

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50557619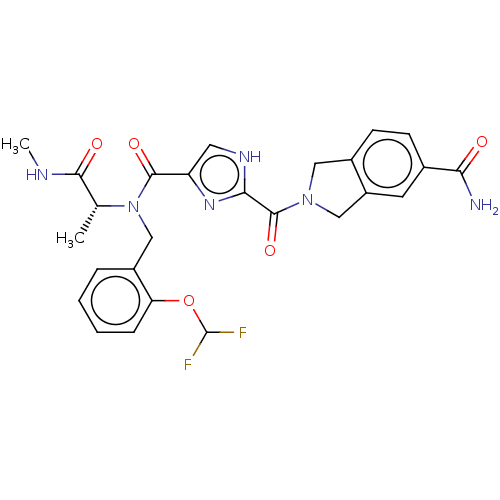 (CHEMBL4796239) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00547 BindingDB Entry DOI: 10.7270/Q20005S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50557620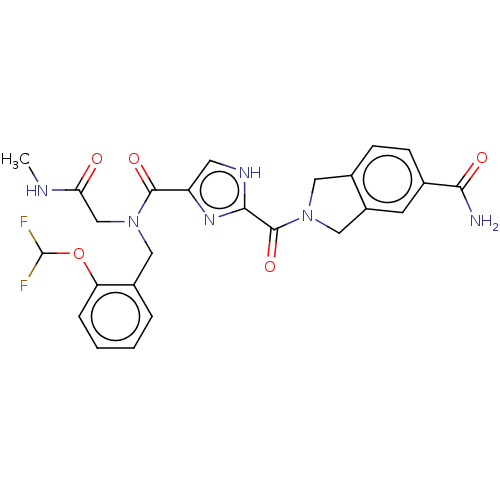 (CHEMBL4752978) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00547 BindingDB Entry DOI: 10.7270/Q20005S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50438317 (CHEMBL2408611) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00547 BindingDB Entry DOI: 10.7270/Q20005S0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50187111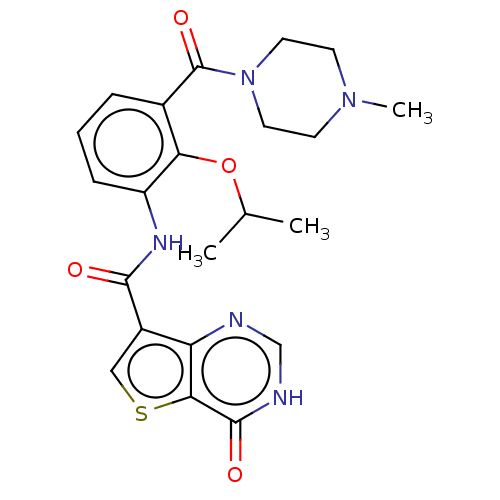 (CHEMBL3823738) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00547 BindingDB Entry DOI: 10.7270/Q20005S0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00547 BindingDB Entry DOI: 10.7270/Q20005S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433293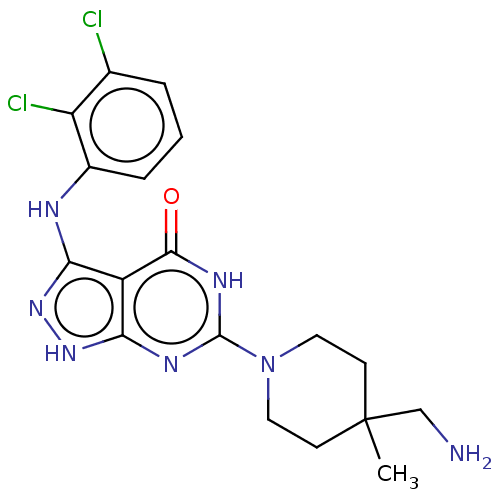 (6-(4-(aminomethyl)-4- methylpiperidin-1-yl)-3- ((2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433294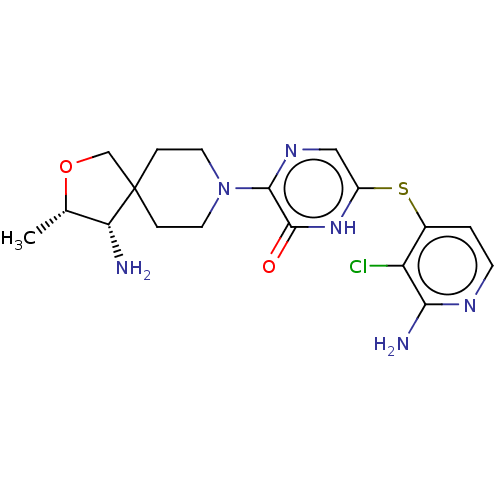 (6-((2-amino-3- chloropyridin-4-yl)thio)-3- ((3S,4S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433295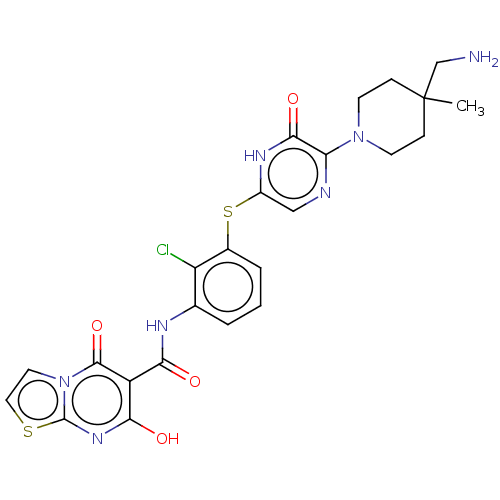 (N-(3-((5-(4- (aminomethyl)-4- methylpiperidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433296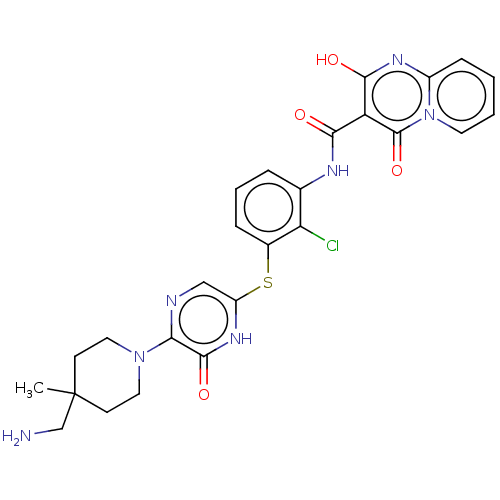 (N-(3-((5-(4- (aminomethyl)-4- methylpiperidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433297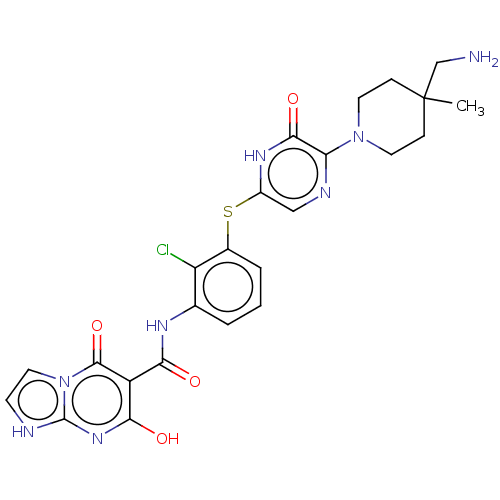 (N-(3-((5-(4- (aminomethyl)-4- methylpiperidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433300 (N-(3-((5-(4- (aminomethyl)-4- methylpiperidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433301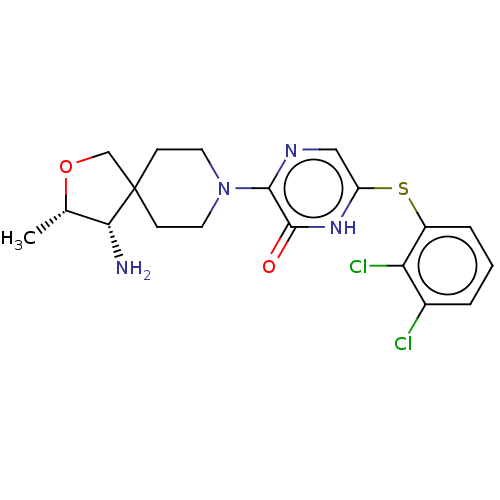 (3-((3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433302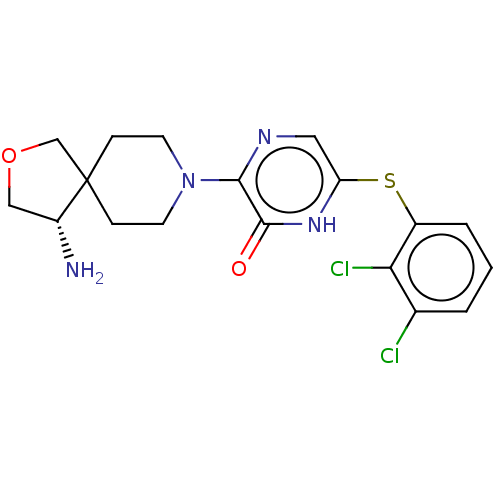 ((S)-3-(4-amino-2-oxa-8- azaspiro[4.5]decan-8-yl)- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433303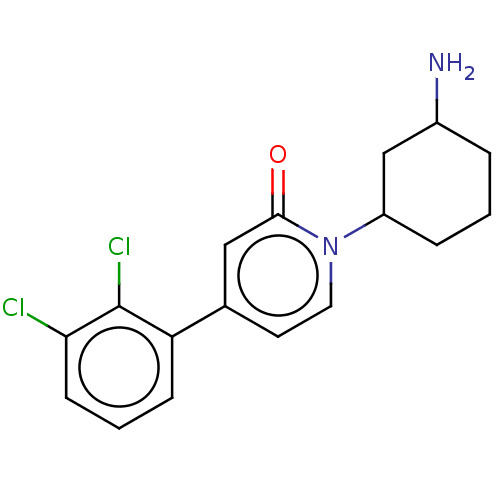 (1-(3-aminocyclohexyl)-4- (2,3- dichlorophenyl)pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433304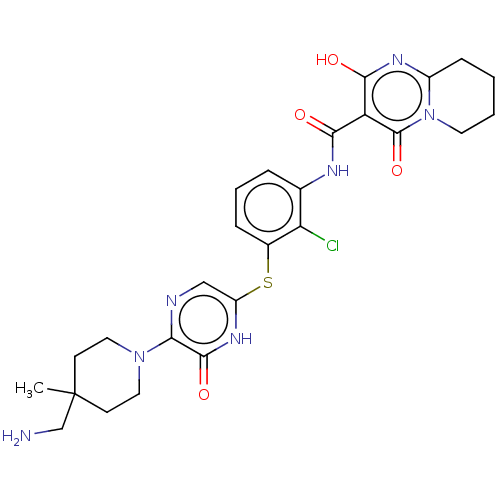 (N-(3-((5-(4- (aminomethyl)-4- methylpiperidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433305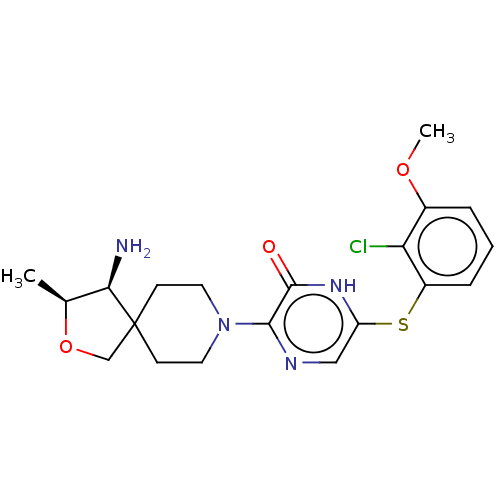 (3-((3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433306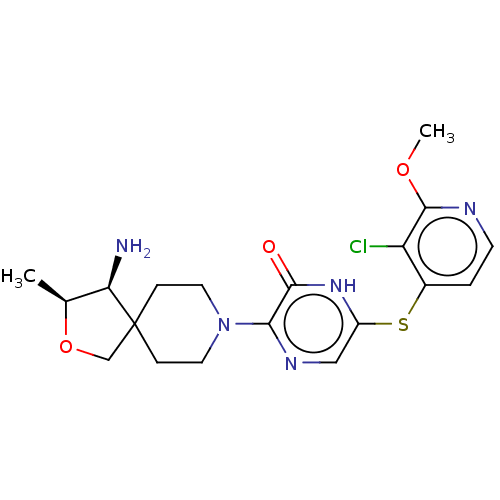 (3-((3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433307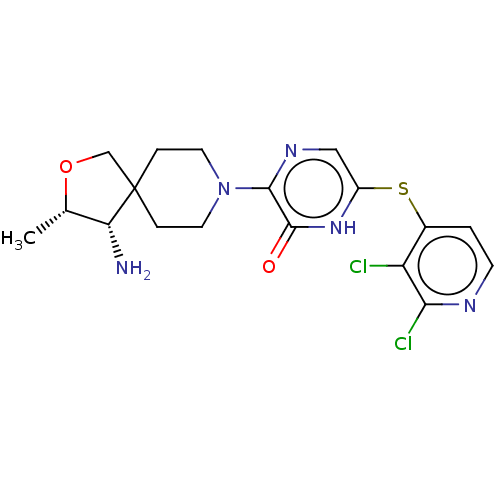 (3-((3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433308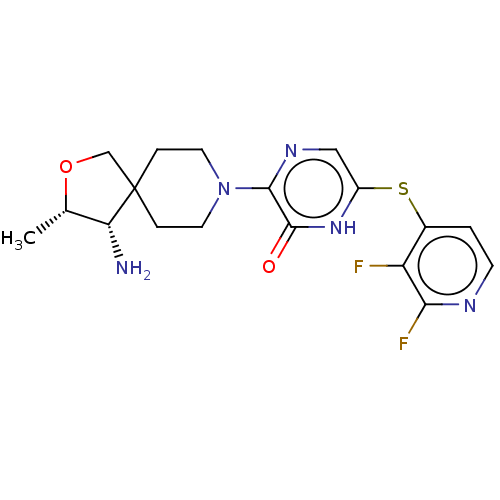 (4-((5-(4-(aminomethyl)-4- methylpiperidin-1-yl)-6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433309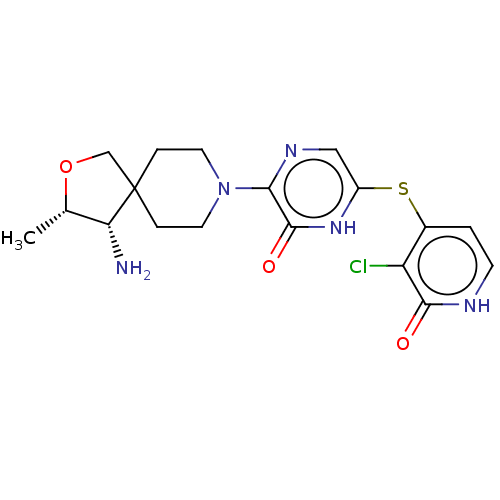 (3-((3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433310 (3-((3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433311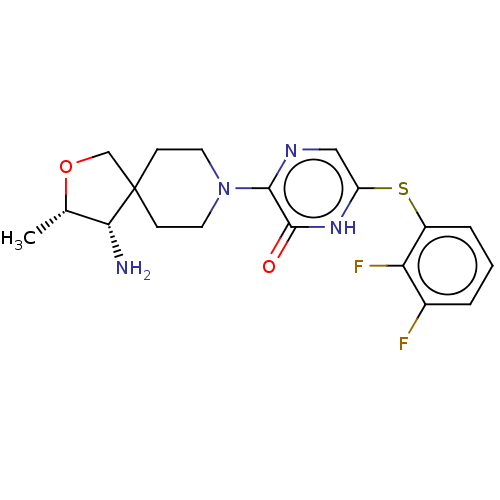 (3-((3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433314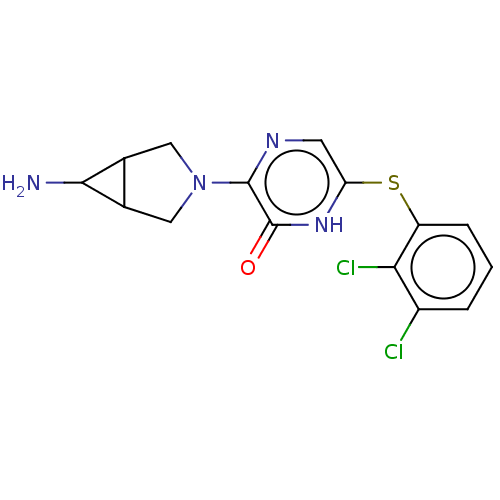 (3-(6-amino-3- azabicyclo[3.1.0]hexan-3- yl)-6-((2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433316 ((R)-3-(3-amino-3H- spiro[benzofuran-2,4'- piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433317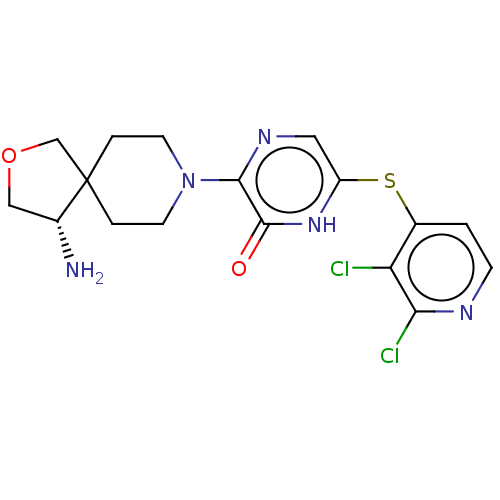 ((S)-3-(4-amino-2-oxa-8- azaspiro[4.5]decan-8-yl)- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433315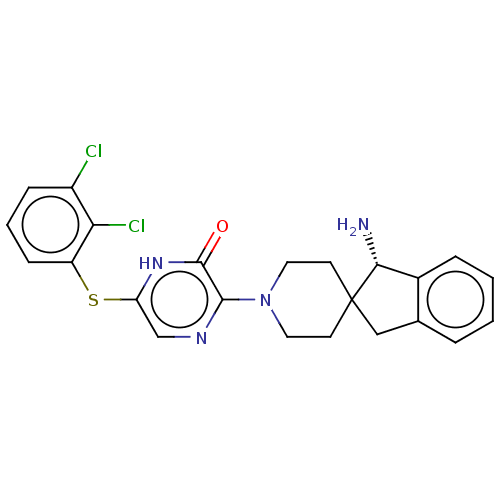 ((S)-3-(1-amino-1,3- dihydrospiro[indene-2,4'- pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433291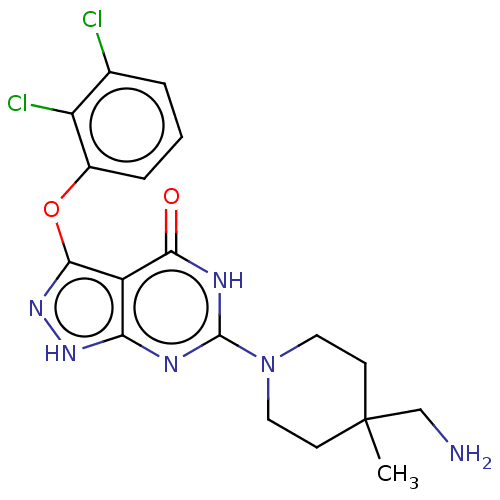 (6-(4-(aminomethyl)-4- methylpiperidin-1-yl)-3- (2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433313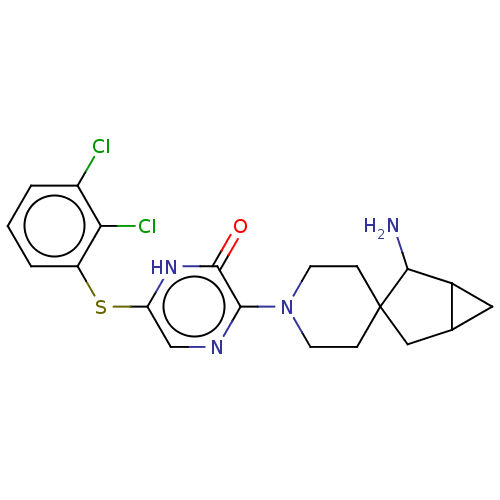 (3-(2- aminospiro[bicyclo[3.1.0] hexane-3,4'-piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433312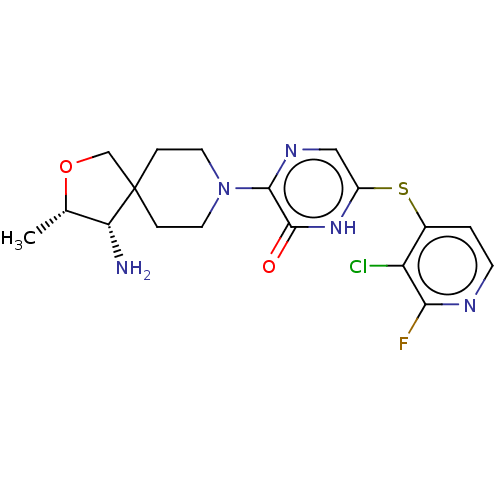 (3-((3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433298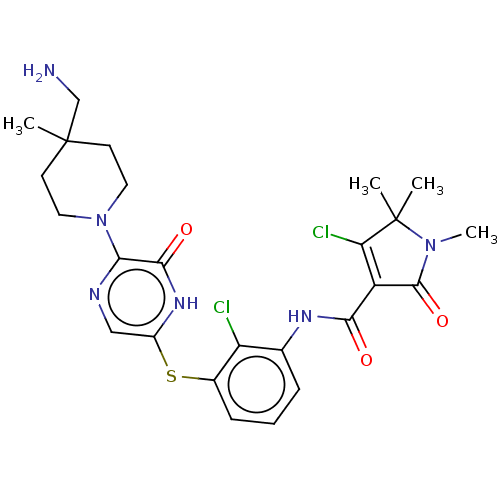 (N-(3-((5-(4- (aminomethyl)-4- methylpiperidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433290 (3-(4-(aminomethyl)-4- methylpiperidin-1-yl)-6- ((2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM433292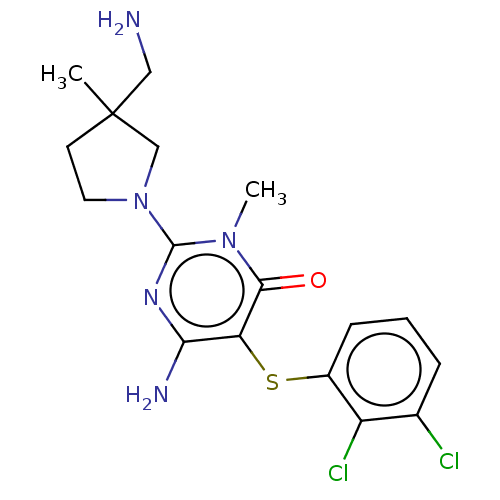 (6-amino-2-(3- (aminomethyl)-3- methylpyrrolidin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
SYNBLia Therapeutics, Inc. US Patent | Assay Description IC50 values were determined at room temperature in 384-well black polystyrene plate, using a final reaction volume of 15 μl and the following as... | US Patent US10561655 (2020) BindingDB Entry DOI: 10.7270/Q23F4S2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50557622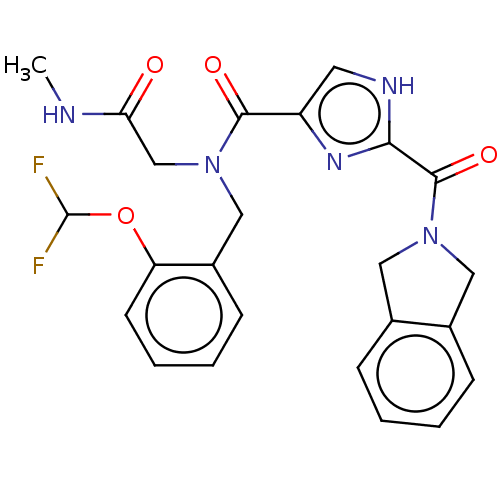 (CHEMBL4786426) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00547 BindingDB Entry DOI: 10.7270/Q20005S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50557633 (CHEMBL4795442) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00547 BindingDB Entry DOI: 10.7270/Q20005S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369905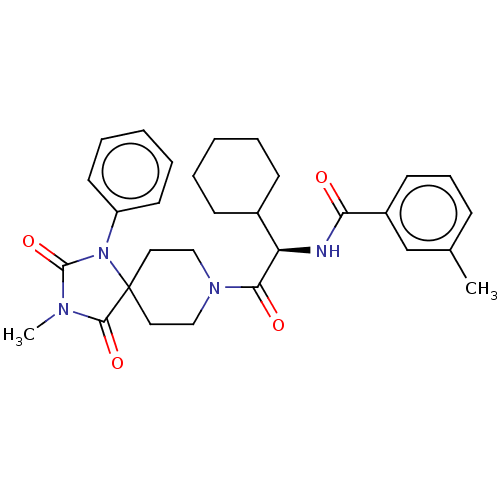 ((R)—N-(1-Cyclohexyl-2-(3-methyl-2,4-dioxo-1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369906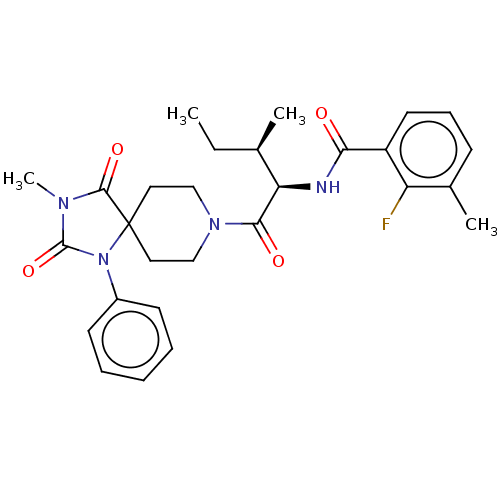 (2-Fluoro-3-methyl-N-((2R,3R)-3-methyl-1-(3-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369909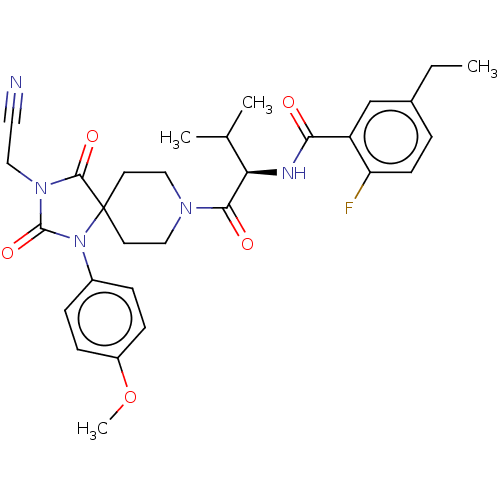 ((R)—N-(1-(3-(Cyanomethyl)-1-(4-methoxyphenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369910 ((R)-2-Fluoro-N-(1-(1-(3-fluoro-4-methoxyphenyl)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369911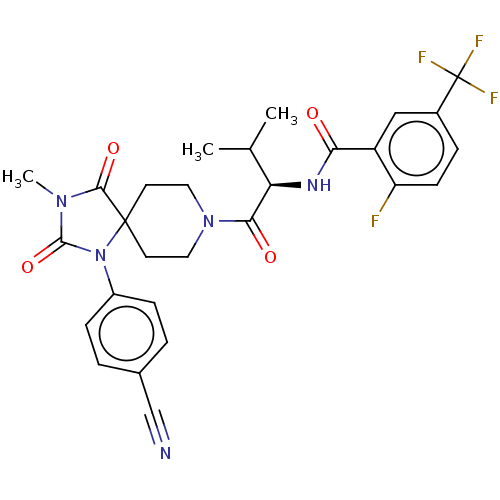 ((R)—N-(1-(1-(4-Cyanophenyl)-3-methyl-2,4-diox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369912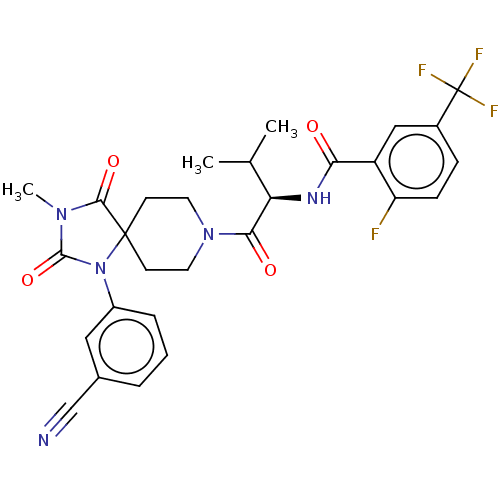 ((R)—N-(1-(1-(3-Cyanophenyl)-3-methyl-2,4-diox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369913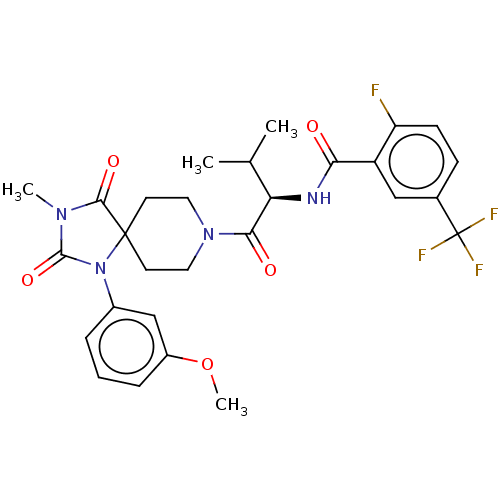 ((R)-2-Fluoro-N-(1-(1-(3-methoxyphenyl)-3-methyl-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369914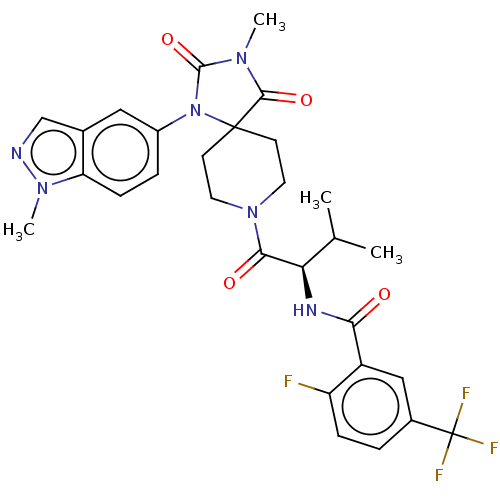 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-1-(1-methyl-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369915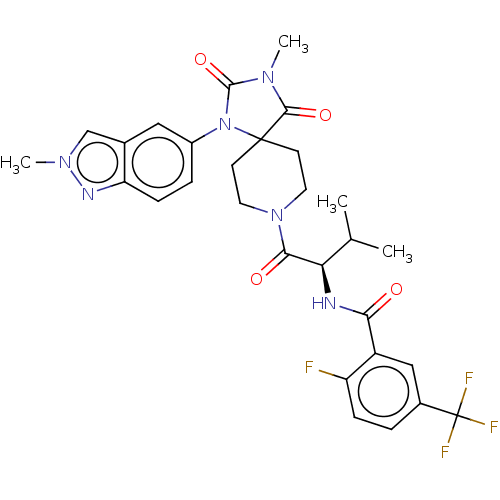 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-1-(2-methyl-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369916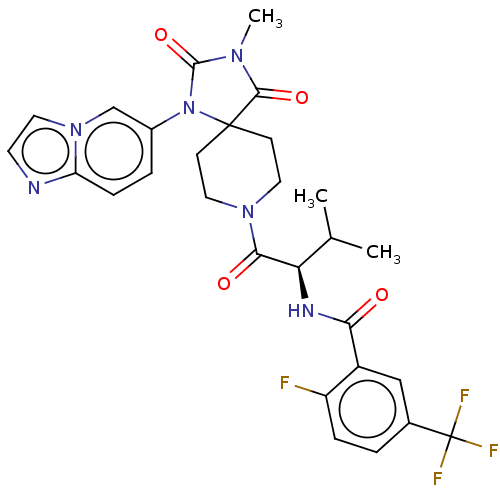 ((R)-2-Fluoro-N-(1-(1-(imidazo[1,2-a]pyridin-6-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369918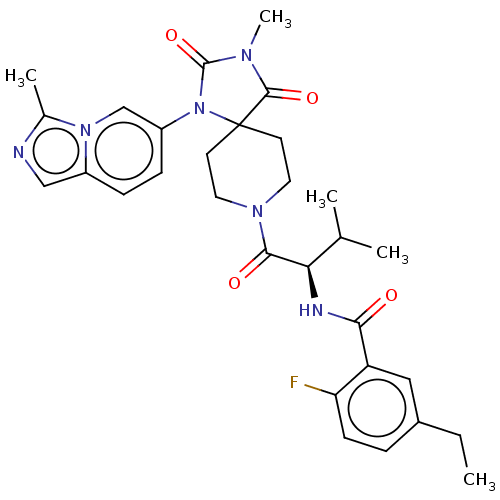 ((R)-5-Ethyl-2-fluoro-N-(3-methyl-1-(3-methyl-1-(3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369919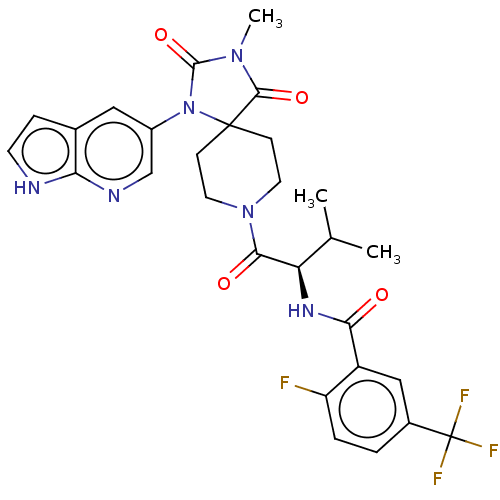 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-2,4-dioxo-1-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369920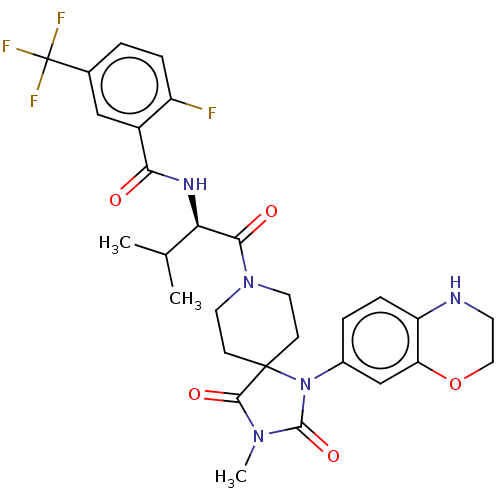 ((R)—N-(1-(1-(3,4-Dihydro-2H-benzo[b][1,4]oxaz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369921 ((R)—N-(1-Cyclopentyl-2-(3-methyl-1-(1-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369922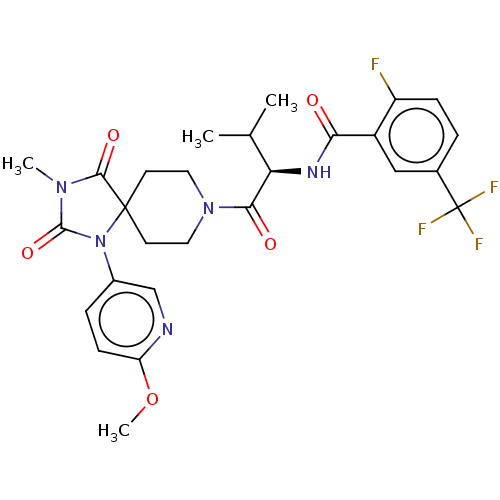 ((R)-2-Fluoro-N-(1-(1-(6-methoxypyridin-3-yl)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369923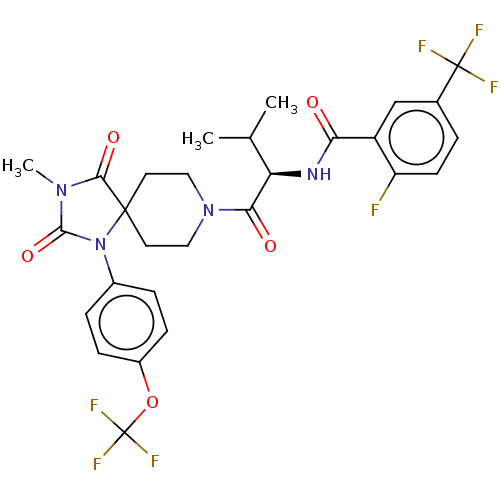 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-2,4-dioxo-1-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 317 total ) | Next | Last >> |