 Found 13401 hits with Last Name = 'yang' and Initial = 'x'
Found 13401 hits with Last Name = 'yang' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456924
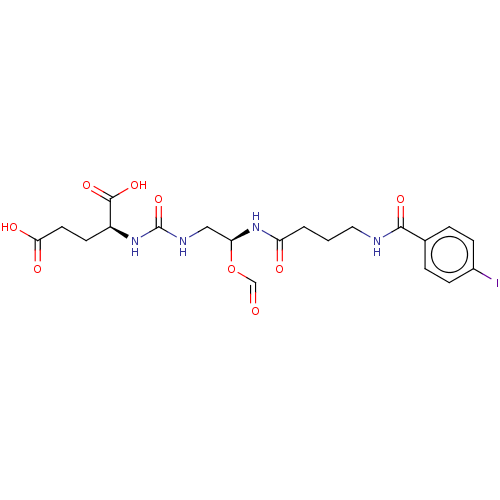
(US10736974, Compound YC-I-27)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(I)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H25IN4O9/c21-13-5-3-12(4-6-13)18(30)22-9-1-2-15(27)25-16(34-11-26)10-23-20(33)24-14(19(31)32)7-8-17(28)29/h3-6,11,14,16H,1-2,7-10H2,(H,22,30)(H,25,27)(H,28,29)(H,31,32)(H2,23,24,33)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456928
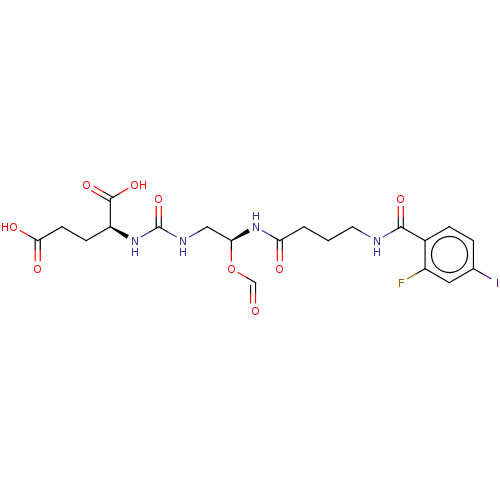
(US10736974, Compound XY-44)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(I)cc1F)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H24FIN4O9/c21-13-8-11(22)3-4-12(13)18(31)23-7-1-2-15(28)26-16(35-10-27)9-24-20(34)25-14(19(32)33)5-6-17(29)30/h3-4,8,10,14,16H,1-2,5-7,9H2,(H,23,31)(H,26,28)(H,29,30)(H,32,33)(H2,24,25,34)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50507816
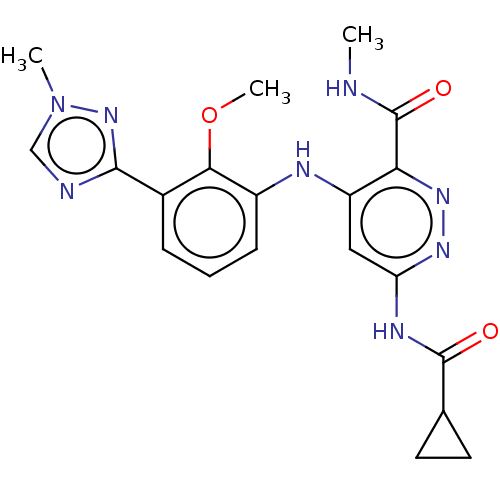
(Bms-986165 | Deucravacitinib)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1cccc(-c2ncn(C)n2)c1OC Show InChI InChI=1S/C20H22N8O3/c1-21-20(30)16-14(9-15(25-26-16)24-19(29)11-7-8-11)23-13-6-4-5-12(17(13)31-3)18-22-10-28(2)27-18/h4-6,9-11H,7-8H2,1-3H3,(H,21,30)(H2,23,24,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein labeled probe binding to His-tagged human TYK2 pseudokinase domain (575-869 residues) by Morrison titration based HTRF assa... |
J Med Chem 62: 8973-8995 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00444
BindingDB Entry DOI: 10.7270/Q2930XJS |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456930

(US10736974, Compound XY-59)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(Br)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H25BrN4O9/c21-13-5-3-12(4-6-13)18(30)22-9-1-2-15(27)25-16(34-11-26)10-23-20(33)24-14(19(31)32)7-8-17(28)29/h3-6,11,14,16H,1-2,7-10H2,(H,22,30)(H,25,27)(H,28,29)(H,31,32)(H2,23,24,33)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0269 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456923
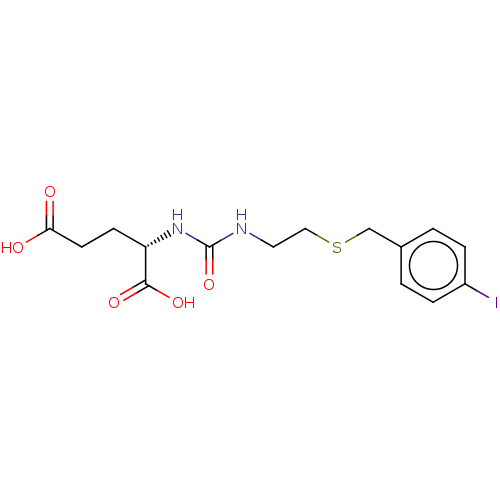
(US10736974, Compound DCIBC)Show SMILES OC(=O)CC[C@H](NC(=O)NCCSCc1ccc(I)cc1)C(O)=O |r| Show InChI InChI=1S/C15H19IN2O5S/c16-11-3-1-10(2-4-11)9-24-8-7-17-15(23)18-12(14(21)22)5-6-13(19)20/h1-4,12H,5-9H2,(H,19,20)(H,21,22)(H2,17,18,23)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456931

(US10736974, Compound XY-58)Show SMILES Cc1cc(Br)ccc1C(=O)NCCCC(=O)N[C@H](CNC(=O)N[C@@H](CCC(O)=O)C(O)=O)OC=O |r| Show InChI InChI=1S/C21H27BrN4O9/c1-12-9-13(22)4-5-14(12)19(31)23-8-2-3-16(28)26-17(35-11-27)10-24-21(34)25-15(20(32)33)6-7-18(29)30/h4-5,9,11,15,17H,2-3,6-8,10H2,1H3,(H,23,31)(H,26,28)(H,29,30)(H,32,33)(H2,24,25,34)/t15-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530394
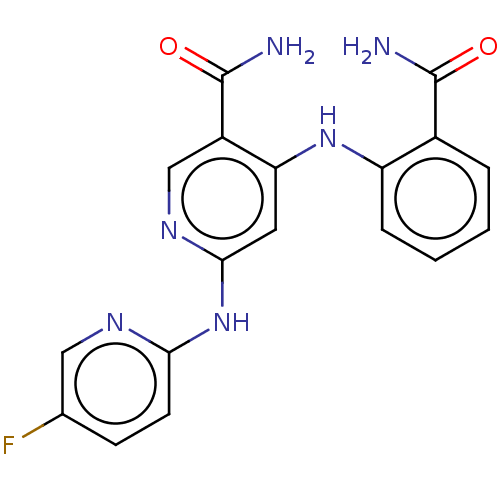
(CHEMBL4561663)Show SMILES NC(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H15FN6O2/c19-10-5-6-15(22-8-10)25-16-7-14(12(9-23-16)18(21)27)24-13-4-2-1-3-11(13)17(20)26/h1-9H,(H2,20,26)(H2,21,27)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530394

(CHEMBL4561663)Show SMILES NC(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H15FN6O2/c19-10-5-6-15(22-8-10)25-16-7-14(12(9-23-16)18(21)27)24-13-4-2-1-3-11(13)17(20)26/h1-9H,(H2,20,26)(H2,21,27)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50519523
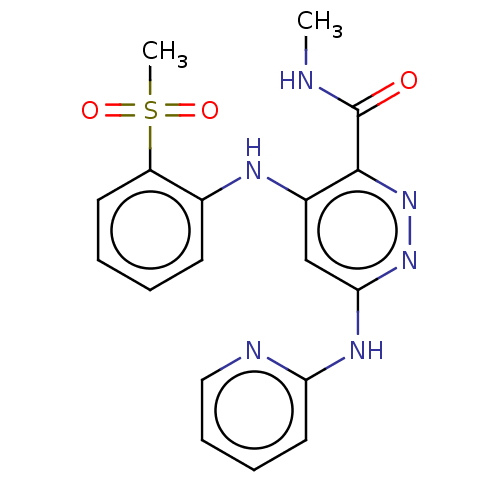
(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50519523

(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM642537
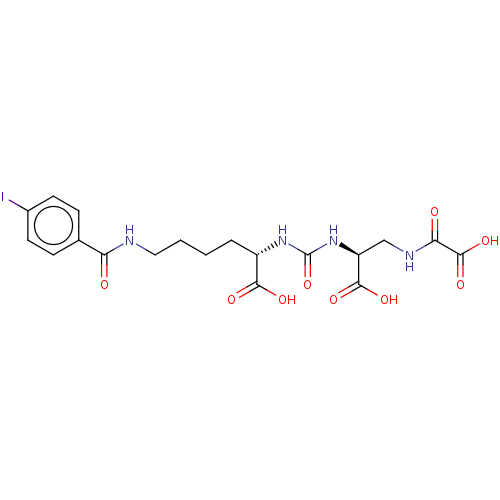
(US20230414794, Compound S2)Show SMILES OC(=O)[C@H](CCCCNC(=O)c1ccc(I)cc1)NC(=O)N[C@@H](CNC(=O)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456929
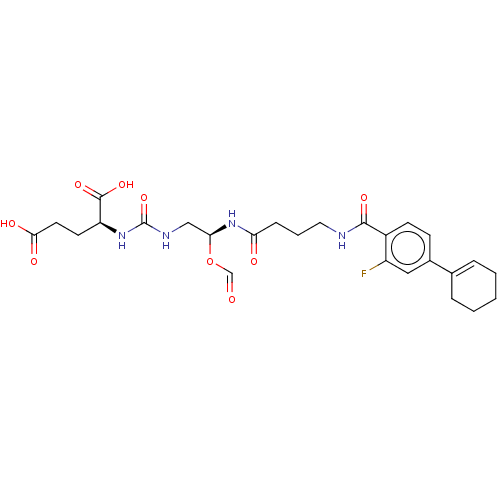
(US10736974, Compound XY-45)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(cc1F)C1=CCCCC1)OC=O)C(O)=O |r,t:29| Show InChI InChI=1S/C26H33FN4O9/c27-19-13-17(16-5-2-1-3-6-16)8-9-18(19)24(36)28-12-4-7-21(33)31-22(40-15-32)14-29-26(39)30-20(25(37)38)10-11-23(34)35/h5,8-9,13,15,20,22H,1-4,6-7,10-12,14H2,(H,28,36)(H,31,33)(H,34,35)(H,37,38)(H2,29,30,39)/t20-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50550643
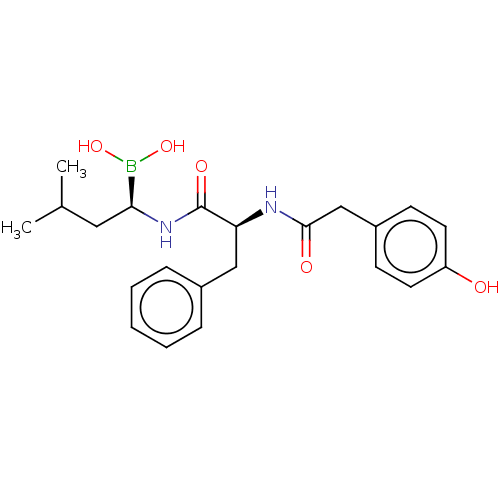
(CHEMBL4749207)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(O)cc1)B(O)O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 20S constitutive proteasome beta 5 subunit assessed as equilibrium constant using fluorogenic peptide Ac-WLA-AMC as substra... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01161
BindingDB Entry DOI: 10.7270/Q2K077W3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50546246
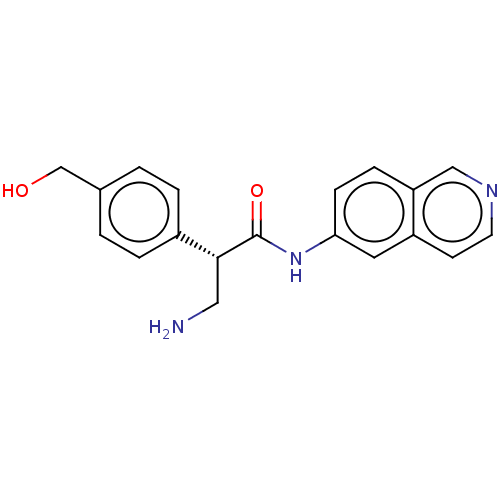
(CHEMBL4753043 | US11608319, Compound AR-13503)Show SMILES NC[C@@H](C(=O)Nc1ccc2cnccc2c1)c1ccc(CO)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50546246

(CHEMBL4753043 | US11608319, Compound AR-13503)Show SMILES NC[C@@H](C(=O)Nc1ccc2cnccc2c1)c1ccc(CO)cc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM210759

(US9290454, 4.4)Show SMILES C[C@@H](NC(=O)c1ccc2c(c1)cc(CCCCC(O)=O)n(-c1ccc(F)cc1)c2=O)c1ccc(F)cc1 Show InChI InChI=1S/C29H26F2N2O4/c1-18(19-6-9-22(30)10-7-19)32-28(36)20-8-15-26-21(16-20)17-25(4-2-3-5-27(34)35)33(29(26)37)24-13-11-23(31)12-14-24/h6-18H,2-5H2,1H3,(H,32,36)(H,34,35)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation |
Bioorg Med Chem Lett 27: 5344-5348 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.064
BindingDB Entry DOI: 10.7270/Q2HX1G7W |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456920
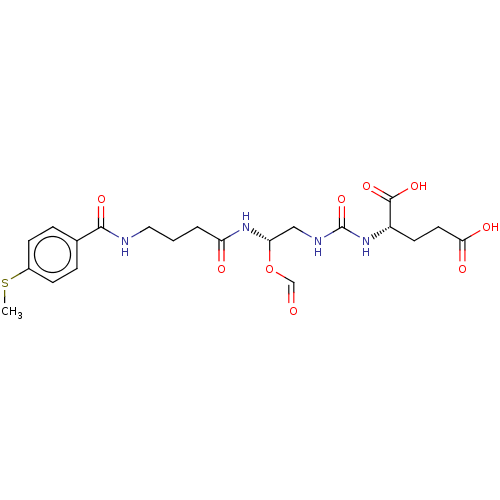
(US10736974, Entry 23)Show SMILES CSc1ccc(cc1)C(=O)NCCCC(=O)N[C@H](CNC(=O)N[C@@H](CCC(O)=O)C(O)=O)OC=O |r| Show InChI InChI=1S/C21H28N4O9S/c1-35-14-6-4-13(5-7-14)19(30)22-10-2-3-16(27)25-17(34-12-26)11-23-21(33)24-15(20(31)32)8-9-18(28)29/h4-7,12,15,17H,2-3,8-11H2,1H3,(H,22,30)(H,25,27)(H,28,29)(H,31,32)(H2,23,24,33)/t15-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456925

(US10736974, Compound YC-I-26)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(F)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H25FN4O9/c21-13-5-3-12(4-6-13)18(30)22-9-1-2-15(27)25-16(34-11-26)10-23-20(33)24-14(19(31)32)7-8-17(28)29/h3-6,11,14,16H,1-2,7-10H2,(H,22,30)(H,25,27)(H,28,29)(H,31,32)(H2,23,24,33)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456914
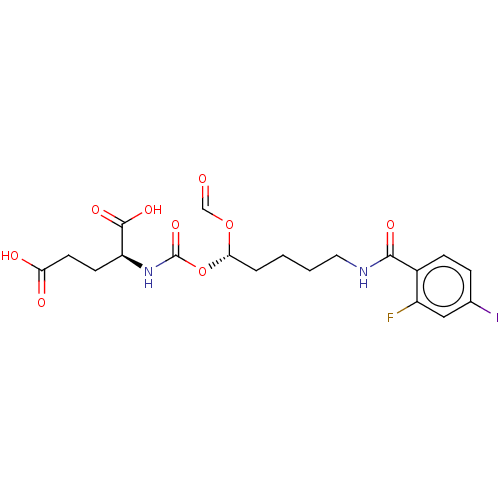
(US10736974, Compound XY-43 | US10736974, Entry 17)Show SMILES OC(=O)CC[C@H](NC(=O)O[C@@H](CCCCNC(=O)c1ccc(I)cc1F)OC=O)C(O)=O |r| Show InChI InChI=1S/C19H22FIN2O9/c20-13-9-11(21)4-5-12(13)17(27)22-8-2-1-3-16(31-10-24)32-19(30)23-14(18(28)29)6-7-15(25)26/h4-5,9-10,14,16H,1-3,6-8H2,(H,22,27)(H,23,30)(H,25,26)(H,28,29)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456914

(US10736974, Compound XY-43 | US10736974, Entry 17)Show SMILES OC(=O)CC[C@H](NC(=O)O[C@@H](CCCCNC(=O)c1ccc(I)cc1F)OC=O)C(O)=O |r| Show InChI InChI=1S/C19H22FIN2O9/c20-13-9-11(21)4-5-12(13)17(27)22-8-2-1-3-16(31-10-24)32-19(30)23-14(18(28)29)6-7-15(25)26/h4-5,9-10,14,16H,1-3,6-8H2,(H,22,27)(H,23,30)(H,25,26)(H,28,29)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456927
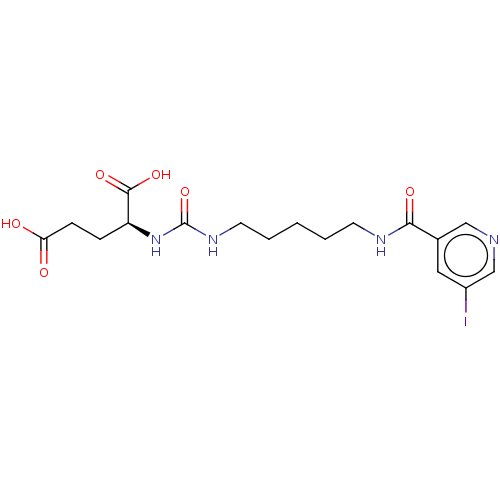
(US10736974, Compound C8)Show SMILES OC(=O)CC[C@H](NC(=O)NCCCCCNC(=O)c1cncc(I)c1)C(O)=O |r| Show InChI InChI=1S/C17H23IN4O6/c18-12-8-11(9-19-10-12)15(25)20-6-2-1-3-7-21-17(28)22-13(16(26)27)4-5-14(23)24/h8-10,13H,1-7H2,(H,20,25)(H,23,24)(H,26,27)(H2,21,22,28)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456921
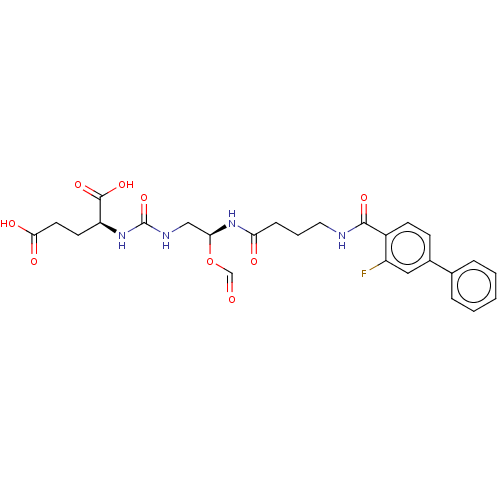
(US10736974, Entry 24)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(cc1F)-c1ccccc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C26H29FN4O9/c27-19-13-17(16-5-2-1-3-6-16)8-9-18(19)24(36)28-12-4-7-21(33)31-22(40-15-32)14-29-26(39)30-20(25(37)38)10-11-23(34)35/h1-3,5-6,8-9,13,15,20,22H,4,7,10-12,14H2,(H,28,36)(H,31,33)(H,34,35)(H,37,38)(H2,29,30,39)/t20-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM210749
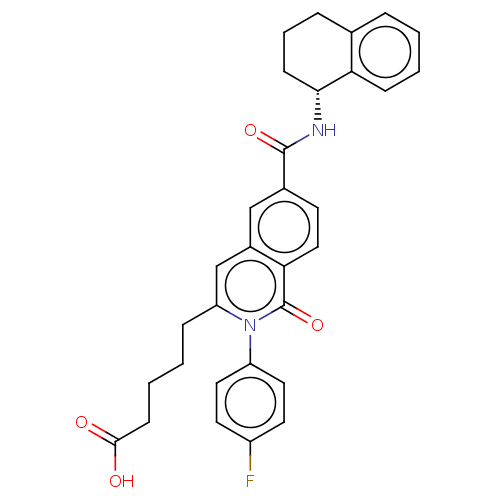
(US9290454, 3.1)Show SMILES OC(=O)CCCCc1cc2cc(ccc2c(=O)n1-c1ccc(F)cc1)C(=O)N[C@@H]1CCCc2ccccc12 Show InChI InChI=1S/C31H29FN2O4/c32-23-13-15-24(16-14-23)34-25(8-2-4-11-29(35)36)19-22-18-21(12-17-27(22)31(34)38)30(37)33-28-10-5-7-20-6-1-3-9-26(20)28/h1,3,6,9,12-19,28H,2,4-5,7-8,10-11H2,(H,33,37)(H,35,36)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation |
Bioorg Med Chem Lett 27: 5344-5348 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.064
BindingDB Entry DOI: 10.7270/Q2HX1G7W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463294

(CHEMBL4249256)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@H](C(=O)c4ccccc4)[C@]2(OC)C=C1)ccc3OC |r,wU:16.16,1.0,wD:17.38,28.36,7.7,19.23,c:37,THB:10:9:17:5.6.4,(9.78,-11.07,;9.03,-9.74,;7.65,-10.81,;5.94,-9.74,;6.72,-8.4,;5.95,-7.07,;6.71,-5.74,;8.25,-5.74,;9.79,-5.74,;9.03,-4.41,;9.78,-3.07,;11.32,-3.05,;12.66,-3.81,;12.64,-2.27,;7.47,-5.14,;7.41,-7.18,;8.25,-8.41,;9.02,-7.07,;10.56,-7.06,;11.34,-8.4,;12.88,-8.4,;13.65,-7.07,;13.65,-9.73,;12.87,-11.06,;13.64,-12.4,;15.18,-12.4,;15.95,-11.05,;15.18,-9.72,;10.59,-9.7,;11.34,-11.07,;10.57,-12.41,;9.25,-8.93,;10.35,-7.84,;4.41,-7.06,;3.64,-8.38,;4.4,-9.72,;3.62,-11.05,;2.08,-11.04,)| Show InChI InChI=1S/C31H33NO4/c1-34-23-11-10-21-16-24-29-12-13-31(35-2,22(17-29)26(33)20-6-4-3-5-7-20)28-30(29,25(21)27(23)36-28)14-15-32(24)18-19-8-9-19/h3-7,10-13,19,22,24,28H,8-9,14-18H2,1-2H3/t22-,24-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50541592
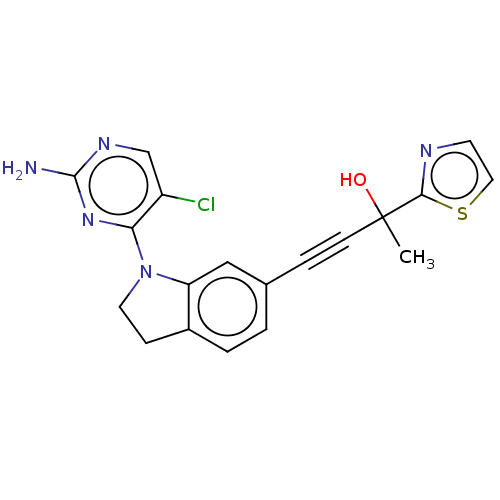
(CHEMBL3187788)Show SMILES CC(O)(C#Cc1ccc2CCN(c2c1)c1nc(N)ncc1Cl)c1nccs1 Show InChI InChI=1S/C19H16ClN5OS/c1-19(26,17-22-7-9-27-17)6-4-12-2-3-13-5-8-25(15(13)10-12)16-14(20)11-23-18(21)24-16/h2-3,7,9-11,26H,5,8H2,1H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506108

(CHEMBL4449252)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC(=O)c2ccccc2)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C37H40N2O4/c1-41-29-15-12-26-20-30-35-16-17-37(42-2,34-36(35,31(26)32(29)43-34)18-19-39(30)22-23-8-9-23)28(21-35)24-10-13-27(14-11-24)38-33(40)25-6-4-3-5-7-25/h3-7,10-15,23,28,30,34H,8-9,16-22H2,1-2H3,(H,38,40)/t28-,30-,34-,35-,36+,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
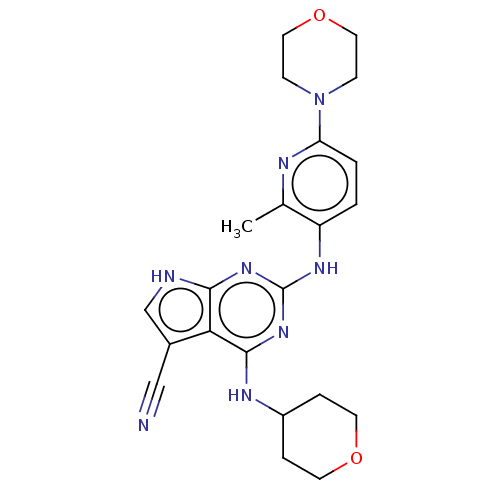
(Homo sapiens (Human)) | BDBM50512458
(CHEMBL4450240)Show SMILES Cc1nc(ccc1Nc1nc(NC2CCOCC2)c2c(c[nH]c2n1)C#N)N1CCOCC1 Show InChI InChI=1S/C22H26N8O2/c1-14-17(2-3-18(25-14)30-6-10-32-11-7-30)27-22-28-20-19(15(12-23)13-24-20)21(29-22)26-16-4-8-31-9-5-16/h2-3,13,16H,4-11H2,1H3,(H3,24,26,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 (510 to 857 residues) catalytic domain (unknown origin) expressed in Escherichia coli after 15 mins by mass-spectrometry analysis |
Eur J Med Chem 175: 247-268 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.047
BindingDB Entry DOI: 10.7270/Q21V5J8N |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
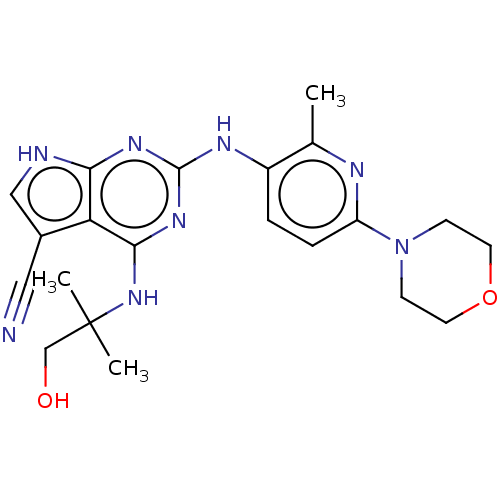
(Homo sapiens (Human)) | BDBM50512455
(CHEMBL4436510)Show SMILES Cc1nc(ccc1Nc1nc(NC(C)(C)CO)c2c(c[nH]c2n1)C#N)N1CCOCC1 Show InChI InChI=1S/C21H26N8O2/c1-13-15(4-5-16(24-13)29-6-8-31-9-7-29)25-20-26-18-17(14(10-22)11-23-18)19(27-20)28-21(2,3)12-30/h4-5,11,30H,6-9,12H2,1-3H3,(H3,23,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 (510 to 857 residues) catalytic domain (unknown origin) expressed in Escherichia coli after 15 mins by mass-spectrometry analysis |
Eur J Med Chem 175: 247-268 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.047
BindingDB Entry DOI: 10.7270/Q21V5J8N |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.545 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50368791
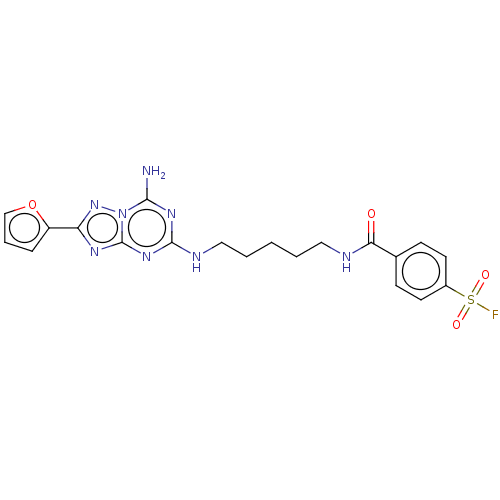
(CHEMBL4171931)Show SMILES Nc1nc(NCCCCCNC(=O)c2ccc(cc2)S(F)(=O)=O)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C20H21FN8O4S/c21-34(31,32)14-8-6-13(7-9-14)17(30)23-10-2-1-3-11-24-19-26-18(22)29-20(27-19)25-16(28-29)15-5-4-12-33-15/h4-9,12H,1-3,10-11H2,(H,23,30)(H3,22,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 human A2AR (1 to 316 residues) expressed in Pichia pastoris SMD1168 cell membranes incubated for 3 hrs |
J Med Chem 61: 7892-7901 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00860
BindingDB Entry DOI: 10.7270/Q2RF5XJ5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50369890
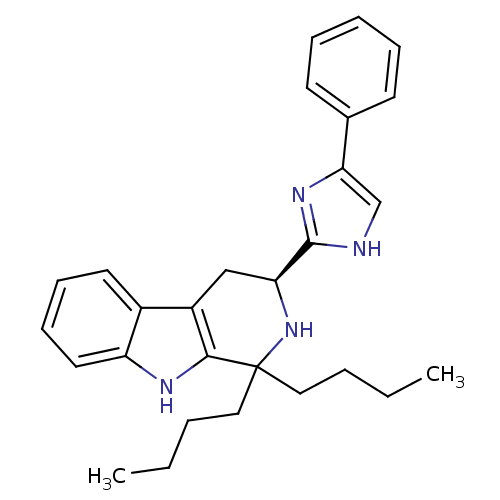
(CHEMBL1237140 | CHEMBL1788167)Show SMILES CCCCC1(CCCC)N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.679 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530395
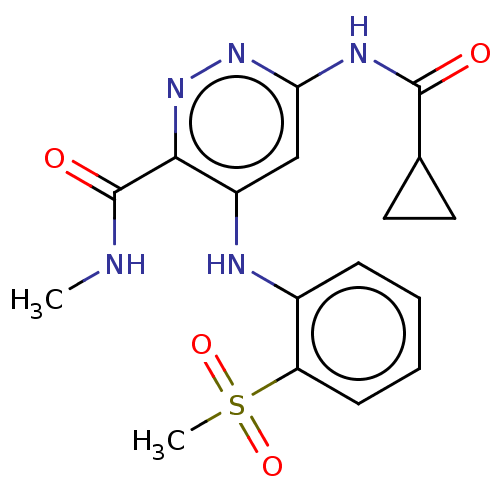
(CHEMBL4444178)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C17H19N5O4S/c1-18-17(24)15-12(9-14(21-22-15)20-16(23)10-7-8-10)19-11-5-3-4-6-13(11)27(2,25)26/h3-6,9-10H,7-8H2,1-2H3,(H,18,24)(H2,19,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530395

(CHEMBL4444178)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C17H19N5O4S/c1-18-17(24)15-12(9-14(21-22-15)20-16(23)10-7-8-10)19-11-5-3-4-6-13(11)27(2,25)26/h3-6,9-10H,7-8H2,1-2H3,(H,18,24)(H2,19,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021074

(CHEMBL3287628)Show SMILES Clc1ccc(CN[C@H]2CCCC[C@H]2NC(=O)c2ccc3ccccc3c2)cc1Cl |r| Show InChI InChI=1S/C24H24Cl2N2O/c25-20-12-9-16(13-21(20)26)15-27-22-7-3-4-8-23(22)28-24(29)19-11-10-17-5-1-2-6-18(17)14-19/h1-2,5-6,9-14,22-23,27H,3-4,7-8,15H2,(H,28,29)/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50520722
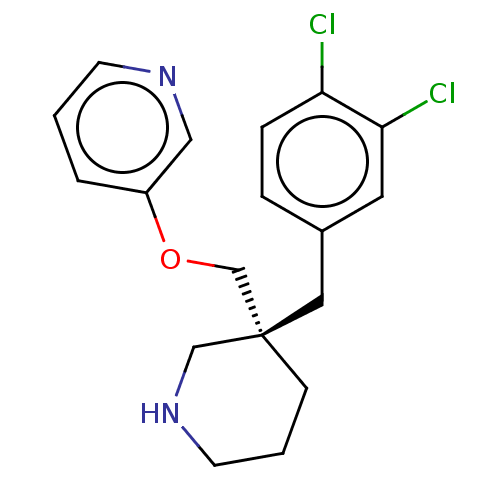
(CHEMBL4554047)Show SMILES Clc1ccc(C[C@@]2(COc3cccnc3)CCCNC2)cc1Cl |r| Show InChI InChI=1S/C18H20Cl2N2O/c19-16-5-4-14(9-17(16)20)10-18(6-2-8-22-12-18)13-23-15-3-1-7-21-11-15/h1,3-5,7,9,11,22H,2,6,8,10,12-13H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Lymnaea stagnalis acetylcholine-binding protein incubated for 60 mins followed by 3 hrs incubation in dark condi... |
Eur J Med Chem 160: 37-48 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.073
BindingDB Entry DOI: 10.7270/Q2QV3QXP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50368789

(CHEMBL4164058)Show SMILES Nc1nc(NCCCCNC(=O)c2ccc(cc2)S(F)(=O)=O)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H19FN8O4S/c20-33(30,31)13-7-5-12(6-8-13)16(29)22-9-1-2-10-23-18-25-17(21)28-19(26-18)24-15(27-28)14-4-3-11-32-14/h3-8,11H,1-2,9-10H2,(H,22,29)(H3,21,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 human A2AR (1 to 316 residues) expressed in Pichia pastoris SMD1168 cell membranes incubated for 3 hrs |
J Med Chem 61: 7892-7901 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00860
BindingDB Entry DOI: 10.7270/Q2RF5XJ5 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456906
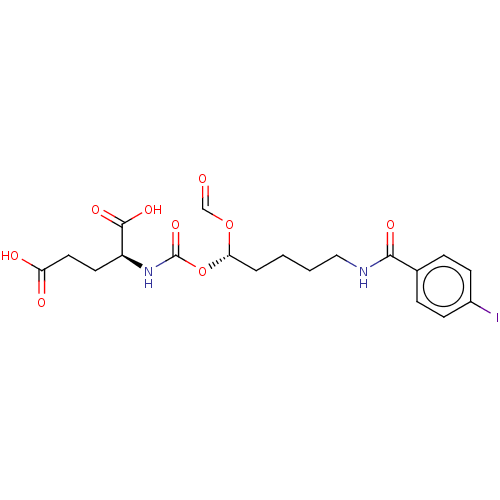
(US10736974, Compound XY-26 | US10736974, Entry 9)Show SMILES OC(=O)CC[C@H](NC(=O)O[C@@H](CCCCNC(=O)c1ccc(I)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C19H23IN2O9/c20-13-6-4-12(5-7-13)17(26)21-10-2-1-3-16(30-11-23)31-19(29)22-14(18(27)28)8-9-15(24)25/h4-7,11,14,16H,1-3,8-10H2,(H,21,26)(H,22,29)(H,24,25)(H,27,28)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456906

(US10736974, Compound XY-26 | US10736974, Entry 9)Show SMILES OC(=O)CC[C@H](NC(=O)O[C@@H](CCCCNC(=O)c1ccc(I)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C19H23IN2O9/c20-13-6-4-12(5-7-13)17(26)21-10-2-1-3-16(30-11-23)31-19(29)22-14(18(27)28)8-9-15(24)25/h4-7,11,14,16H,1-3,8-10H2,(H,21,26)(H,22,29)(H,24,25)(H,27,28)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50515430
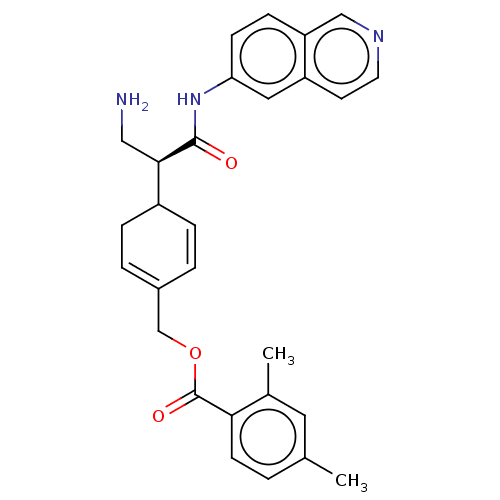
(NETARSUDIL | US11608319, Compound AR-13324)Show SMILES Cc1ccc(C(=O)OCC2=CCC(C=C2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r,c:13,t:9| Show InChI InChI=1S/C28H29N3O3/c1-18-3-10-25(19(2)13-18)28(33)34-17-20-4-6-21(7-5-20)26(15-29)27(32)31-24-9-8-23-16-30-12-11-22(23)14-24/h3-6,8-14,16,21,26H,7,15,17,29H2,1-2H3,(H,31,32)/t21?,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50515430

(NETARSUDIL | US11608319, Compound AR-13324)Show SMILES Cc1ccc(C(=O)OCC2=CCC(C=C2)[C@@H](CN)C(=O)Nc2ccc3cnccc3c2)c(C)c1 |r,c:13,t:9| Show InChI InChI=1S/C28H29N3O3/c1-18-3-10-25(19(2)13-18)28(33)34-17-20-4-6-21(7-5-20)26(15-29)27(32)31-24-9-8-23-16-30-12-11-22(23)14-24/h3-6,8-14,16,21,26H,7,15,17,29H2,1-2H3,(H,31,32)/t21?,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02190
BindingDB Entry DOI: 10.7270/Q2BV7MPD |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM510127
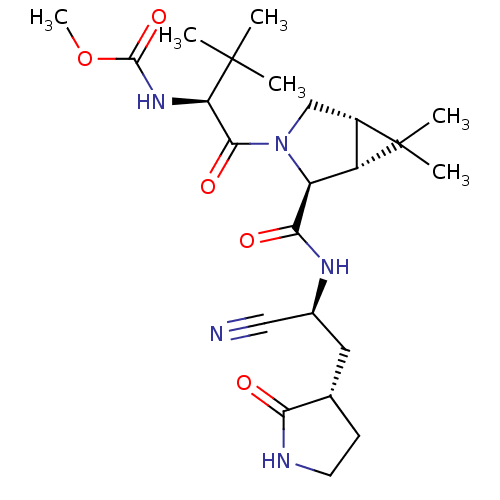
(Methyl {(2S)-1-[(1R,2S,5S)-2-({(1S)-1-cyano-2-[(3S...)Show SMILES COC(=O)N[C@H](C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C)C(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2222Z08 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data