,18-21H2,1H3,(H,28,30)/b17-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Michaelis-Menten plot analysis |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256915

(CHEMBL4068851)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C23H28ClN5O5/c1-13(28-23(34)18(25)10-14-4-8-17(30)9-5-14)22(33)27-12-20(31)29-19(21(26)32)11-15-2-6-16(24)7-3-15/h2-9,13,18-19,30H,10-12,25H2,1H3,(H2,26,32)(H,27,33)(H,28,34)(H,29,31)/t13-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta-opioid receptor in Wistar rat brain membrane after 3 hrs by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499205

(CHEMBL3734814)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2ccc(CO)cc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.81,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-3.72,-1.53,;-3.72,-2.76,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C23H24F3N3O3/c24-23(25,26)17-3-1-2-16(11-17)21(32)28-22-27-19-9-6-15(13-31)10-20(19)29(22)18-7-4-14(12-30)5-8-18/h1-3,6,9-11,14,18,30-31H,4-5,7-8,12-13H2,(H,27,28,32)/t14-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50045060
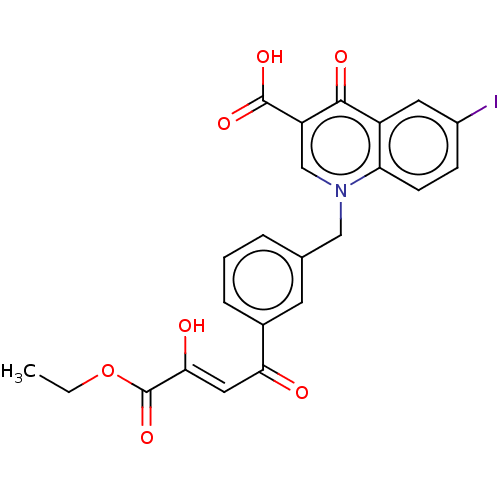
(CHEMBL3309789)Show SMILES CCOC(=O)C(\O)=C\C(=O)c1cccc(Cn2cc(C(O)=O)c(=O)c3cc(I)ccc23)c1 Show InChI InChI=1S/C23H18INO7/c1-2-32-23(31)20(27)10-19(26)14-5-3-4-13(8-14)11-25-12-17(22(29)30)21(28)16-9-15(24)6-7-18(16)25/h3-10,12,27H,2,11H2,1H3,(H,29,30)/b20-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of PTP1B (unknown origin) using pNPP as substrate by Lineweaver-Burk plot |
Bioorg Med Chem 22: 3670-83 (2014)
Article DOI: 10.1016/j.bmc.2014.05.028
BindingDB Entry DOI: 10.7270/Q2G44RX2 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50453765

(CHEMBL4215224)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C35H40ClN9O6/c1-19(42-33(50)28(45-35(38)39)15-21-8-12-24(46)13-9-21)32(49)41-18-30(47)43-29(16-22-17-40-26-5-3-2-4-25(22)26)34(51)44-27(31(37)48)14-20-6-10-23(36)11-7-20/h2-13,17,19,27-29,40,46H,14-16,18H2,1H3,(H2,37,48)(H,41,49)(H,42,50)(H,43,47)(H,44,51)(H4,38,39,45)/t19-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPDPE from delta opioid receptor in Wistar rat brain membranes preincubated for 3 hrs measured after 1 hr by scintillation count... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499195

(CHEMBL3735504)Show SMILES CC(C)(C)C(=O)Nc1ccc2n([C@@H]3CC[C@H](CO)CC3)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r,wD:12.11,15.15,(-7.45,.88,;-6.39,1.5,;-7.46,2.11,;-6.4,2.74,;-5.05,.74,;-5.04,-.49,;-3.72,1.53,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;3.72,-3.12,;4.09,-4.62,;2.98,-5.68,;3.36,-7.18,;4.54,-7.52,;1.5,-5.26,;1.13,-3.76,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,)| Show InChI InChI=1S/C27H31F3N4O3/c1-26(2,3)24(37)31-19-9-12-22-21(14-19)32-25(34(22)20-10-7-16(15-35)8-11-20)33-23(36)17-5-4-6-18(13-17)27(28,29)30/h4-6,9,12-14,16,20,35H,7-8,10-11,15H2,1-3H3,(H,31,37)(H,32,33,36)/t16-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data