

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351401 (CHEMBL1819091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351399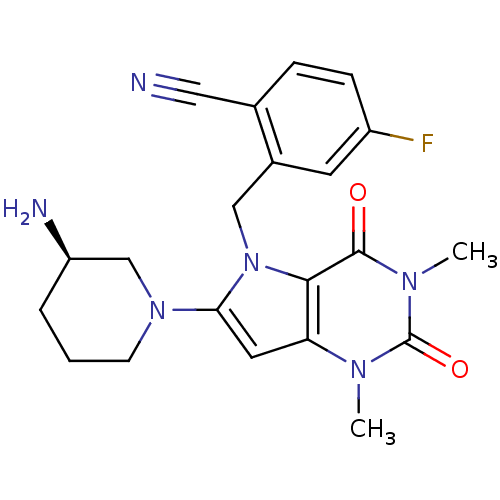 (CHEMBL1819089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278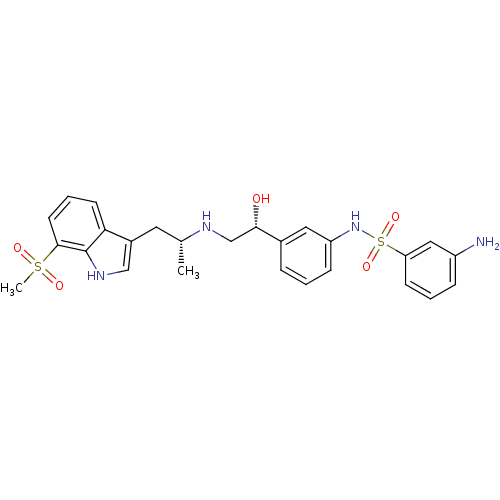 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267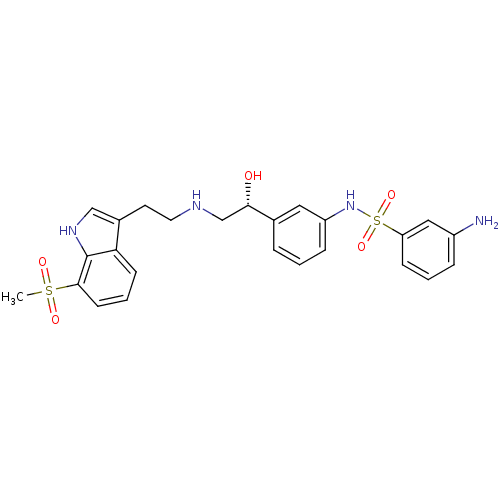 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring cAMP accumulation in CHO cells expressing cloned human Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250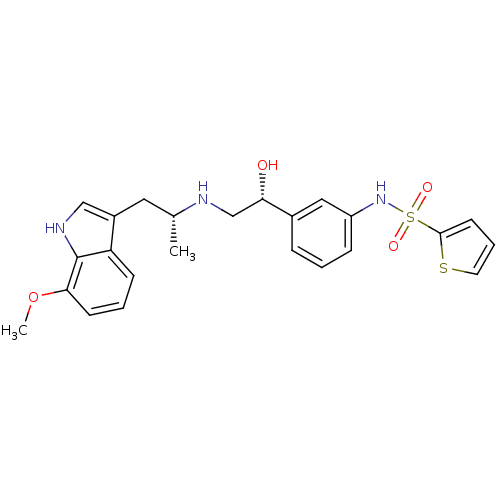 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257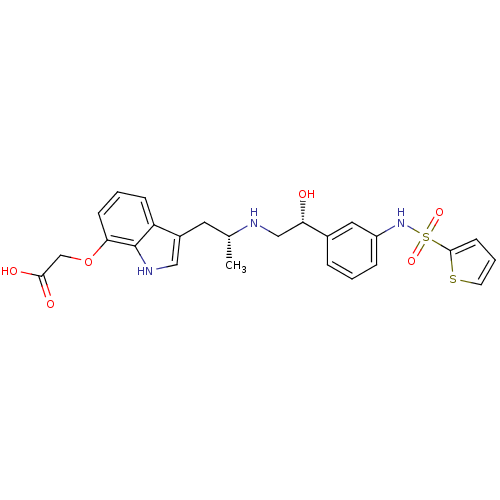 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252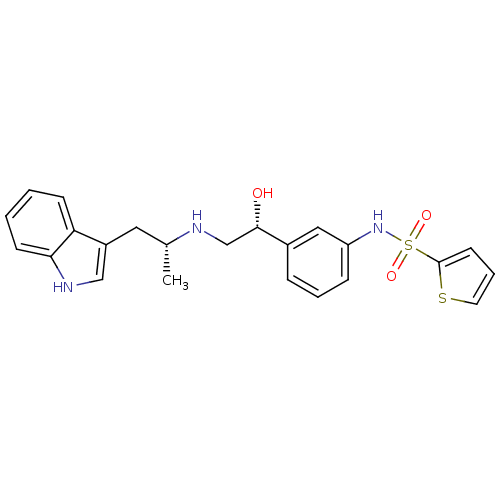 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270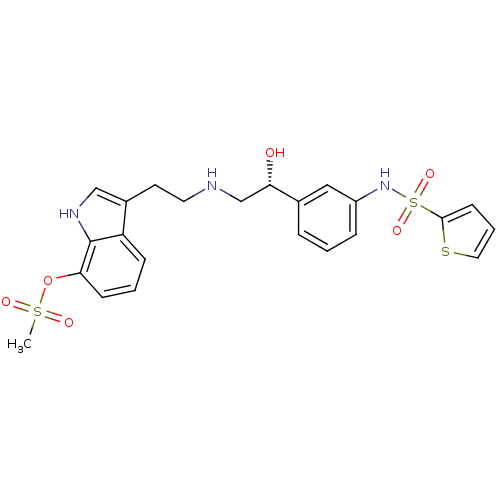 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP9 | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP8 | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50318885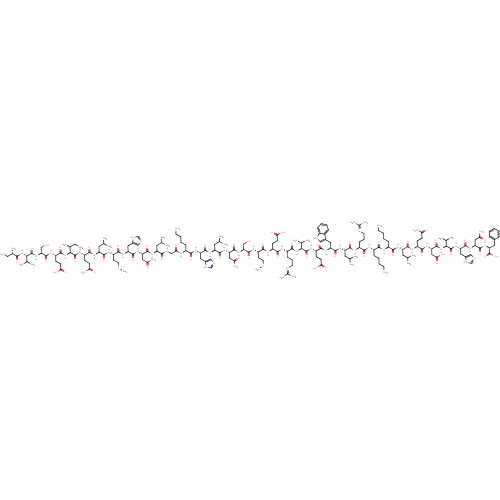 (CHEMBL525610 | teriparatide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of 125I-PTH (1 to 15 residues) from human PTHR1 expressed in African green monkey COS7 cell membranes at 300 uM after 90 mins by gamma c... | J Med Chem 61: 5949-5962 (2018) Article DOI: 10.1021/acs.jmedchem.8b00182 BindingDB Entry DOI: 10.7270/Q2ZC85GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50351399 (CHEMBL1819089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391433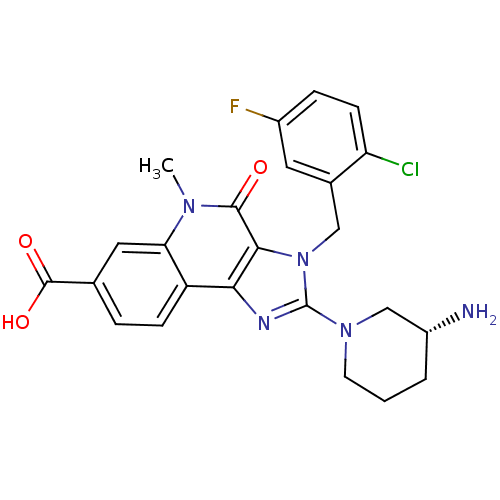 (CHEMBL2147070) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391470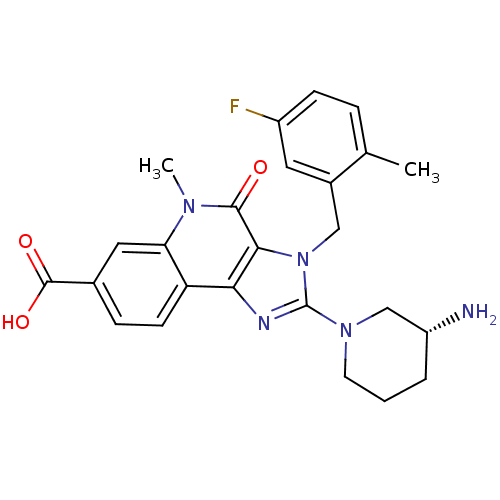 (CHEMBL2147056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM16285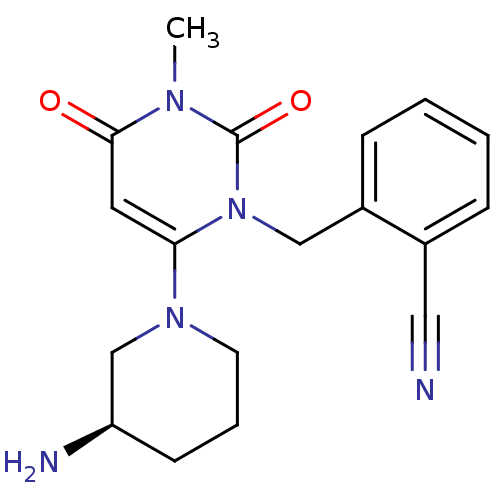 (2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50351402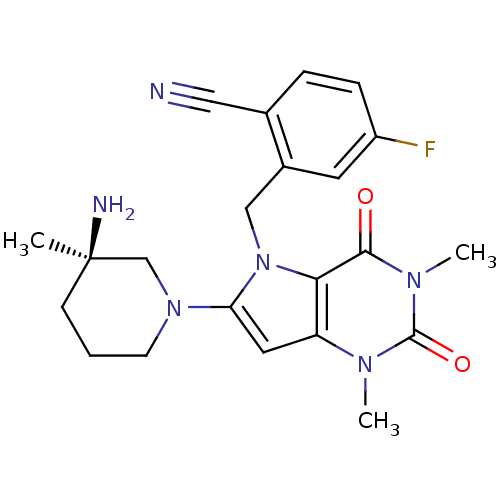 (CHEMBL1819090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391443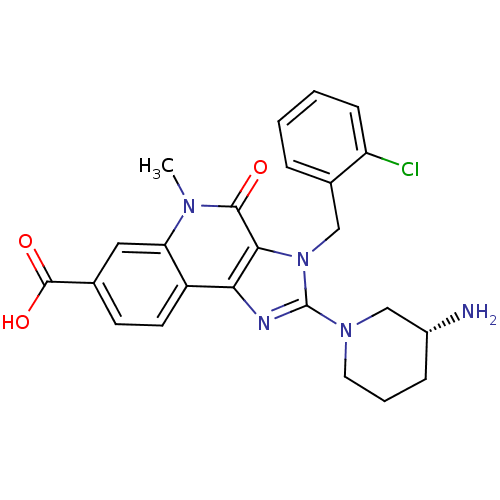 (CHEMBL2147073) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50225074 ((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391460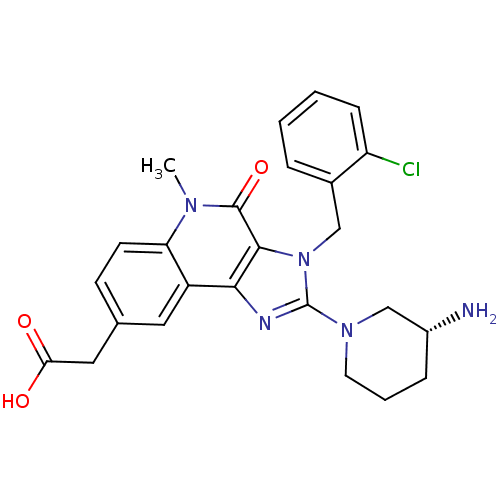 (CHEMBL2147083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391449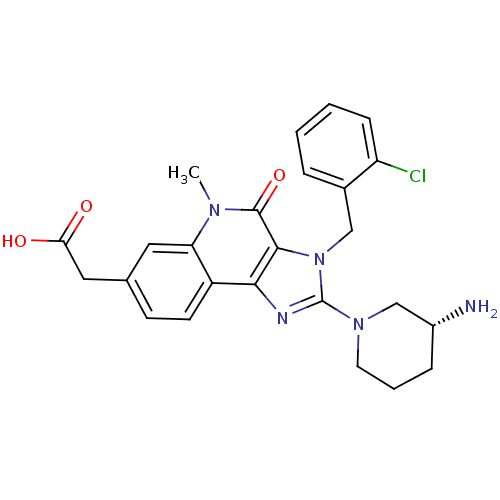 (CHEMBL2147074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM16285 (2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391478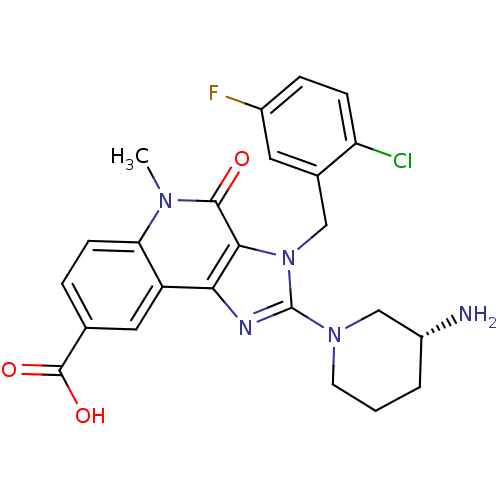 (CHEMBL2147069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391453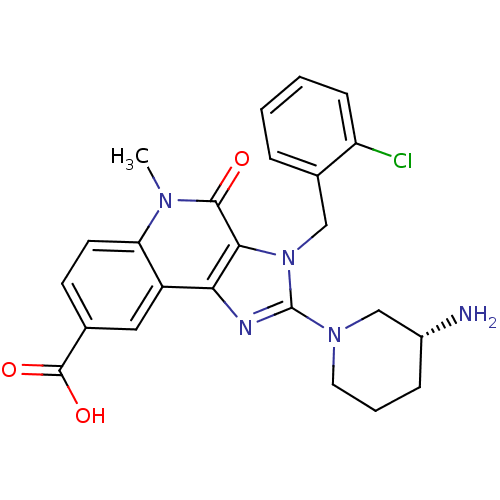 (CHEMBL2147082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391442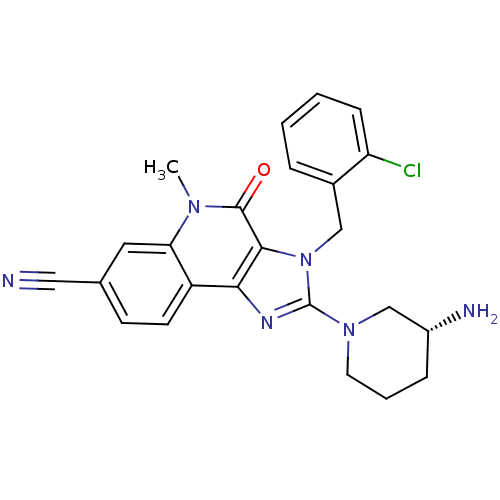 (CHEMBL2147072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391480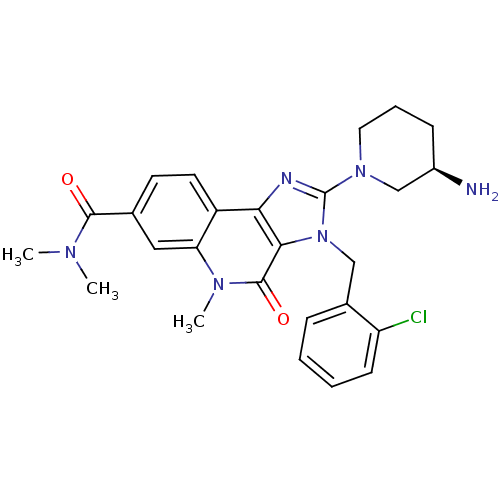 (CHEMBL2147071) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391463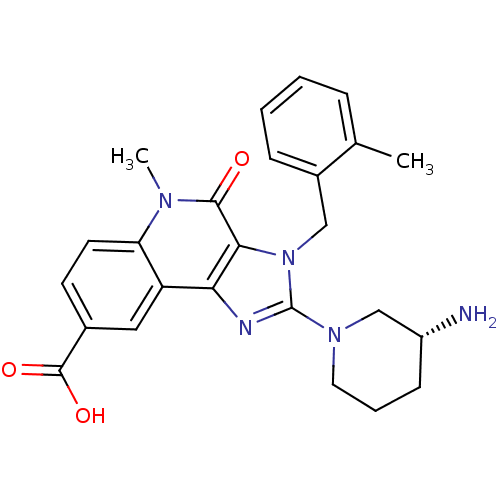 (CHEMBL2147058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391479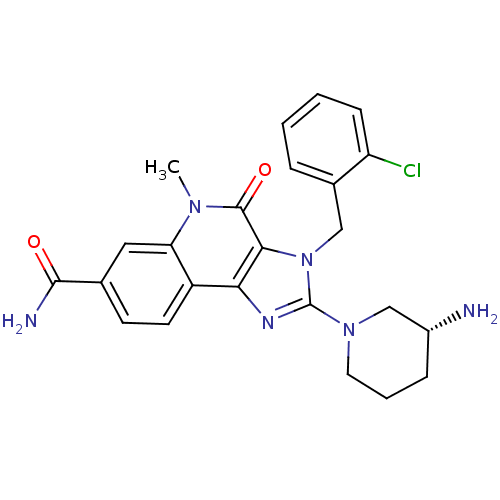 (CHEMBL2147196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391471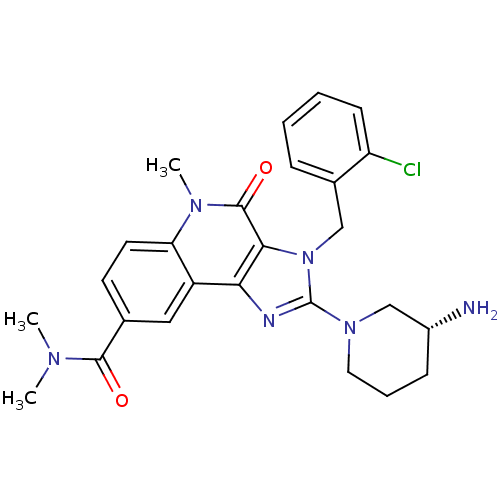 (CHEMBL2147081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis | Bioorg Med Chem 20: 5864-83 (2012) Article DOI: 10.1016/j.bmc.2012.07.046 BindingDB Entry DOI: 10.7270/Q2FX7BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 486 total ) | Next | Last >> |