

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351401 (CHEMBL1819091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351399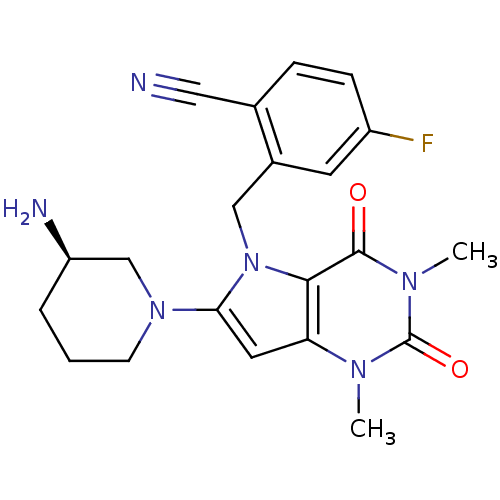 (CHEMBL1819089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145527 ((S)-5-{4-[3-(5-Fluoro-1,4,5,6-tetrahydro-pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145533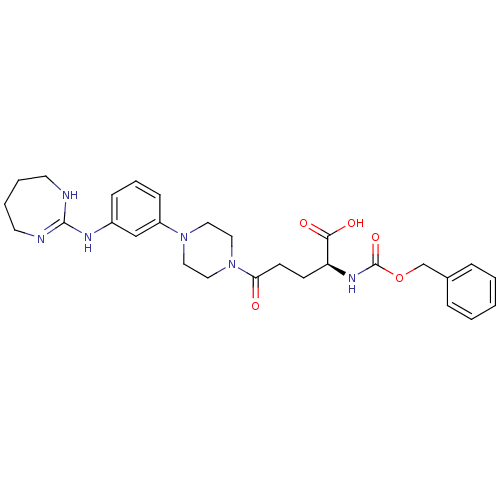 ((S)-2-Benzyloxycarbonylamino-5-oxo-5-{4-[3-(4,5,6,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of binding to human alphaV-beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145528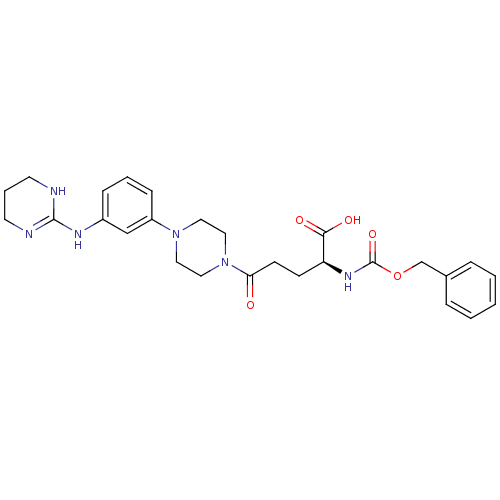 ((S)-2-Benzyloxycarbonylamino-5-oxo-5-{4-[3-(1,4,5,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145530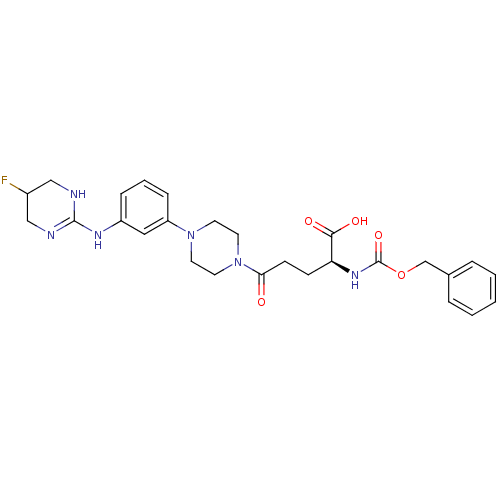 ((S)-2-Benzyloxycarbonylamino-5-{4-[3-(5-fluoro-1,4...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145526 ((S)-2-Benzyloxycarbonylamino-5-{4-[3-(5-hydroxy-1,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145525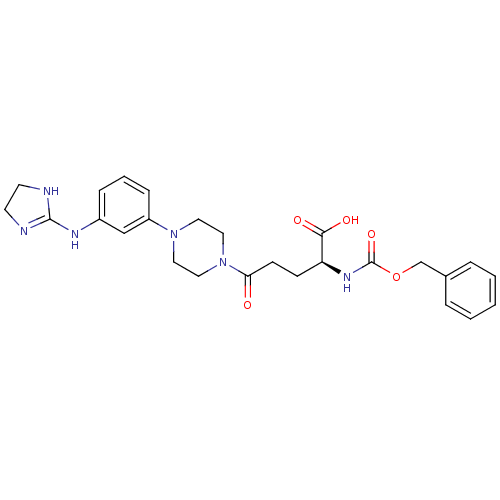 (2-Benzyloxycarbonylamino-5-{4-[3-(4,5-dihydro-1H-i...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of binding to human alphaV-beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145523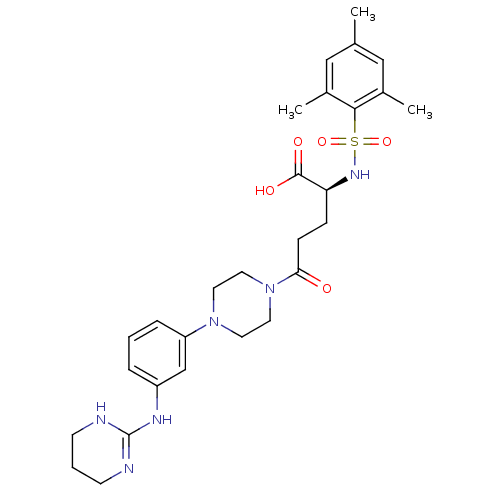 ((S)-5-Oxo-5-{4-[3-(1,4,5,6-tetrahydro-pyrimidin-2-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145522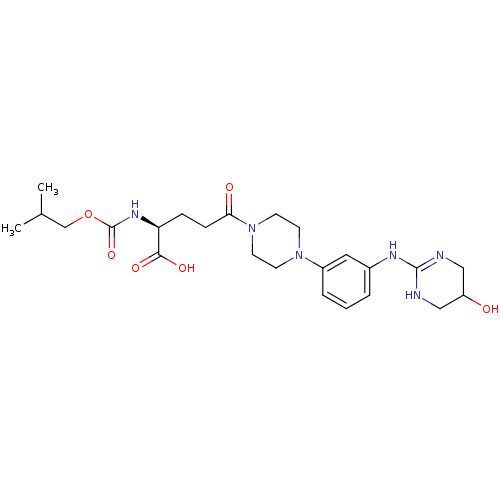 ((S)-5-{4-[3-(5-Hydroxy-1,4,5,6-tetrahydro-pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145529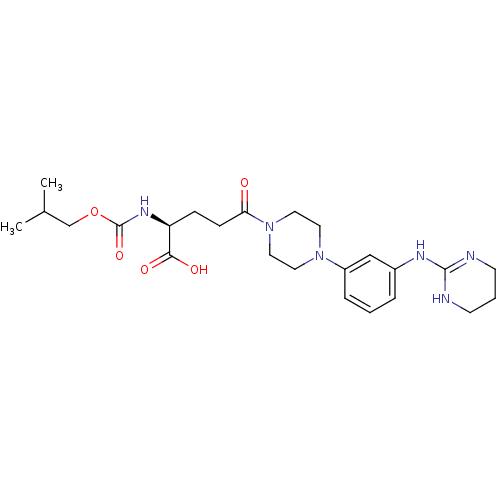 ((S)-2-Isobutoxycarbonylamino-5-oxo-5-{4-[3-(1,4,5,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50351399 (CHEMBL1819089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145532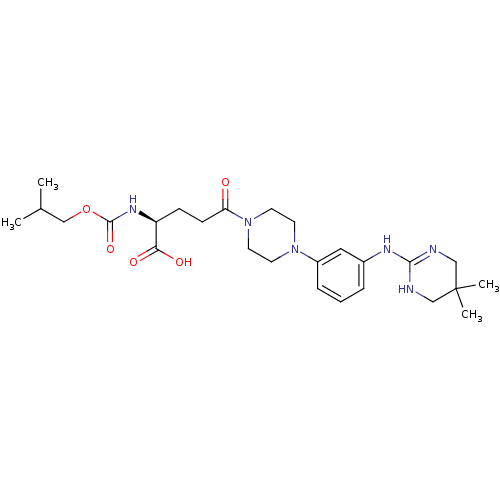 ((S)-5-{4-[3-(5,5-Dimethyl-1,4,5,6-tetrahydro-pyrim...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280297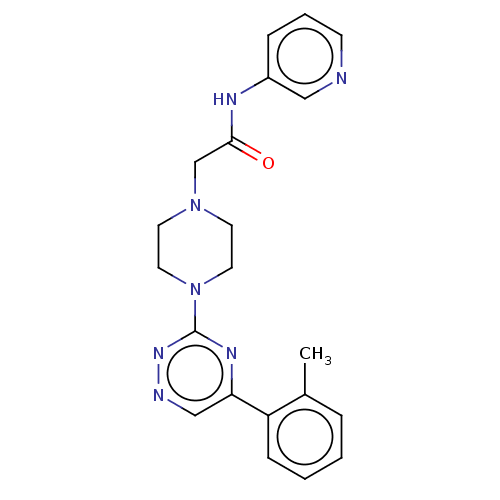 (US10029993, Example 86) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145524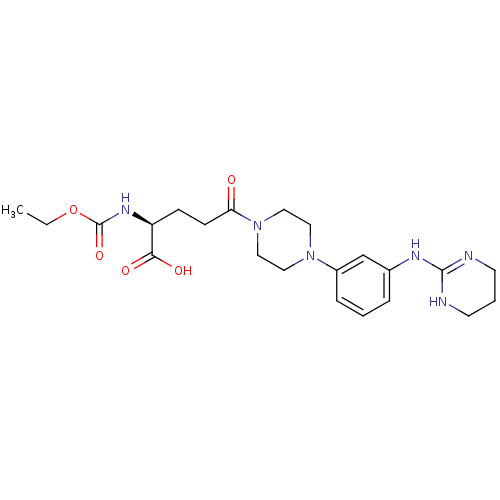 ((S)-2-Ethoxycarbonylamino-5-oxo-5-{4-[3-(1,4,5,6-t...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280316 (US10029993, Example 183) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM16285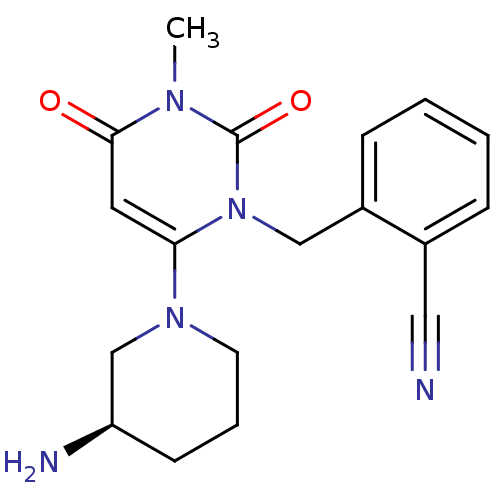 (2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280305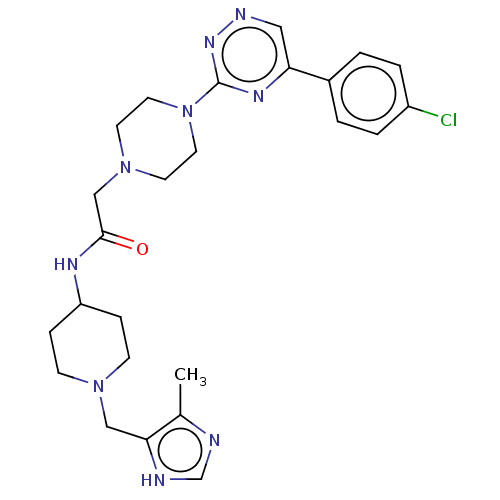 (US10029993, Example 154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50351402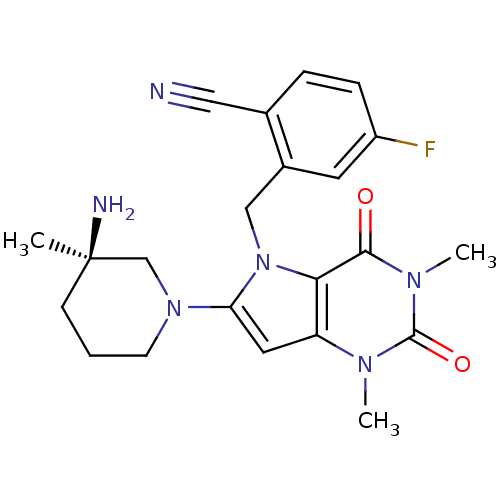 (CHEMBL1819090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292016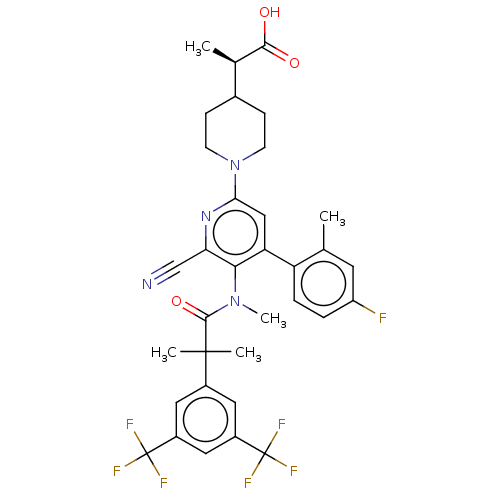 (US10100030, Example 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145521 ((S)-2-(3-Benzyl-ureido)-5-oxo-5-{4-[3-(1,4,5,6-tet...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145520 ((S)-2-Benzenesulfonylamino-5-oxo-5-{4-[3-(1,4,5,6-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292006 (US10100030, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.11 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50275544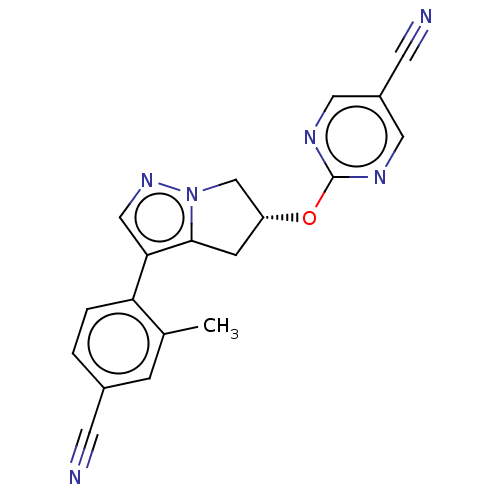 (CHEMBL4127675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50275535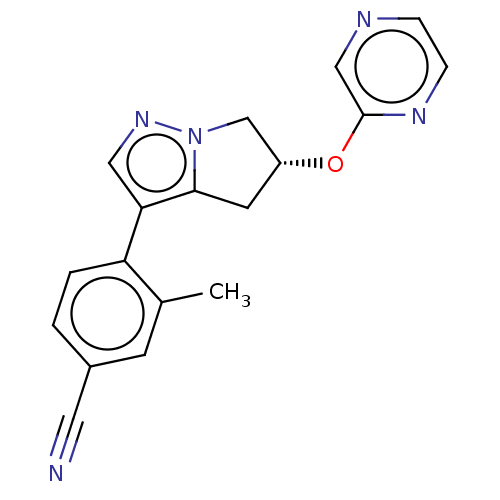 (CHEMBL4126893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292013 (US10100030, Example 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.25 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50275496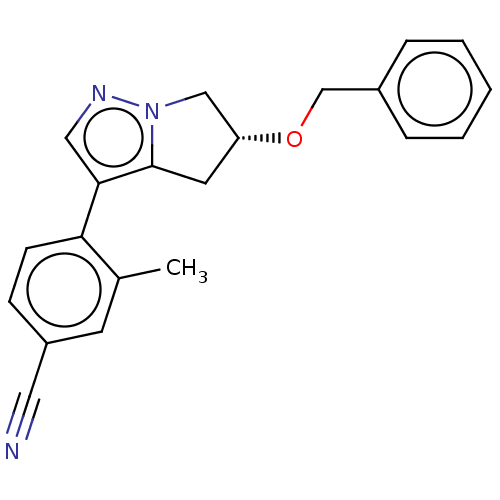 (CHEMBL4127904) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280321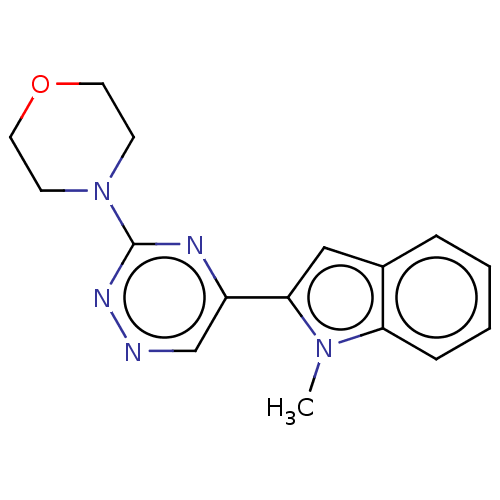 (US10029993, Example 211) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280326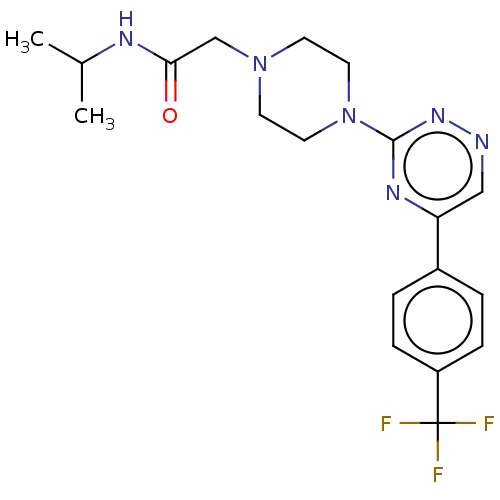 (US10029993, Example 221) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50275497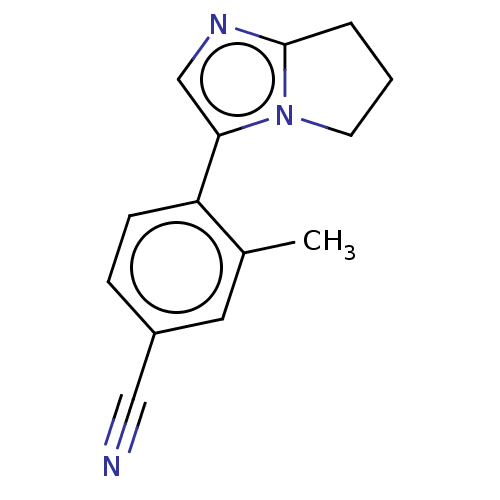 (CHEMBL4125850) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292007 (US10100030, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.53 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292011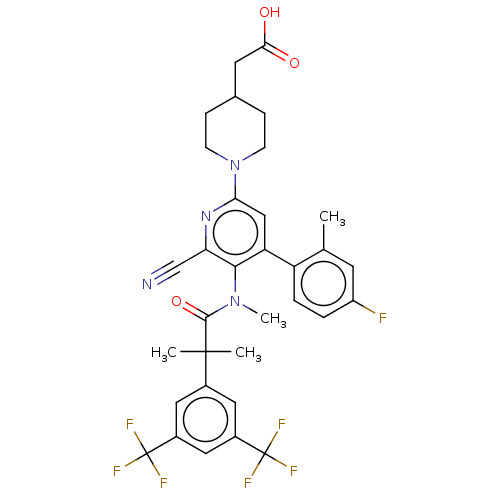 (US10100030, Example 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280292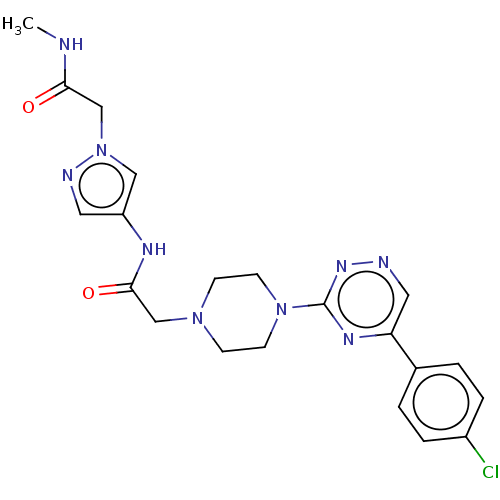 (US10029993, Example 81) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292014 (US10100030, Example 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.83 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280324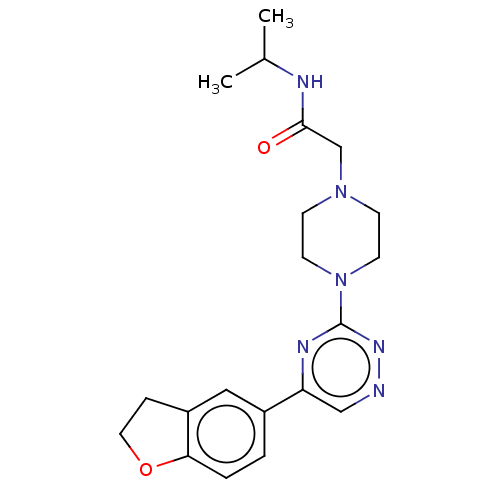 (US10029993, Example 219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50275533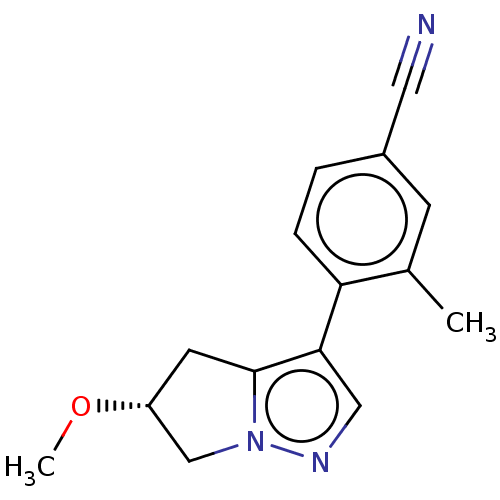 (CHEMBL4129296) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292010 (US10100030, Example 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.29 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292015 (US10100030, Example 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.32 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma assessed as formation of 7-amino-4-methylcoumarin from glycyl-L-proline 4-methylcoumaryl-7-amide by fluorescence a... | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292009 (US10100030, Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.54 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145531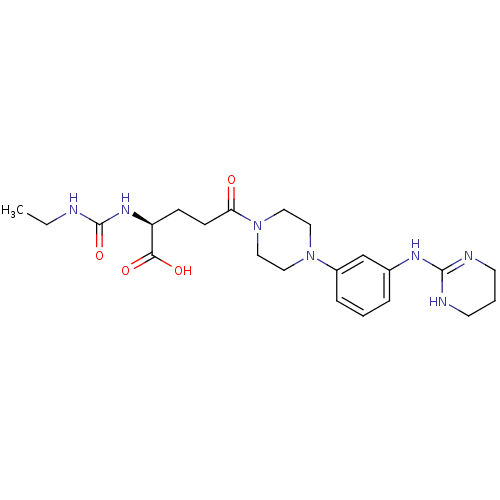 ((S)-2-(3-Ethyl-ureido)-5-oxo-5-{4-[3-(1,4,5,6-tetr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50275511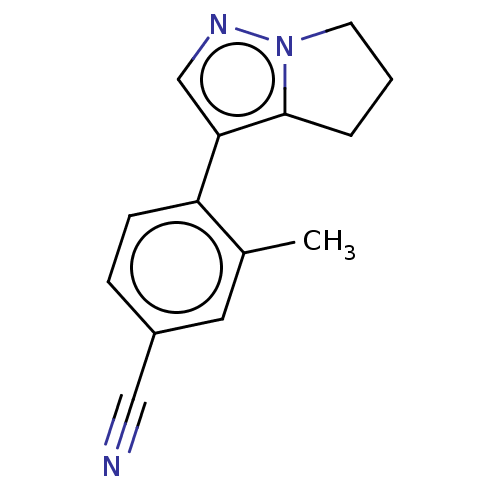 (CHEMBL4128315) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50275494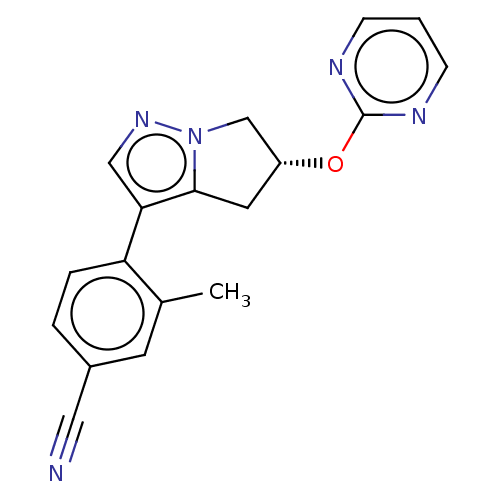 (CHEMBL4129177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50275543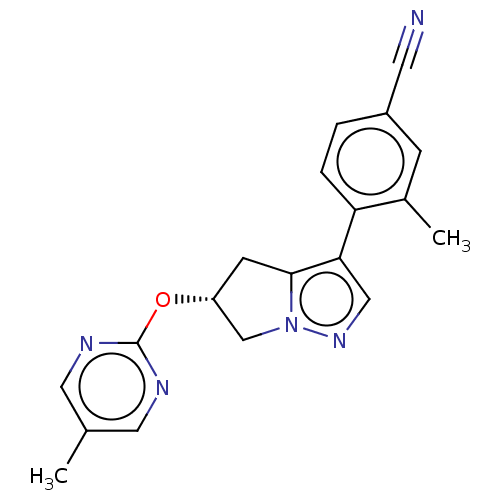 (CHEMBL4125736) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79 cell mitochondria assessed as reduction in aldosterone production using deoxycorticoster... | J Med Chem 61: 5594-5608 (2018) Article DOI: 10.1021/acs.jmedchem.8b00328 BindingDB Entry DOI: 10.7270/Q2F47RN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280291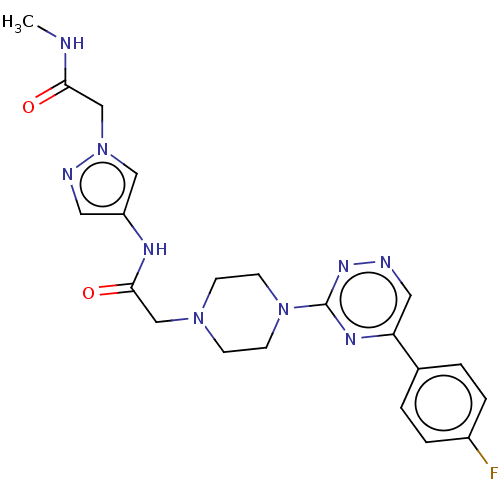 (US10029993, Example 80) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280289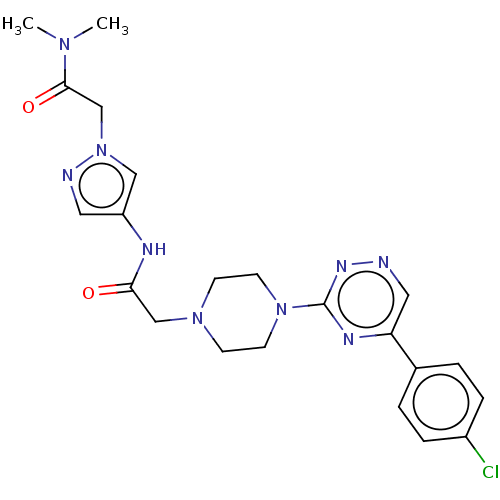 (US10029993, Example 76) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM280290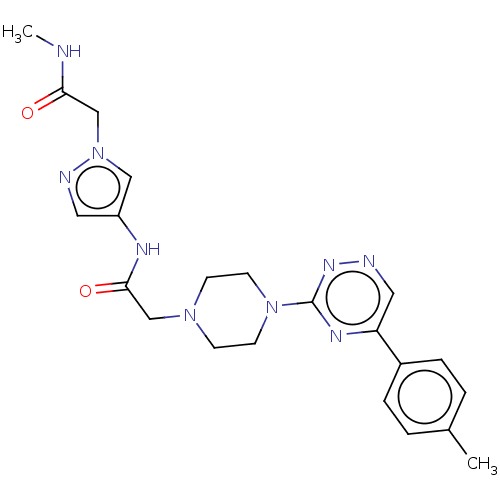 (US10029993, Example 79) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The pcDNA3.1-human CYP11B2 plasmid was transfected into a Chinese hamster lung fibroblast V79 cell line to produce a cell line stably expressing huma... | US Patent US10029993 (2018) BindingDB Entry DOI: 10.7270/Q2V40X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292008 (US10100030, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.61 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 224 total ) | Next | Last >> |