

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435960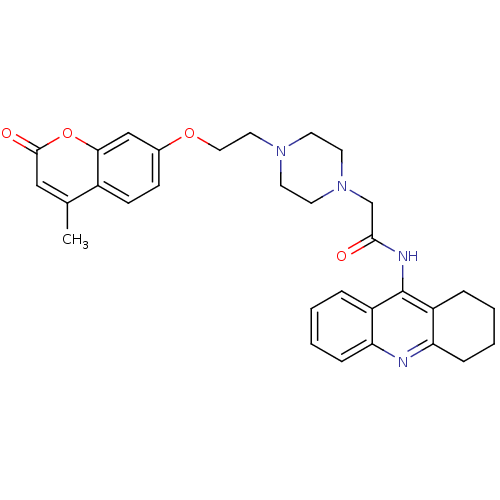 (CHEMBL2391486) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469009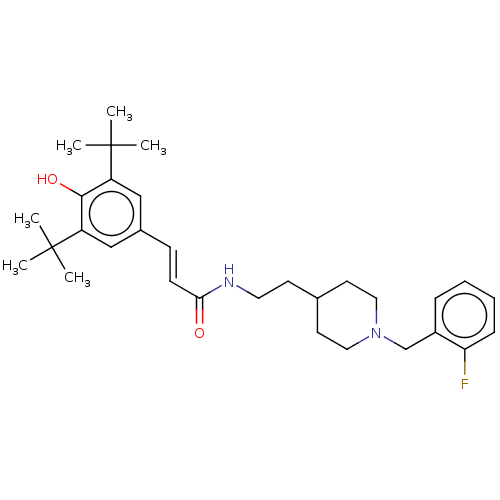 (CHEMBL4292766) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469008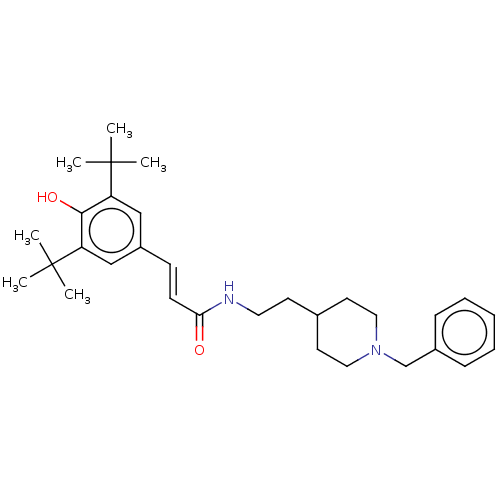 (CHEMBL4283390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099601 (CHEMBL3343930) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073116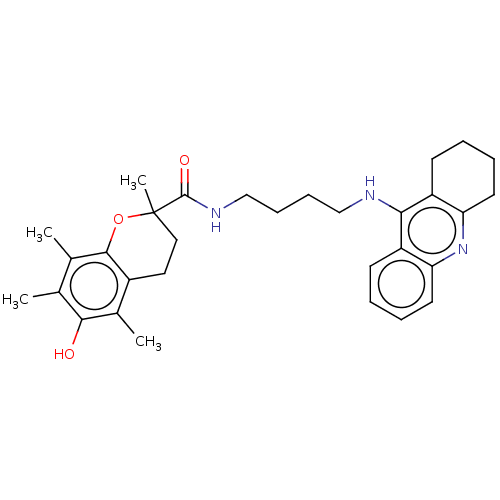 (CHEMBL3410952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099603 (CHEMBL3343932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099597 (CHEMBL3343926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756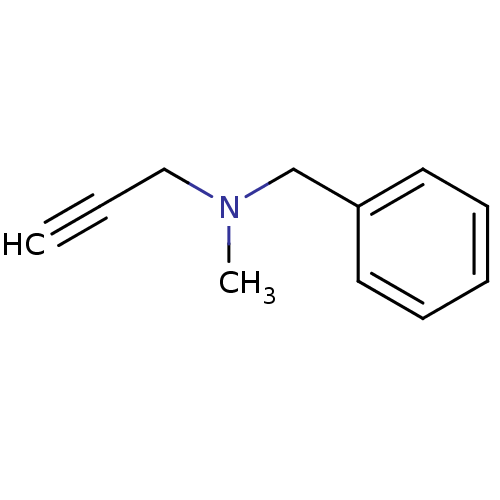 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-B (unknown origin) | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441841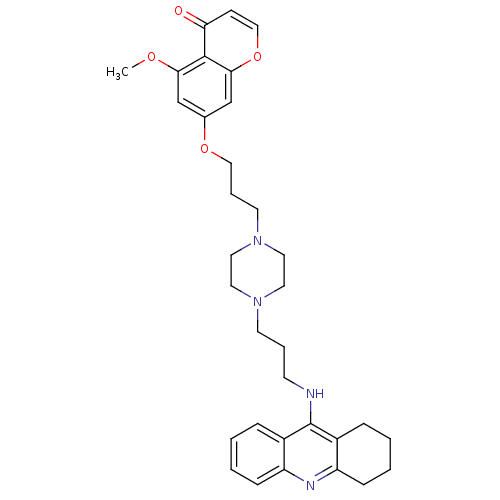 (CHEMBL2436691) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073114 (CHEMBL3410954) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073115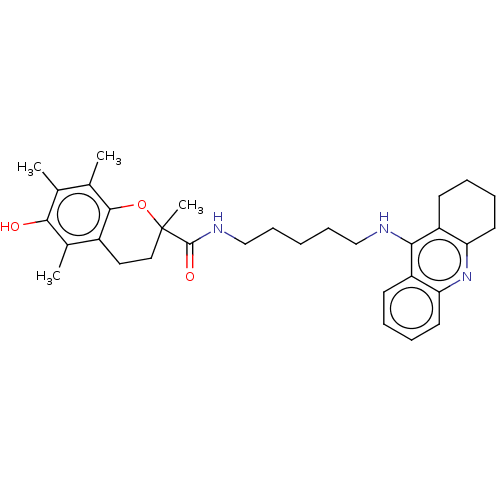 (CHEMBL3410953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441840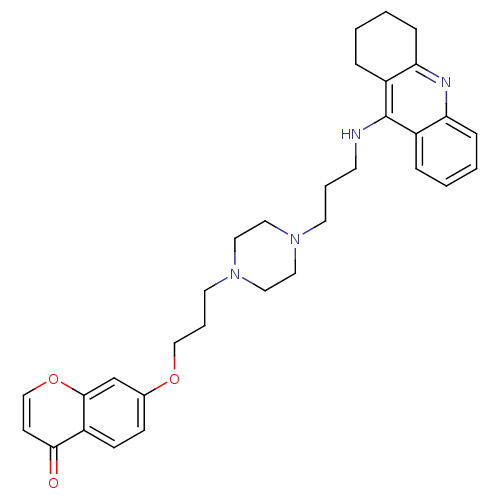 (CHEMBL2436692) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 180... | Eur J Med Chem 139: 68-83 (2017) Article DOI: 10.1016/j.ejmech.2017.07.077 BindingDB Entry DOI: 10.7270/Q2Q52S78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099602 (CHEMBL3343931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50172756 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-A (unknown origin) | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441842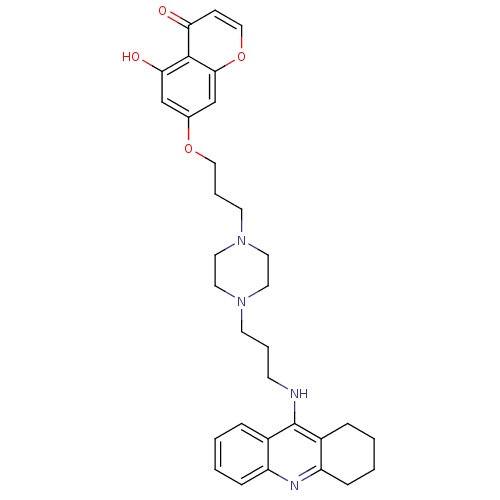 (CHEMBL2436690) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073116 (CHEMBL3410952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073113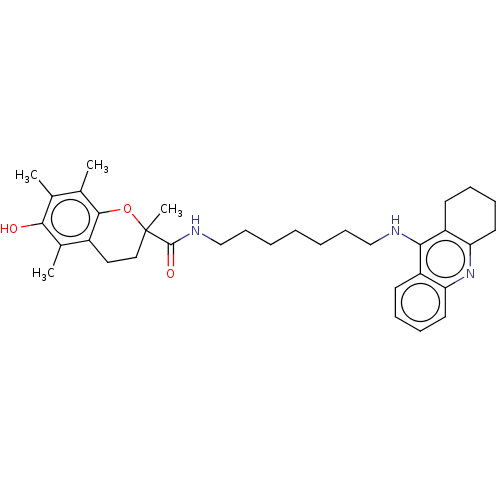 (CHEMBL3410955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099598 (CHEMBL3343927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099599 (CHEMBL3343928) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073114 (CHEMBL3410954) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50441840 (CHEMBL2436692) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylcholinesterase iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans ... | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456663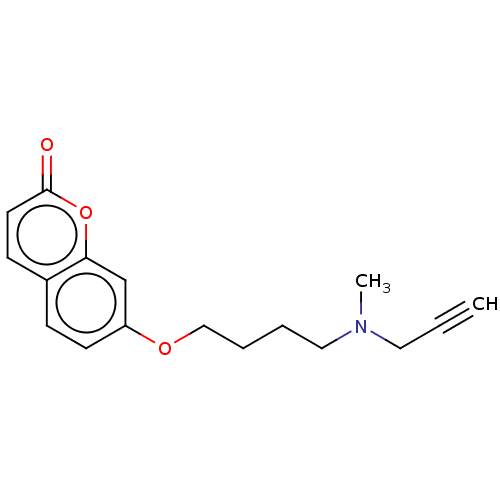 (CHEMBL4215298) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by... | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BCHE preincubated for 6 mins followed by addition of S-butyrylcholine iodide as substrate by Ellman's method | Bioorg Med Chem 24: 4324-4338 (2016) Article DOI: 10.1016/j.bmc.2016.07.025 BindingDB Entry DOI: 10.7270/Q2DV1MV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099591 (CHEMBL3343922) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204751 (CHEMBL3943450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 6 mins followed by substrate addition and me... | Citation and Details Article DOI: 10.1039/c9md00441f BindingDB Entry DOI: 10.7270/Q270852S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50553084 (CHEMBL4798359) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 6 mins followed by substrate addition and me... | Citation and Details Article DOI: 10.1039/c9md00441f BindingDB Entry DOI: 10.7270/Q270852S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073112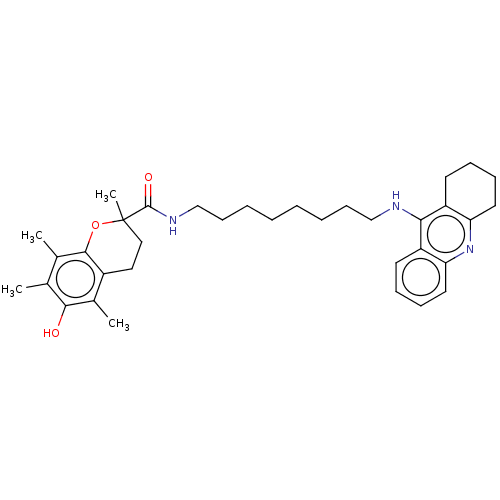 (CHEMBL3410956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50441841 (CHEMBL2436691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylcholinesterase iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans ... | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50553077 (CHEMBL4760340) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate preincubated with enzyme for 15 mins followed incubation with substrate for 20 mi... | Citation and Details Article DOI: 10.1039/c9md00441f BindingDB Entry DOI: 10.7270/Q270852S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073115 (CHEMBL3410953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10949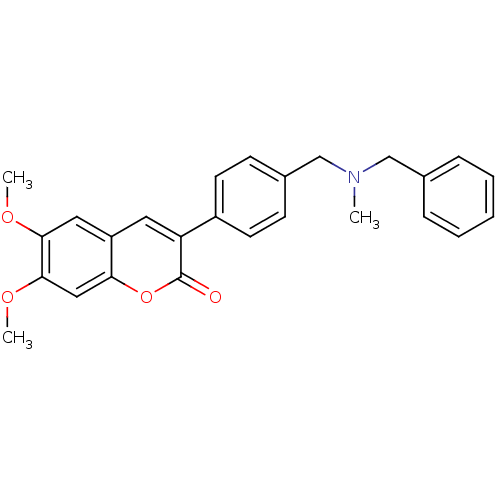 (3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 180... | Eur J Med Chem 139: 68-83 (2017) Article DOI: 10.1016/j.ejmech.2017.07.077 BindingDB Entry DOI: 10.7270/Q2Q52S78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099598 (CHEMBL3343927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel ACHE preincubated for 6 mins followed by addition of acetylcholine iodide as substrate by Ellman's method | Bioorg Med Chem 24: 4324-4338 (2016) Article DOI: 10.1016/j.bmc.2016.07.025 BindingDB Entry DOI: 10.7270/Q2DV1MV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441844 (CHEMBL2436709) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099570 (CHEMBL3343885) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073117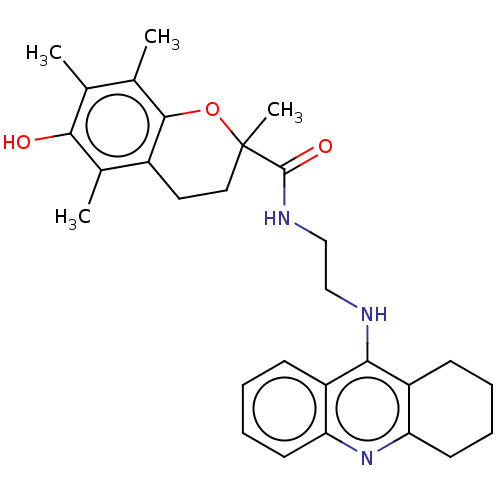 (CHEMBL3410951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099591 (CHEMBL3343922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204754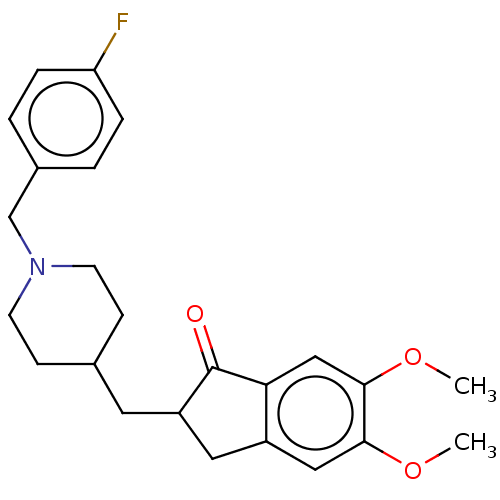 (CHEMBL3985059) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using s-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204751 (CHEMBL3943450) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50441842 (CHEMBL2436690) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylcholinesterase iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans ... | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using s-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 924 total ) | Next | Last >> |