

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50517805 (CHEMBL4454648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343634 (4-((E)-4-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343641 (CHEMBL1774964 | cis-4-((E)-4-((S)-4-methyl-1-oxo-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343632 (4-((E)-4-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50462868 (CHEMBL4251246) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed-type inhibition of Helicobacter pylori ATCC 43504 urease assessed as enzyme-inhibitor complex using urea as substrate preincubated for 1.5 hrs | Bioorg Med Chem 26: 4145-4152 (2018) Article DOI: 10.1016/j.bmc.2018.07.003 BindingDB Entry DOI: 10.7270/Q2N87DFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343644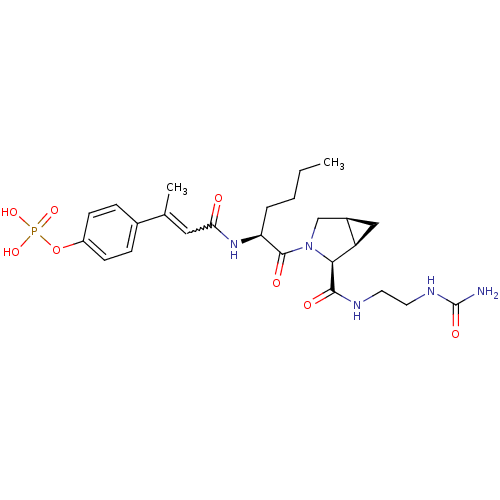 (CHEMBL1774967 | cis-4-((E)-4-oxo-4-((S)-1-oxo-1-((...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343633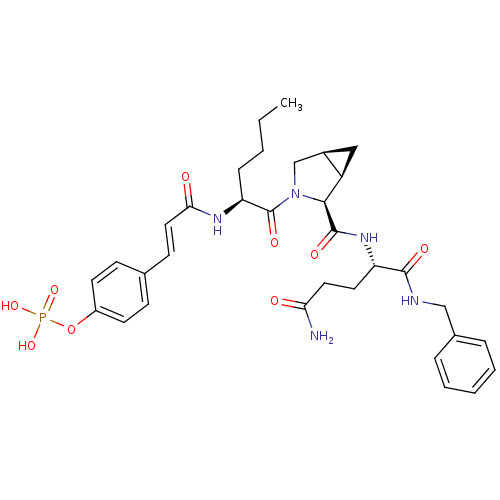 (4-((E)-3-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50462868 (CHEMBL4251246) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed-type inhibition of Helicobacter pylori ATCC 43504 urease assessed as enzyme-substrate-inhibitor complex using urea as substrate preincubated fo... | Bioorg Med Chem 26: 4145-4152 (2018) Article DOI: 10.1016/j.bmc.2018.07.003 BindingDB Entry DOI: 10.7270/Q2N87DFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343635 (4-((E)-4-((3S,6S)-6-((S)-5-amino-1-(benzylamino)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449762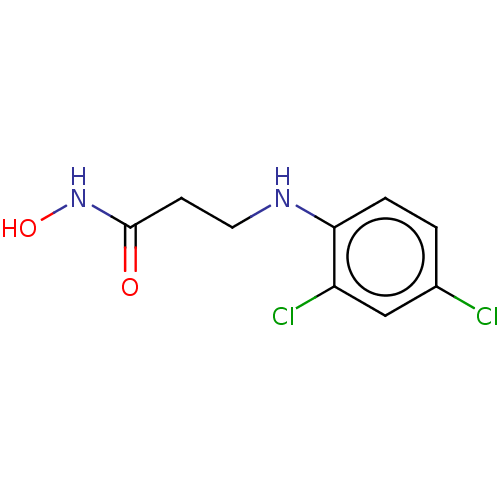 (CHEMBL4172924) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as enzyme-substrate-inhibitor complex constant preincubate... | Eur J Med Chem 156: 126-136 (2018) Article DOI: 10.1016/j.ejmech.2018.06.065 BindingDB Entry DOI: 10.7270/Q2N87DBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343631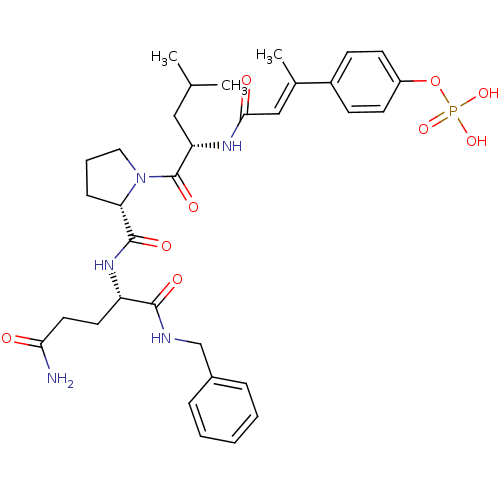 (4-((E)-4-((S)-1-((S)-2-((S)-5-amino-1-(benzylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343642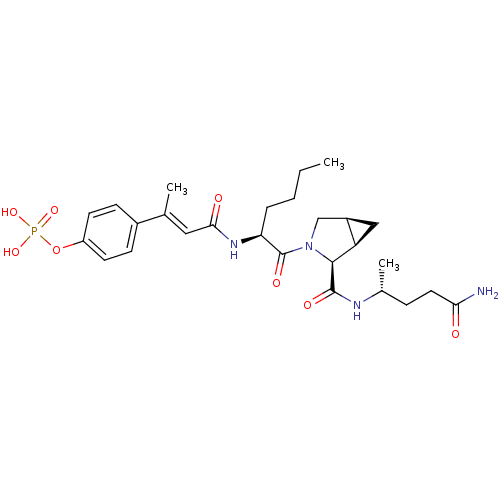 (CHEMBL1774965 | cis-4-((E)-4-((S)-1-((1R,2S,5S)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343639 (CHEMBL1774962 | cis-4-((E)-4-((S)-1-((1R,2S,5S)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343638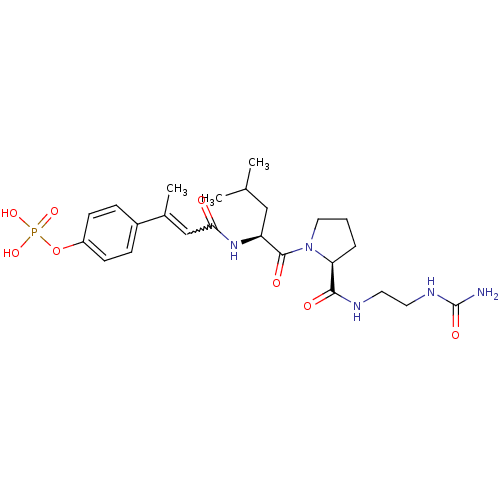 (4-((E)-4-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343645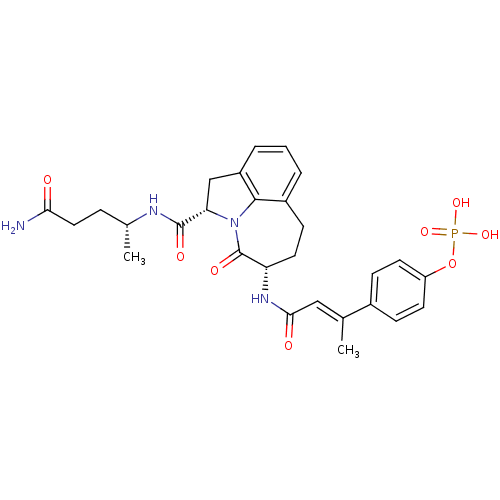 (4-((E)-4-((3S,6S)-6-((R)-5-amino-5-oxopentan-2-ylc...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343643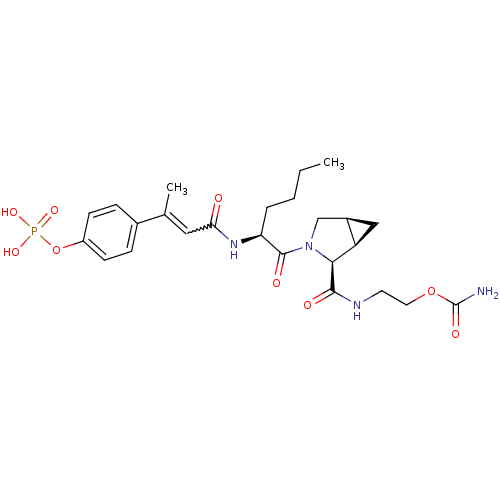 (CHEMBL1774966 | cis-2-((1R,2S,5S)-3-((S)-2-((E)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343636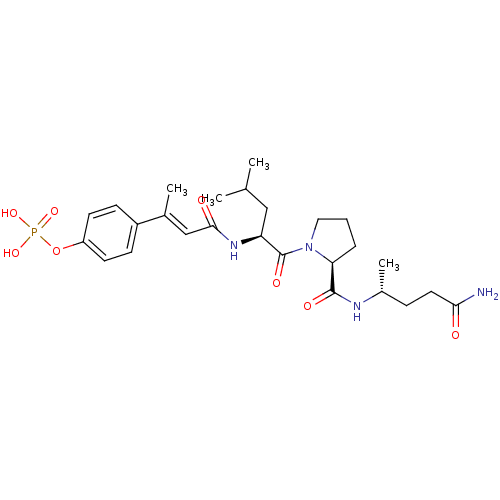 (4-((E)-4-((S)-1-((S)-2-((R)-5-amino-5-oxopentan-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449754 (CHEMBL4167553) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as enzyme-substrate-inhibitor complex constant preincubate... | Eur J Med Chem 156: 126-136 (2018) Article DOI: 10.1016/j.ejmech.2018.06.065 BindingDB Entry DOI: 10.7270/Q2N87DBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343646 (2-((3S,6S)-4-oxo-3-((E)-3-(4-(phosphonooxy)phenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343640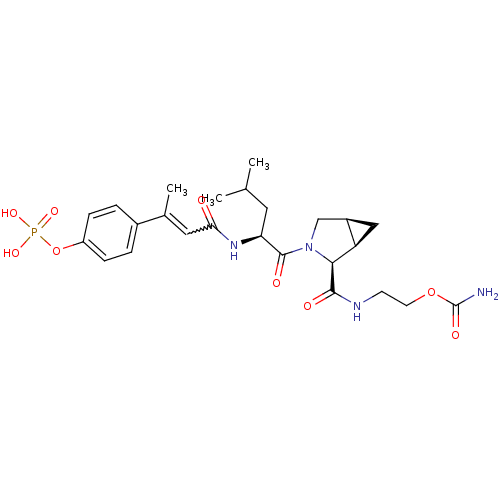 (CHEMBL1774963 | cis-2-((1R,2S,5S)-3-((S)-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin L | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343637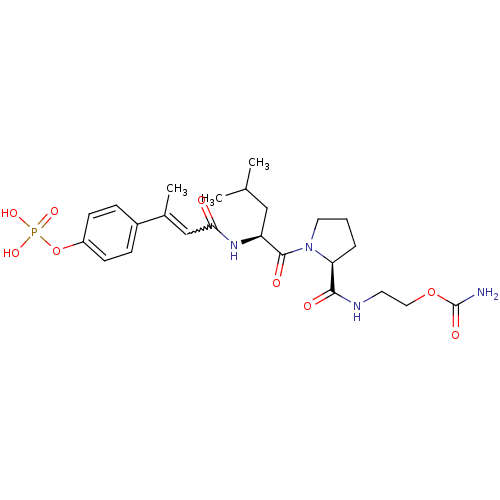 (2-((S)-1-((S)-4-methyl-2-((E)-3-(4-(phosphonooxy)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin S expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449763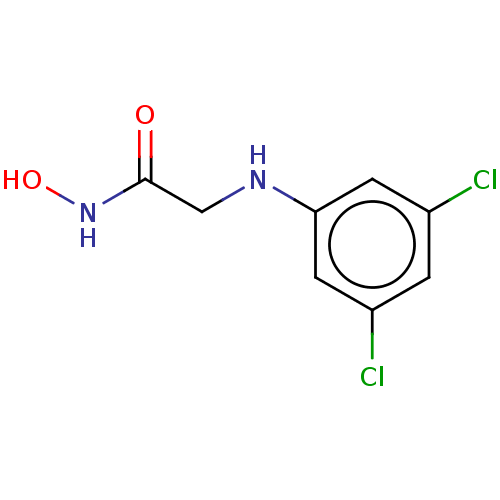 (CHEMBL4176964) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as enzyme-substrate-inhibitor complex constant preincubate... | Eur J Med Chem 156: 126-136 (2018) Article DOI: 10.1016/j.ejmech.2018.06.065 BindingDB Entry DOI: 10.7270/Q2N87DBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343647 (4-((E)-4-oxo-4-((3S,6S)-4-oxo-6-(2-ureidoethylcarb...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50517805 (CHEMBL4454648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 406 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin L | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449762 (CHEMBL4172924) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 441 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as reduction in ammonia production preincubated for 1.5 hr... | Eur J Med Chem 156: 126-136 (2018) Article DOI: 10.1016/j.ejmech.2018.06.065 BindingDB Entry DOI: 10.7270/Q2N87DBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50517805 (CHEMBL4454648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 735 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin S expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 909 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin B | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449754 (CHEMBL4167553) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as reduction in ammonia production preincubated for 1.5 hr... | Eur J Med Chem 156: 126-136 (2018) Article DOI: 10.1016/j.ejmech.2018.06.065 BindingDB Entry DOI: 10.7270/Q2N87DBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50449763 (CHEMBL4176964) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed type non-competitive inhibition of urease in Helicobacter pylori ATCC 43504 assessed as reduction in ammonia production preincubated for 1.5 hr... | Eur J Med Chem 156: 126-136 (2018) Article DOI: 10.1016/j.ejmech.2018.06.065 BindingDB Entry DOI: 10.7270/Q2N87DBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50517805 (CHEMBL4454648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin B | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352620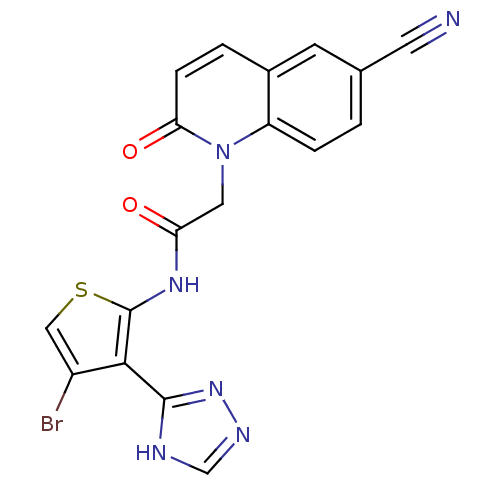 (CHEMBL1822151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428701 (CHEMBL2333115 | US9884828, 2-127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352624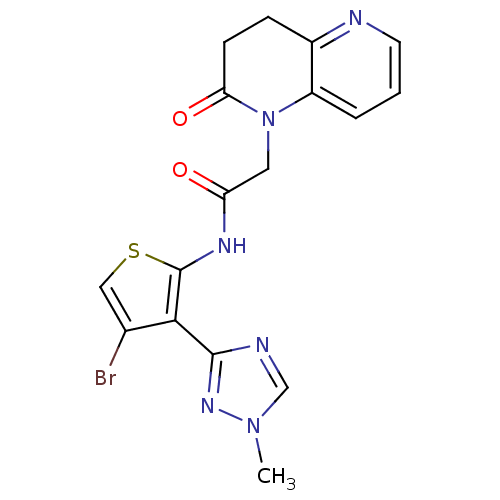 (CHEMBL1822305 | US9796706, Compound 139) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352621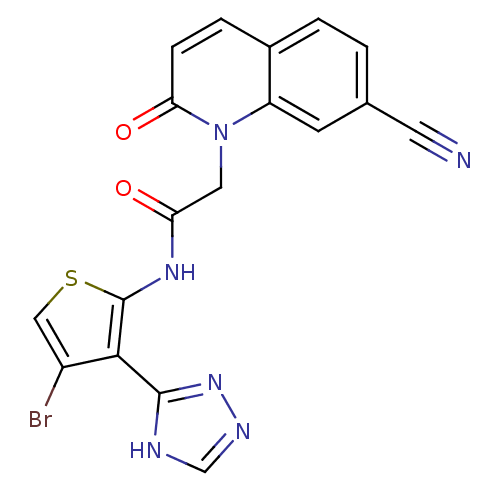 (CHEMBL1822152) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428717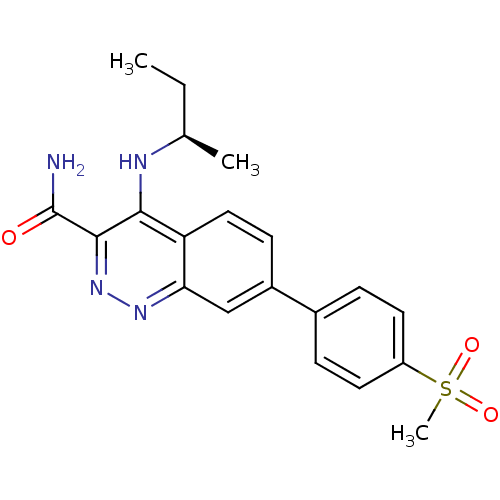 (CHEMBL2333127 | US9884828, 2-37) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428716 (CHEMBL2333128 | US9884828, 2-41) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM81790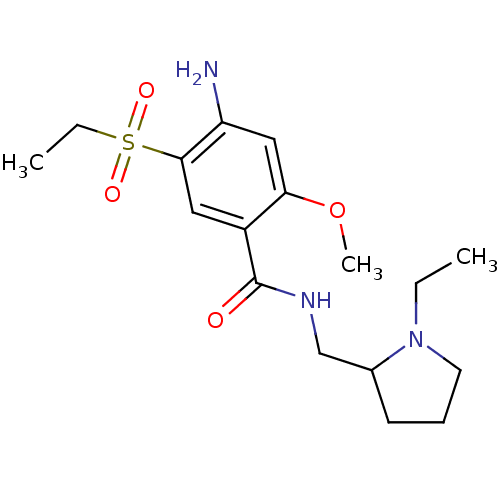 (Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LB PHARMACEUTICALS INC. US Patent | Assay Description The ability of Compound 102 to bind dopamine D2 receptors was measured in a cell-based assay. Dopamine D2 receptor cells were seeded in a half a blac... | US Patent US10167256 (2019) BindingDB Entry DOI: 10.7270/Q2NG4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM81790 (Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Biologie Structurale | Assay Description The ability of Compound 102 to bind dopamine D2 receptors was measured in a cell-based assay. Dopamine D2 receptor cells were seeded in a half a blac... | J Med Chem 51: 1115-25 (2008) BindingDB Entry DOI: 10.7270/Q2377C1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM16016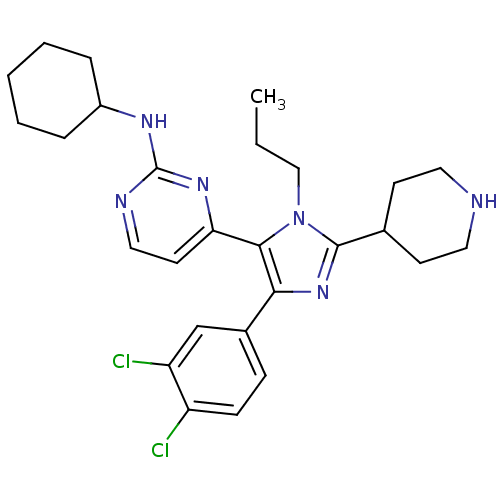 (CHEMBL437747 | N-cyclohexyl-4-[4-(3,4-dichlorophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK3 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 315-9 (2010) Article DOI: 10.1016/j.bmcl.2010.11.010 BindingDB Entry DOI: 10.7270/Q24F1R0T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50142796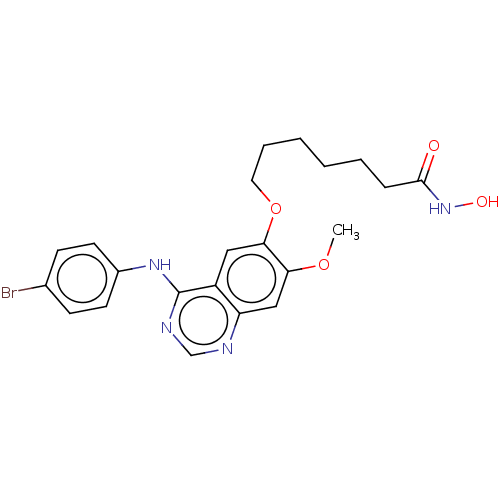 (CHEMBL3759186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorometric assay | Eur J Med Chem 109: 1-12 (2016) Article DOI: 10.1016/j.ejmech.2015.12.033 BindingDB Entry DOI: 10.7270/Q2377BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352615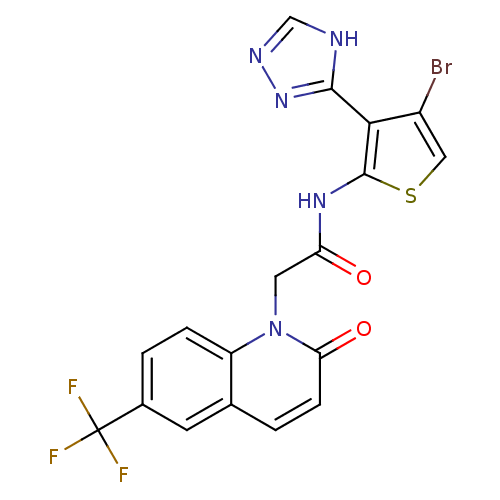 (CHEMBL1822146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352628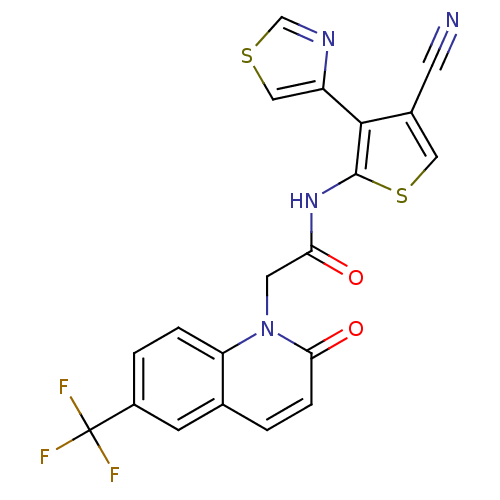 (CHEMBL1822309 | US9796706, Compound 136) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352618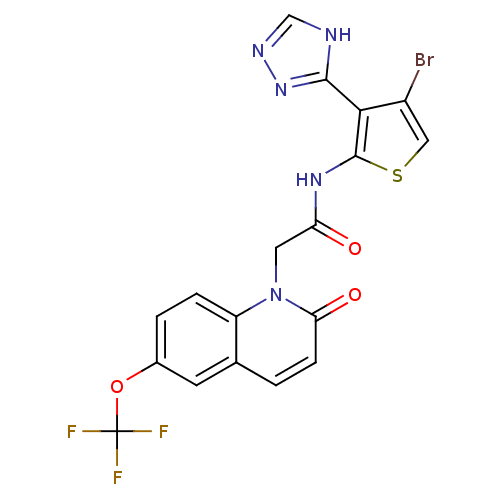 (CHEMBL1822149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50352620 (CHEMBL1822151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50352621 (CHEMBL1822152) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428703 (CHEMBL2333113 | US9884828, 2-100) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50142796 (CHEMBL3759186) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extracts preincubated for 15 mins followed by addition of Fluor de Lys as substrate for 1 hr by fluorom... | Eur J Med Chem 109: 1-12 (2016) Article DOI: 10.1016/j.ejmech.2015.12.033 BindingDB Entry DOI: 10.7270/Q2377BJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2417 total ) | Next | Last >> |