

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM124107 (US8754105, 23) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635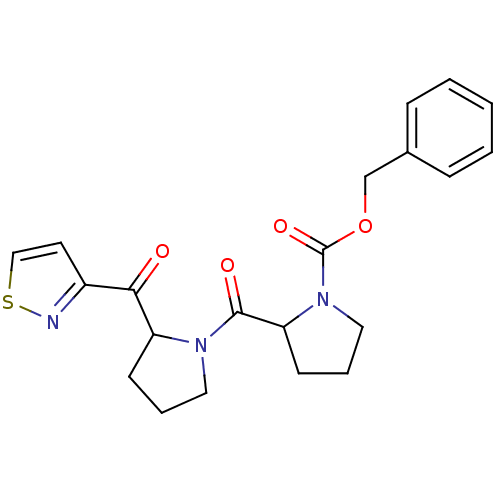 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135637 (1-{(S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135634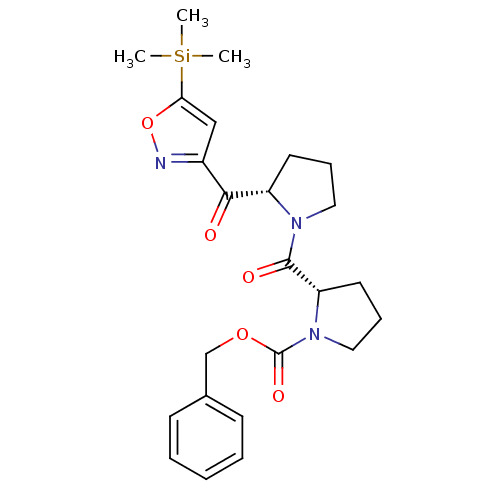 ((S)-2-[(S)-2-(5-Trimethylsilanyl-isoxazole-3-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124112 (US8754105, 25) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of PKD2 ( assessed as residual activity at 1 uM ) by TR-FRET assay | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135636 ((S)-2-[(S)-2-(5-Cyano-isoxazole-3-carbonyl)-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human; Moderately active | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135634 ((S)-2-[(S)-2-(5-Trimethylsilanyl-isoxazole-3-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135632 ((S)-2-[(S)-2-(Isoxazole-3-carbonyl)-pyrrolidine-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135636 ((S)-2-[(S)-2-(5-Cyano-isoxazole-3-carbonyl)-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124111 (US8754105, 24) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 40 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124110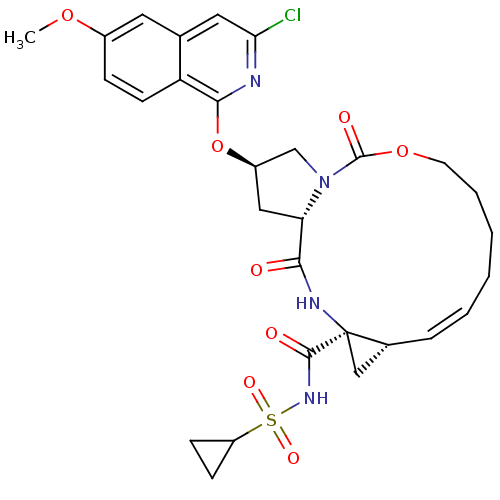 (US8754105, 17) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 43 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135632 ((S)-2-[(S)-2-(Isoxazole-3-carbonyl)-pyrrolidine-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124109 (US8754105, 16) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135631 (3-[(S)-1-((S)-1-Benzyloxycarbonyl-pyrrolidine-2-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi; Moderately active | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135631 (3-[(S)-1-((S)-1-Benzyloxycarbonyl-pyrrolidine-2-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124108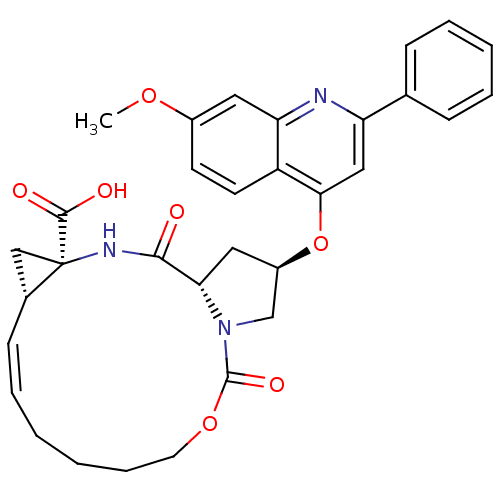 (US8754105, 22) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.07E+3 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||