 Found 96 hits with Last Name = 'babiarz' and Initial = 'je'
Found 96 hits with Last Name = 'babiarz' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021127
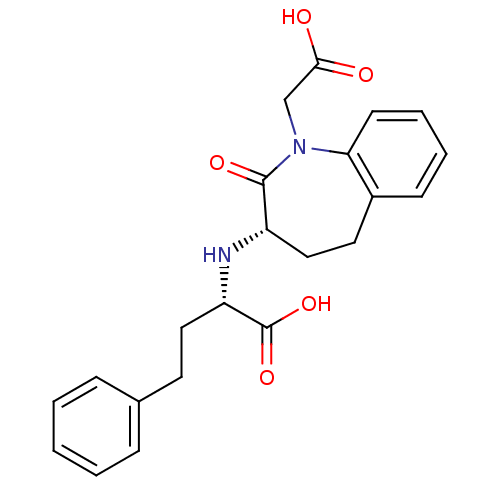
(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5/c25-20(26)14-24-19-9-5-4-8-16(19)11-13-17(21(24)27)23-18(22(28)29)12-10-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 1511-6 (1985)
BindingDB Entry DOI: 10.7270/Q2BR8SRQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021153
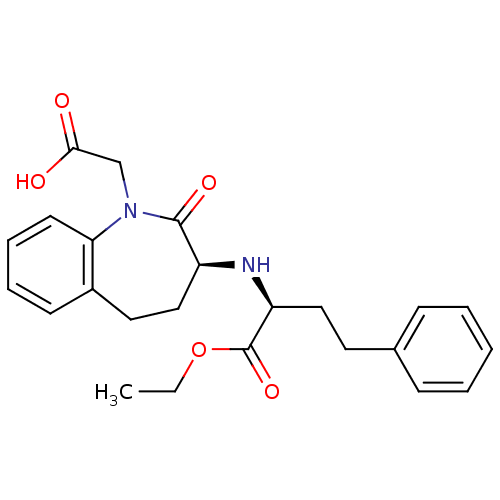
(1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021127

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5/c25-20(26)14-24-19-9-5-4-8-16(19)11-13-17(21(24)27)23-18(22(28)29)12-10-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 1511-6 (1985)
BindingDB Entry DOI: 10.7270/Q2BR8SRQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021790
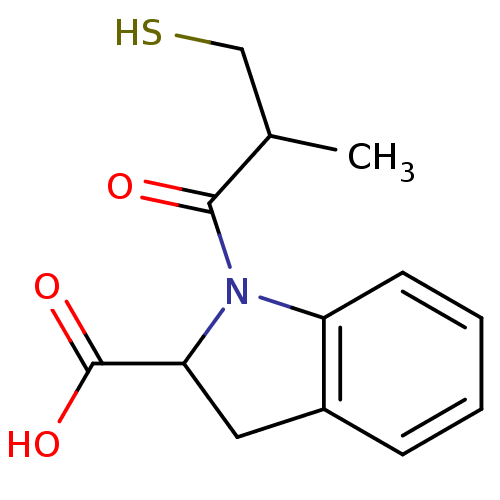
((S,S)1-(3-Mercapto-2-methyl-propionyl)-2,3-dihydro...)Show InChI InChI=1S/C13H15NO3S/c1-8(7-18)12(15)14-10-5-3-2-4-9(10)6-11(14)13(16)17/h2-5,8,11,18H,6-7H2,1H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase in Escherichia coli |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021783

(1-(3-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...)Show InChI InChI=1S/C12H13NO3S/c14-11(5-6-17)13-9-4-2-1-3-8(9)7-10(13)12(15)16/h1-4,10,17H,5-7H2,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021127

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5/c25-20(26)14-24-19-9-5-4-8-16(19)11-13-17(21(24)27)23-18(22(28)29)12-10-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 1511-6 (1985)
BindingDB Entry DOI: 10.7270/Q2BR8SRQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021148
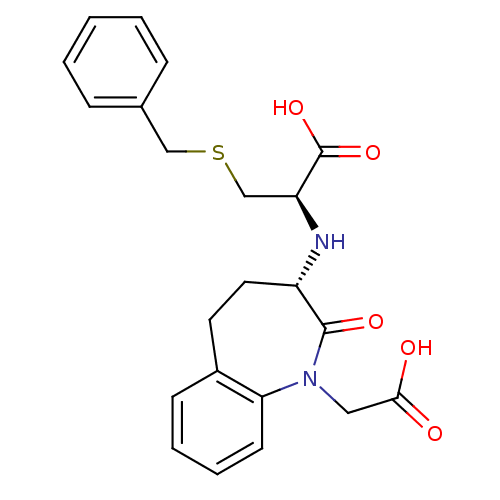
(3-Benzylsulfanyl-2-(1-carboxymethyl-2-oxo-2,3,4,5-...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CSCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5S/c25-20(26)12-24-19-9-5-4-8-16(19)10-11-17(21(24)27)23-18(22(28)29)14-30-13-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021151
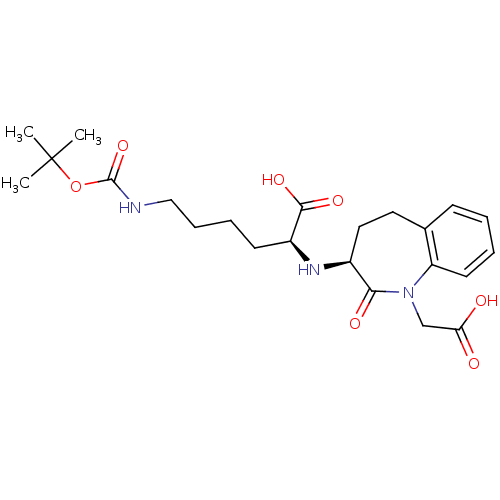
(6-tert-Butoxycarbonylamino-2-(1-carboxymethyl-2-ox...)Show SMILES CC(C)(C)OC(=O)NCCCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C23H33N3O7/c1-23(2,3)33-22(32)24-13-7-6-9-17(21(30)31)25-16-12-11-15-8-4-5-10-18(15)26(20(16)29)14-19(27)28/h4-5,8,10,16-17,25H,6-7,9,11-14H2,1-3H3,(H,24,32)(H,27,28)(H,30,31)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021138

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CSc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C21H22N2O5S/c24-19(25)12-23-18-9-5-4-6-14(18)10-11-16(20(23)26)22-17(21(27)28)13-29-15-7-2-1-3-8-15/h1-9,16-17,22H,10-13H2,(H,24,25)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367173

(CHEMBL1744333)Show InChI InChI=1S/C13H17NO3S/c1-9(8-18)12(15)14(10(2)13(16)17)11-6-4-3-5-7-11/h3-7,9-10,18H,8H2,1-2H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021129
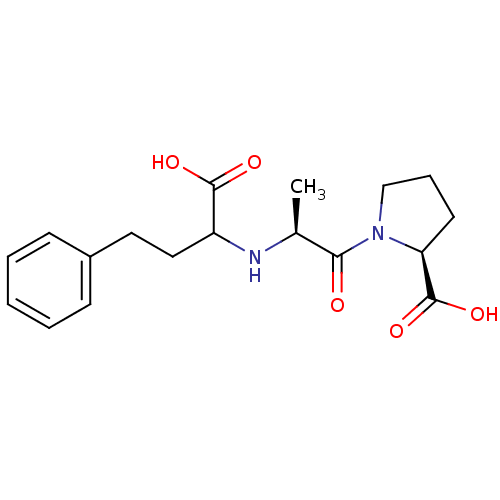
(1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-p...)Show SMILES C[C@H](NC(CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14?,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 1511-6 (1985)
BindingDB Entry DOI: 10.7270/Q2BR8SRQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017129

((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027697

(6-Fluoro-1-(3-mercapto-propionyl)-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14FNO3S/c14-9-2-4-10-8(7-9)1-3-11(13(17)18)15(10)12(16)5-6-19/h2,4,7,11,19H,1,3,5-6H2,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021154

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CCCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C18H24N2O5/c1-2-3-7-14(18(24)25)19-13-10-9-12-6-4-5-8-15(12)20(17(13)23)11-16(21)22/h4-6,8,13-14,19H,2-3,7,9-11H2,1H3,(H,21,22)(H,24,25)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367155

(CHEMBL1744329)Show InChI InChI=1S/C13H14ClNO3S/c14-9-2-4-10-8(7-9)1-3-11(13(17)18)15(10)12(16)5-6-19/h2,4,7,11,19H,1,3,5-6H2,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021127

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5/c25-20(26)14-24-19-9-5-4-8-16(19)11-13-17(21(24)27)23-18(22(28)29)12-10-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 1511-6 (1985)
BindingDB Entry DOI: 10.7270/Q2BR8SRQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367149
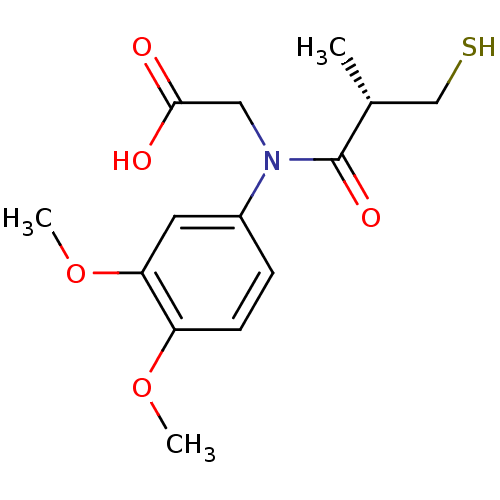
(CHEMBL1169540)Show SMILES COc1ccc(cc1OC)N(CC(O)=O)C(=O)[C@H](C)CS |r| Show InChI InChI=1S/C14H19NO5S/c1-9(8-21)14(18)15(7-13(16)17)10-4-5-11(19-2)12(6-10)20-3/h4-6,9,21H,7-8H2,1-3H3,(H,16,17)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021149

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C17H22N2O5/c1-2-5-13(17(23)24)18-12-9-8-11-6-3-4-7-14(11)19(16(12)22)10-15(20)21/h3-4,6-7,12-13,18H,2,5,8-10H2,1H3,(H,20,21)(H,23,24)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021140

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CSCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C17H22N2O5S/c1-25-9-8-13(17(23)24)18-12-7-6-11-4-2-3-5-14(11)19(16(12)22)10-15(20)21/h2-5,12-13,18H,6-10H2,1H3,(H,20,21)(H,23,24)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367146
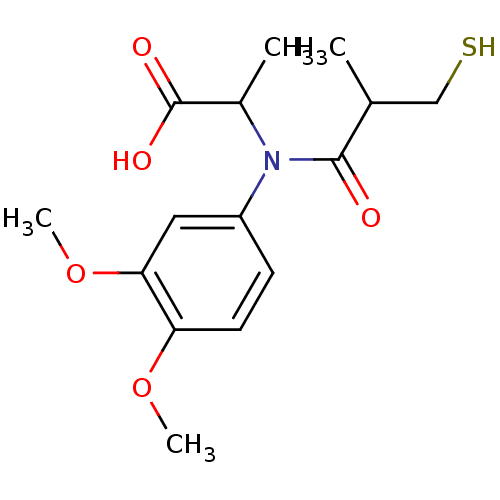
(CHEMBL1744332)Show InChI InChI=1S/C15H21NO5S/c1-9(8-22)14(17)16(10(2)15(18)19)11-5-6-12(20-3)13(7-11)21-4/h5-7,9-10,22H,8H2,1-4H3,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021137

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CSCCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C23H26N2O5S/c26-21(27)14-25-20-9-5-4-8-17(20)10-11-18(22(25)28)24-19(23(29)30)15-31-13-12-16-6-2-1-3-7-16/h1-9,18-19,24H,10-15H2,(H,26,27)(H,29,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50020766
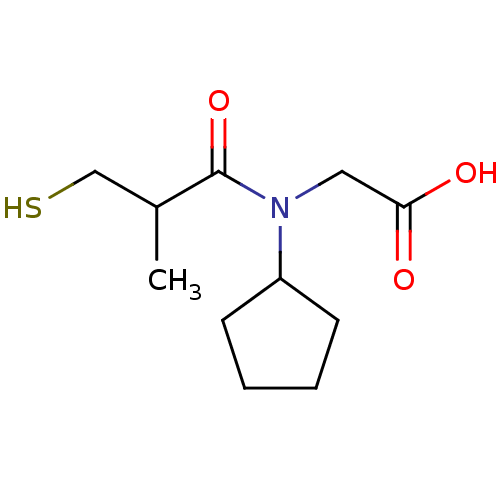
((R+S)-[Cyclopentyl-(3-mercapto-2-methyl-propionyl)...)Show InChI InChI=1S/C11H19NO3S/c1-8(7-16)11(15)12(6-10(13)14)9-4-2-3-5-9/h8-9,16H,2-7H2,1H3,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021142

(6-Amino-2-(1-carboxymethyl-2-oxo-2,3,4,5-tetrahydr...)Show SMILES NCCCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C18H25N3O5/c19-10-4-3-6-14(18(25)26)20-13-9-8-12-5-1-2-7-15(12)21(17(13)24)11-16(22)23/h1-2,5,7,13-14,20H,3-4,6,8-11,19H2,(H,22,23)(H,25,26)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50020798
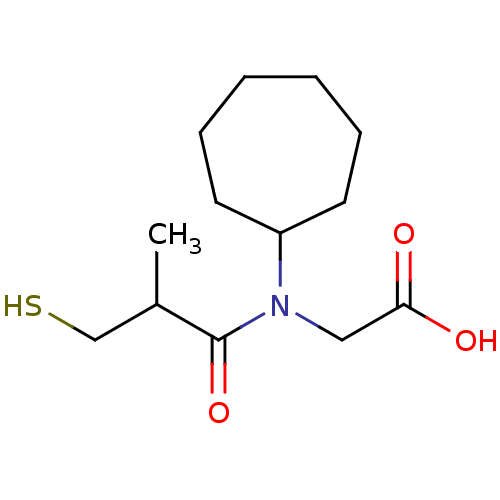
(CHEMBL1788148 | CHEMBL23841 | [Cycloheptyl-(3-merc...)Show InChI InChI=1S/C13H23NO3S/c1-10(9-18)13(17)14(8-12(15)16)11-6-4-2-3-5-7-11/h10-11,18H,2-9H2,1H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027736
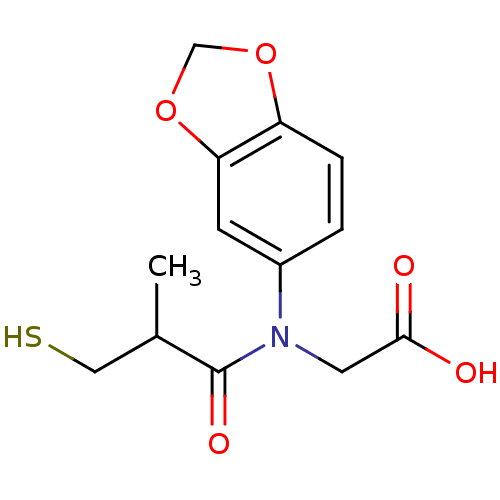
(CHEMBL22654 | [Benzo[1,3]dioxol-5-yl-(3-mercapto-2...)Show InChI InChI=1S/C13H15NO5S/c1-8(6-20)13(17)14(5-12(15)16)9-2-3-10-11(4-9)19-7-18-10/h2-4,8,20H,5-7H2,1H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50421710
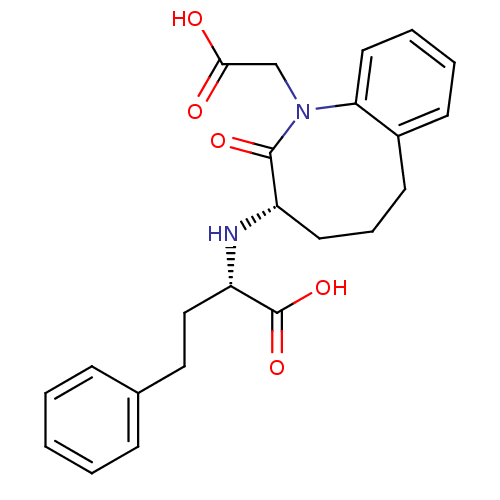
(CHEMBL2093973)Show SMILES OC(=O)CN1c2ccccc2CCC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O |r| Show InChI InChI=1S/C23H26N2O5/c26-21(27)15-25-20-12-5-4-9-17(20)10-6-11-18(22(25)28)24-19(23(29)30)14-13-16-7-2-1-3-8-16/h1-5,7-9,12,18-19,24H,6,10-11,13-15H2,(H,26,27)(H,29,30)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 1511-6 (1985)
BindingDB Entry DOI: 10.7270/Q2BR8SRQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50421710

(CHEMBL2093973)Show SMILES OC(=O)CN1c2ccccc2CCC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O |r| Show InChI InChI=1S/C23H26N2O5/c26-21(27)15-25-20-12-5-4-9-17(20)10-6-11-18(22(25)28)24-19(23(29)30)14-13-16-7-2-1-3-8-16/h1-5,7-9,12,18-19,24H,6,10-11,13-15H2,(H,26,27)(H,29,30)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 1511-6 (1985)
BindingDB Entry DOI: 10.7270/Q2BR8SRQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50020768

(CHEMBL1788152 | CHEMBL23518 | [Cyclohexyl-(3-merca...)Show InChI InChI=1S/C12H21NO3S/c1-9(8-17)12(16)13(7-11(14)15)10-5-3-2-4-6-10/h9-10,17H,2-8H2,1H3,(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367157

(CHEMBL1744325)Show InChI InChI=1S/C11H19NO3S3/c1-8(5-16)11(15)12(4-10(13)14)9-6-17-2-3-18-7-9/h8-9,16H,2-7H2,1H3,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367169
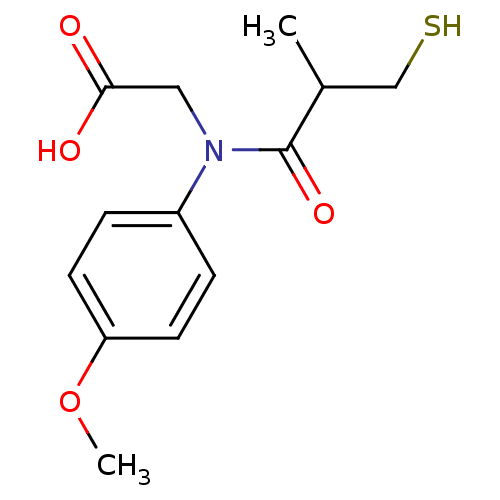
(CHEMBL1744334)Show InChI InChI=1S/C13H17NO4S/c1-9(8-19)13(17)14(7-12(15)16)10-3-5-11(18-2)6-4-10/h3-6,9,19H,7-8H2,1-2H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021783

(1-(3-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...)Show InChI InChI=1S/C12H13NO3S/c14-11(5-6-17)13-9-4-2-1-3-8(9)7-10(13)12(15)16/h1-4,10,17H,5-7H2,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase in Lactobacillus casei |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021150

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](Cc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C21H22N2O5/c24-19(25)13-23-18-9-5-4-8-15(18)10-11-16(20(23)26)22-17(21(27)28)12-14-6-2-1-3-7-14/h1-9,16-17,22H,10-13H2,(H,24,25)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027731

(1-(3-Mercapto-propionyl)-1,2,3,4-tetrahydro-quinol...)Show InChI InChI=1S/C13H15NO3S/c15-12(7-8-18)14-10-4-2-1-3-9(10)5-6-11(14)13(16)17/h1-4,11,18H,5-8H2,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase in Escherichia coli |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027717
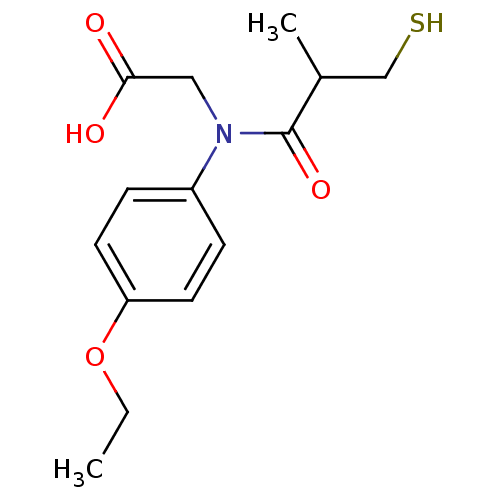
(CHEMBL282730 | [(4-Ethoxy-phenyl)-(3-mercapto-2-me...)Show InChI InChI=1S/C14H19NO4S/c1-3-19-12-6-4-11(5-7-12)15(8-13(16)17)14(18)10(2)9-20/h4-7,10,20H,3,8-9H2,1-2H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50020801

(CHEMBL23641 | [Indan-5-yl-(3-mercapto-2-methyl-pro...)Show InChI InChI=1S/C15H19NO3S/c1-10(9-20)15(19)16(8-14(17)18)13-6-5-11-3-2-4-12(11)7-13/h5-7,10,20H,2-4,8-9H2,1H3,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027716
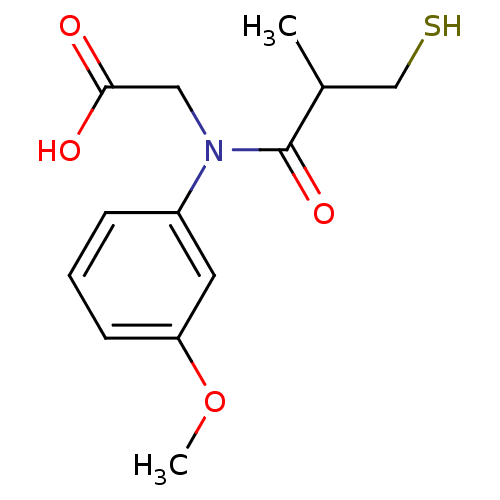
(CHEMBL23347 | [(3-Mercapto-2-methyl-propionyl)-(3-...)Show InChI InChI=1S/C13H17NO4S/c1-9(8-19)13(17)14(7-12(15)16)10-4-3-5-11(6-10)18-2/h3-6,9,19H,7-8H2,1-2H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027696
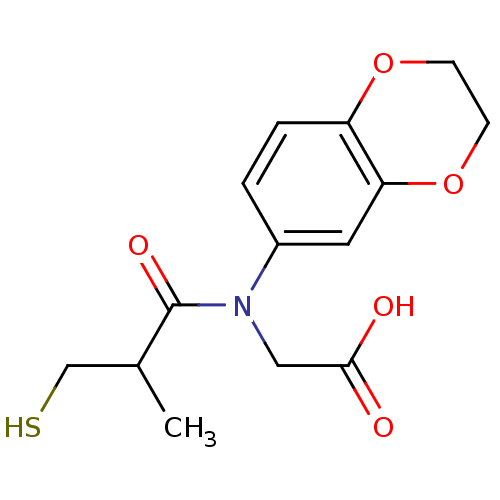
(CHEMBL23379 | [(2,3-Dihydro-benzo[1,4]dioxin-6-yl)...)Show InChI InChI=1S/C14H17NO5S/c1-9(8-21)14(18)15(7-13(16)17)10-2-3-11-12(6-10)20-5-4-19-11/h2-3,6,9,21H,4-5,7-8H2,1H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021152

(3-Benzyloxy-2-(1-carboxymethyl-2-oxo-2,3,4,5-tetra...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](COCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O6/c25-20(26)12-24-19-9-5-4-8-16(19)10-11-17(21(24)27)23-18(22(28)29)14-30-13-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50020793
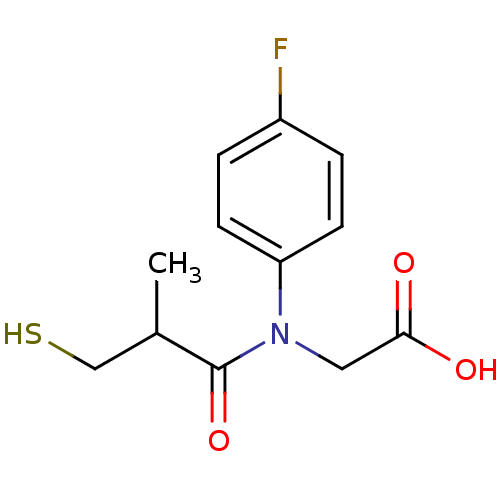
(CHEMBL23727 | [(4-Fluoro-phenyl)-(3-mercapto-2-met...)Show InChI InChI=1S/C12H14FNO3S/c1-8(7-18)12(17)14(6-11(15)16)10-4-2-9(13)3-5-10/h2-5,8,18H,6-7H2,1H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
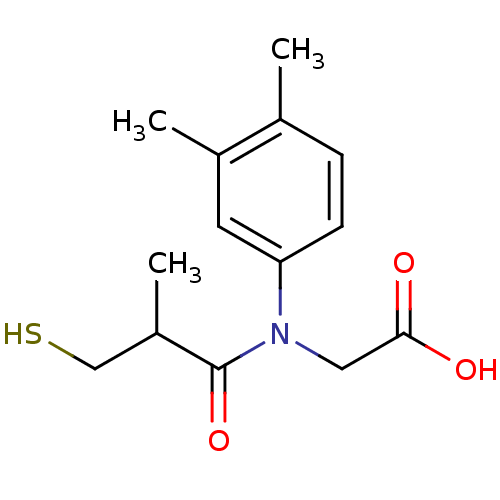
(Oryctolagus cuniculus) | BDBM50027721
(CHEMBL279938 | [(3,4-Dimethyl-phenyl)-(3-mercapto-...)Show InChI InChI=1S/C14H19NO3S/c1-9-4-5-12(6-10(9)2)15(7-13(16)17)14(18)11(3)8-19/h4-6,11,19H,7-8H2,1-3H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
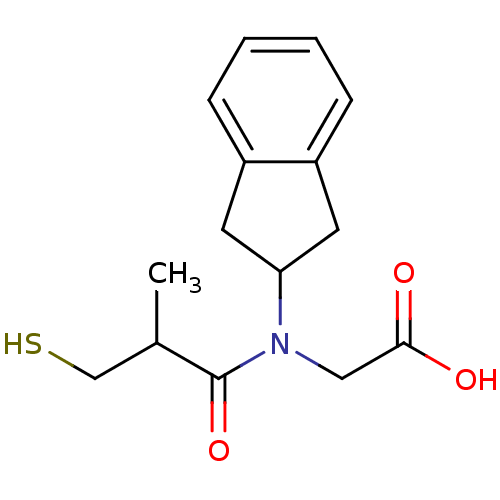
(Oryctolagus cuniculus) | BDBM50020808
(CHEMBL1788147 | CHEMBL278348 | [Indan-2-yl-(3-merc...)Show InChI InChI=1S/C15H19NO3S/c1-10(9-20)15(19)16(8-14(17)18)13-6-11-4-2-3-5-12(11)7-13/h2-5,10,13,20H,6-9H2,1H3,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
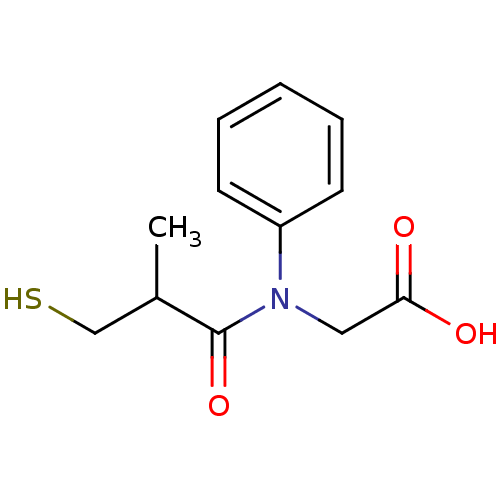
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50020800
(CHEMBL26338 | [(3-Mercapto-2-methyl-propionyl)-phe...)Show InChI InChI=1S/C12H15NO3S/c1-9(8-17)12(16)13(7-11(14)15)10-5-3-2-4-6-10/h2-6,9,17H,7-8H2,1H3,(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021141

(6-Benzyloxycarbonylamino-2-(1-carboxymethyl-2-oxo-...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CCCCNC(=O)OCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C26H31N3O7/c30-23(31)16-29-22-12-5-4-10-19(22)13-14-20(24(29)32)28-21(25(33)34)11-6-7-15-27-26(35)36-17-18-8-2-1-3-9-18/h1-5,8-10,12,20-21,28H,6-7,11,13-17H2,(H,27,35)(H,30,31)(H,33,34)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027731

(1-(3-Mercapto-propionyl)-1,2,3,4-tetrahydro-quinol...)Show InChI InChI=1S/C13H15NO3S/c15-12(7-8-18)14-10-4-2-1-3-9(10)5-6-11(14)13(16)17/h1-4,11,18H,5-8H2,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021144

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](Cc2c[nH]c3ccccc23)C(O)=O)C1=O Show InChI InChI=1S/C23H23N3O5/c27-21(28)13-26-20-8-4-1-5-14(20)9-10-18(22(26)29)25-19(23(30)31)11-15-12-24-17-7-3-2-6-16(15)17/h1-8,12,18-19,24-25H,9-11,13H2,(H,27,28)(H,30,31)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027718
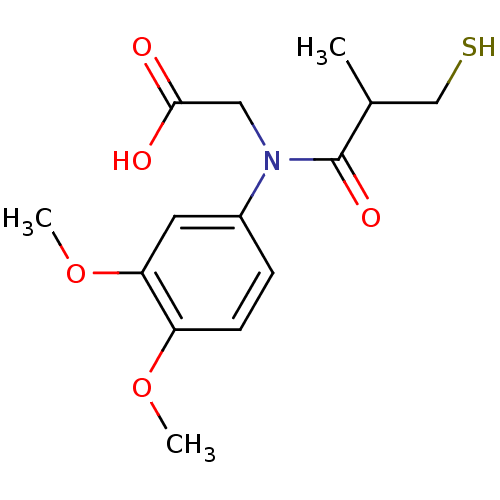
(CHEMBL280420 | [(3,4-Dimethoxy-phenyl)-(3-mercapto...)Show InChI InChI=1S/C14H19NO5S/c1-9(8-21)14(18)15(7-13(16)17)10-4-5-11(19-2)12(6-10)20-3/h4-6,9,21H,7-8H2,1-3H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021143

(6-Benzyloxycarbonylamino-2-(1-carboxymethyl-2-oxo-...)Show SMILES CCOC(=O)[C@H](CCCCNC(=O)OCc1ccccc1)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C28H35N3O7/c1-2-37-27(35)23(13-8-9-17-29-28(36)38-19-20-10-4-3-5-11-20)30-22-16-15-21-12-6-7-14-24(21)31(26(22)34)18-25(32)33/h3-7,10-12,14,22-23,30H,2,8-9,13,15-19H2,1H3,(H,29,36)(H,32,33)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027722
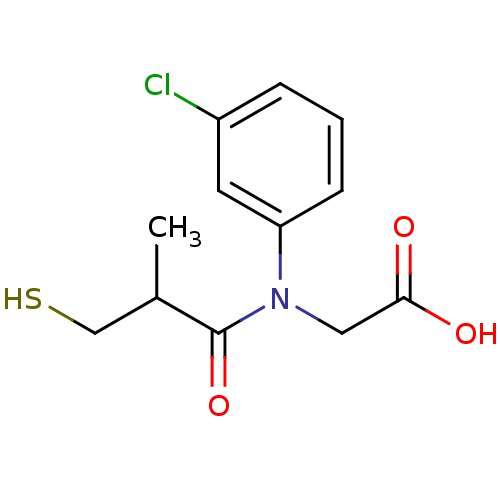
(CHEMBL24814 | [(3-Chloro-phenyl)-(3-mercapto-2-met...)Show InChI InChI=1S/C12H14ClNO3S/c1-8(7-18)12(17)14(6-11(15)16)10-4-2-3-9(13)5-10/h2-5,8,18H,6-7H2,1H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme |
J Med Chem 26: 1267-77 (1983)
BindingDB Entry DOI: 10.7270/Q29024B9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data