

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300504 (CHEMBL574455 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300502 (CHEMBL572682 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300497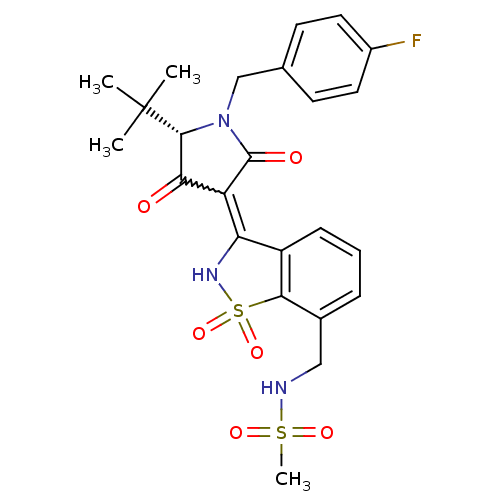 (CHEMBL578433 | N-({3-[(5S)-5-tert-butyl-1-(4-fluor...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300503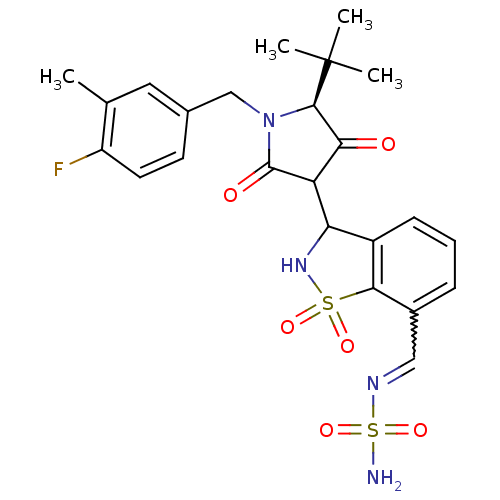 (CHEMBL582995 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300498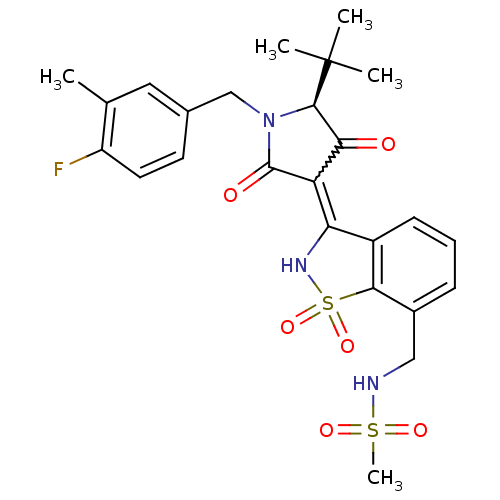 (CHEMBL575777 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300499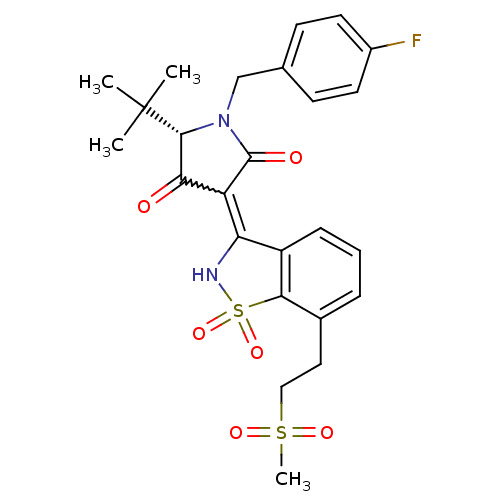 ((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-3-[...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300496 (CHEMBL577404 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300505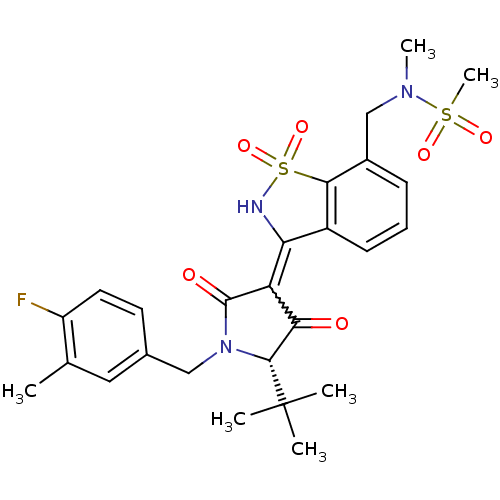 (CHEMBL572683 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300501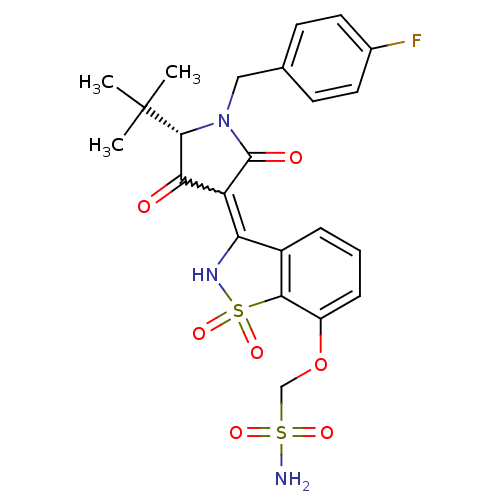 (CHEMBL573175 | {3-[(S)-5-tert-Butyl-1-(4-fluoro-be...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300500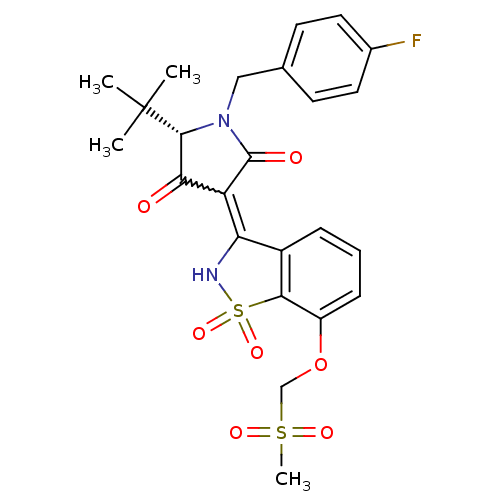 ((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-3-(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM30491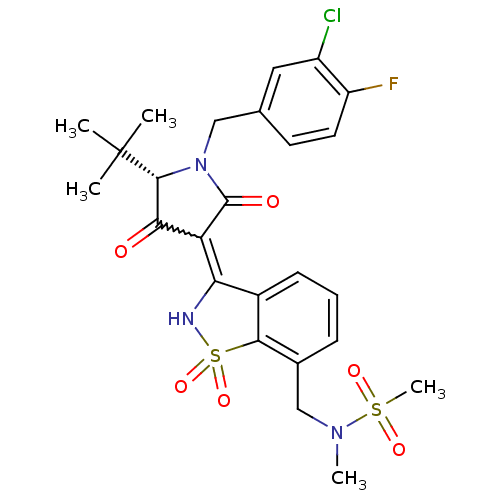 (1,1-dioxoisothiazole analog., 35) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor coactivator 1 (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of Src1 | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of Lyn | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of ErbB4 | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of Btk | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-2B (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of ACTR-2B | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50349213 (CHEMBL1808264 | D3RKN_42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 21: 4436-40 (2011) Article DOI: 10.1016/j.bmcl.2011.06.021 BindingDB Entry DOI: 10.7270/Q2Q240KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300506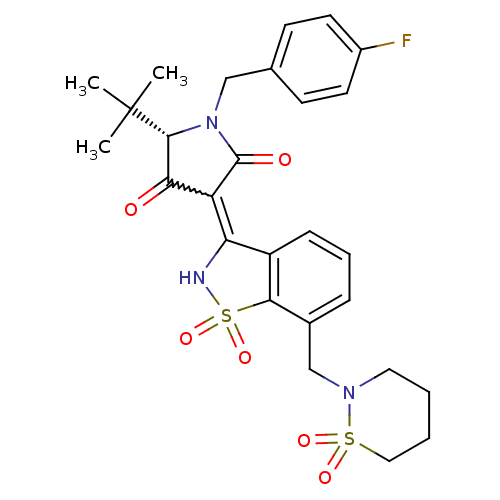 ((S)-5-tert-Butyl-3-[7-(1,1-dioxo-1lambda*6*-[1,2]t...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300495 (CHEMBL572718 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50428286 (DABRAFENIB | GSK2118436A) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Alk5 in TGF-beta-stimulated human HepG2 cells assessed as decrease in Smad2 phosphorylation treated for 45 mins prior to TGF-beta stimu... | ACS Med Chem Lett 4: 358-62 (2013) Article DOI: 10.1021/ml4000063 BindingDB Entry DOI: 10.7270/Q20003FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514765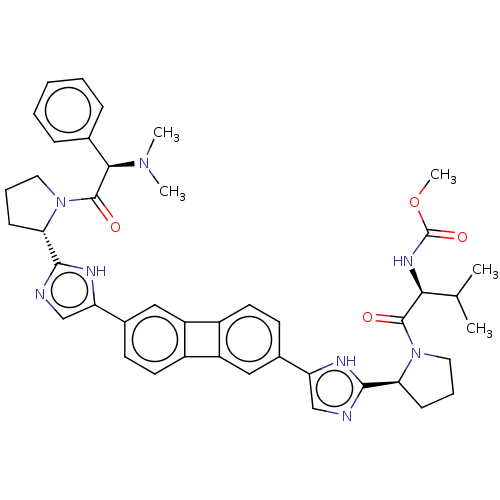 (CHEMBL4549709) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.47 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 2a con infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514766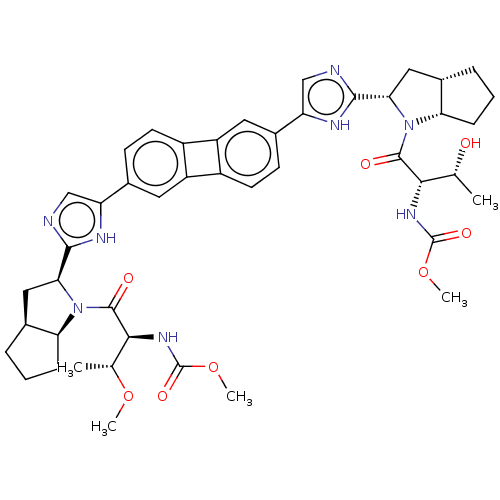 (CHEMBL4531684) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.188 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 2a con infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514767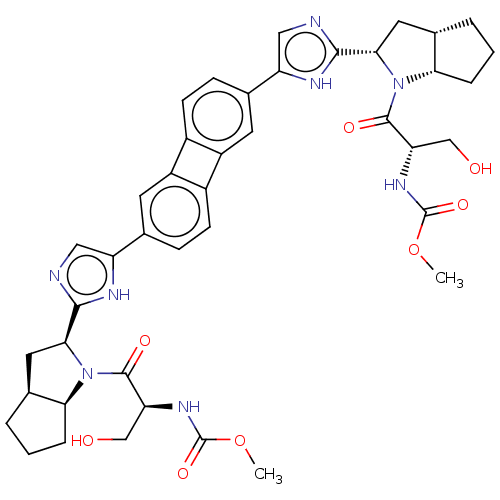 (CHEMBL4448604) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514768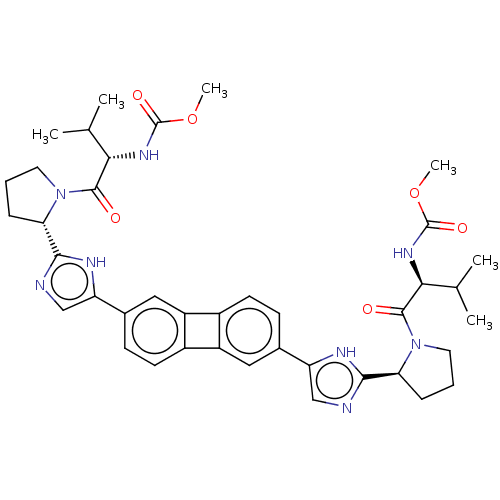 (CHEMBL3360467) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514769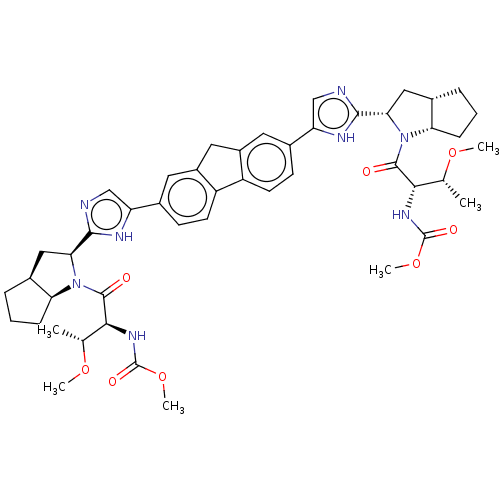 (CHEMBL4548808) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514769 (CHEMBL4548808) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514770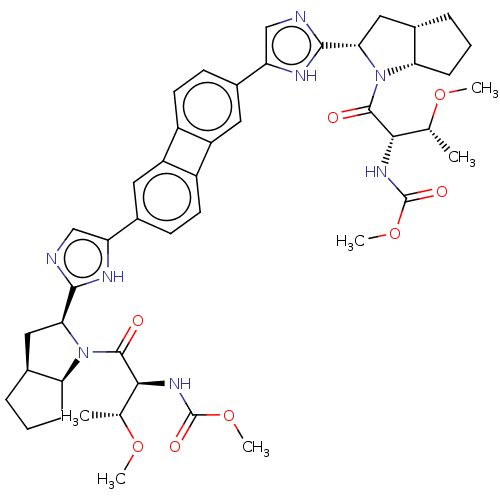 (CHEMBL4476378) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514771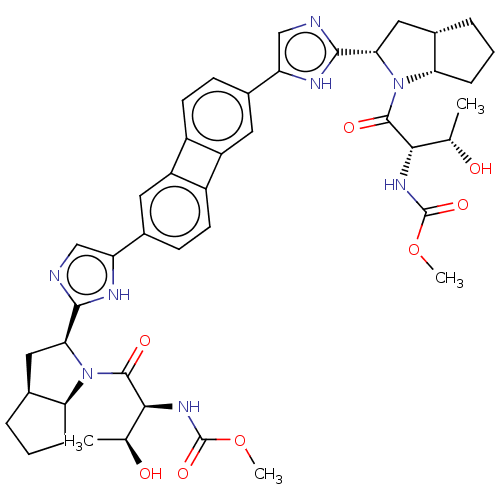 (CHEMBL4579455) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514772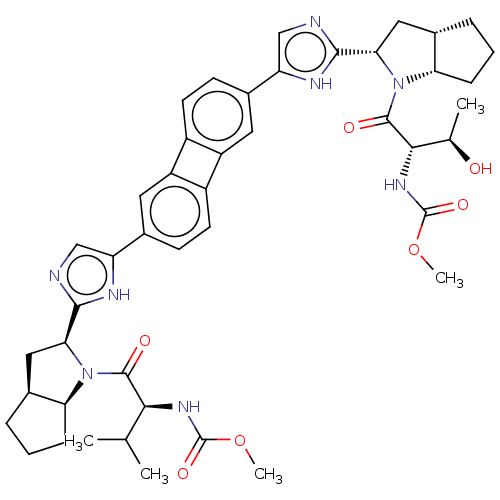 (CHEMBL4533823) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514773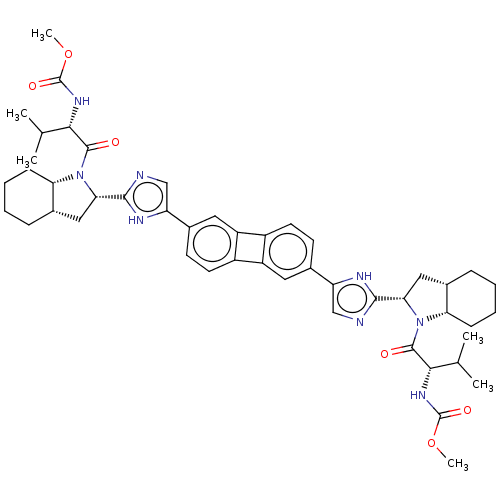 (CHEMBL4483702) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514774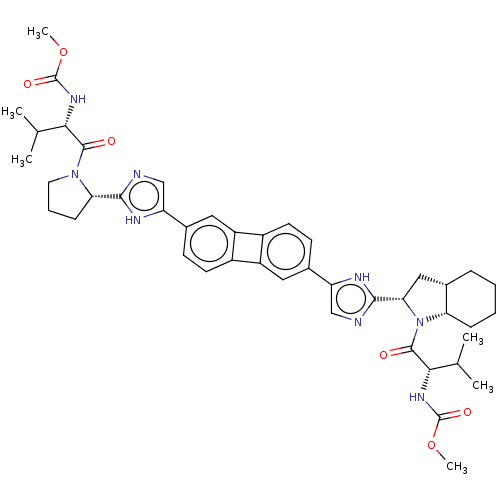 (CHEMBL4444885) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514775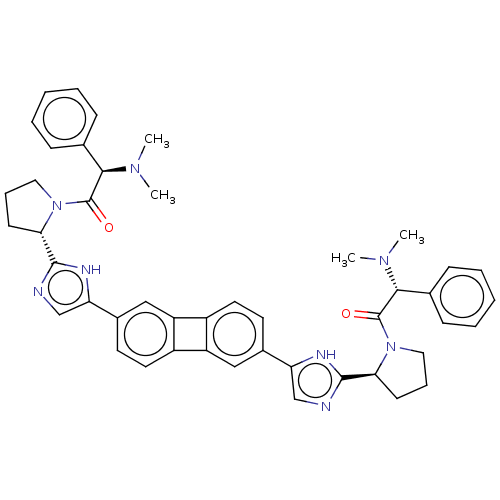 (CHEMBL4446161) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.248 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514768 (CHEMBL3360467) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50387084 (BMS-790052 | DACLATASVIR) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1a infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514775 (CHEMBL4446161) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514774 (CHEMBL4444885) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514776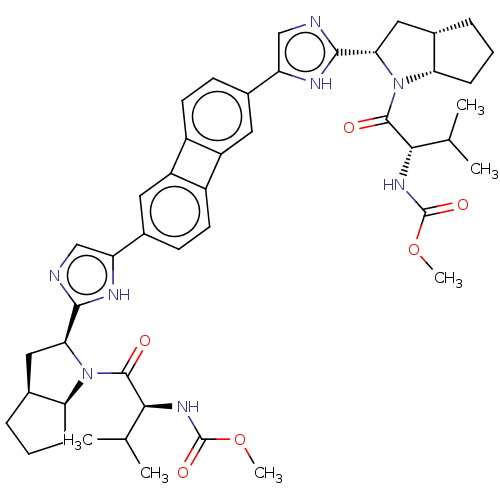 (CHEMBL4465921) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514767 (CHEMBL4448604) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514772 (CHEMBL4533823) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514771 (CHEMBL4579455) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514770 (CHEMBL4476378) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514777 (CHEMBL4561734) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514778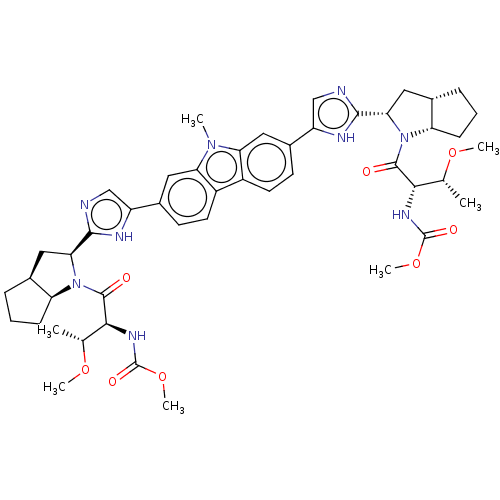 (CHEMBL4466702) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514779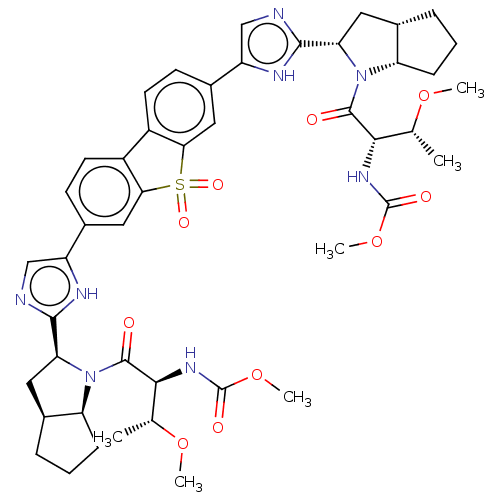 (CHEMBL4472037) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514780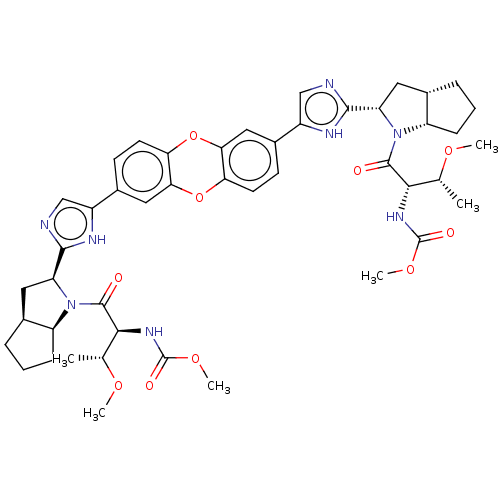 (CHEMBL4471986) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 1b infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase rep... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50387084 (BMS-790052 | DACLATASVIR) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 52.8 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 2a con infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514775 (CHEMBL4446161) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.327 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 2a con infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514774 (CHEMBL4444885) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 2a con infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50514773 (CHEMBL4483702) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.33 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NS5A in HCV genotype 2a con infected in HuH-Lcu-Neo cells assessed as reduction in viral replication incubated for 48 hrs by luciferase... | J Med Chem 63: 4155-4170 (2020) Article DOI: 10.1021/acs.jmedchem.9b02176 BindingDB Entry DOI: 10.7270/Q2222Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 91 total ) | Next | Last >> |