 Found 288 hits with Last Name = 'baker' and Initial = 'k'
Found 288 hits with Last Name = 'baker' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50430584

(CHEMBL2337806)Show SMILES C[C@H](NC(=O)c1sccc1OCc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C20H18ClNO2S/c1-14(16-5-3-2-4-6-16)22-20(23)19-18(11-12-25-19)24-13-15-7-9-17(21)10-8-15/h2-12,14H,13H2,1H3,(H,22,23)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50469035

(CHEMBL4282693)Show InChI InChI=1S/C19H13Cl2F2NO2S/c20-12-5-4-11(14(21)8-12)10-26-17-6-7-27-18(17)19(25)24-9-13-15(22)2-1-3-16(13)23/h1-8H,9-10H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50586357
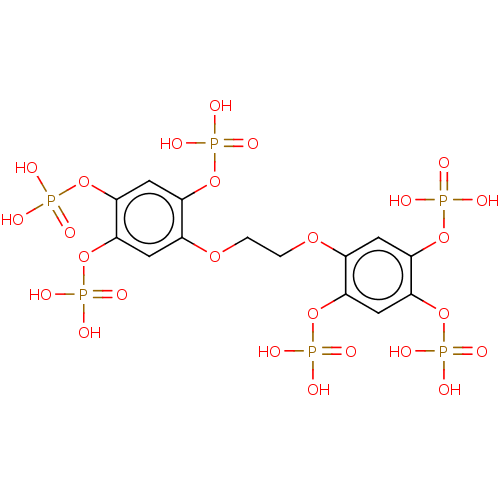
(CHEMBL5080660)Show SMILES OP(O)(=O)Oc1cc(OP(O)(O)=O)c(OP(O)(O)=O)cc1OCCOc1cc(OP(O)(O)=O)c(OP(O)(O)=O)cc1OP(O)(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM400979
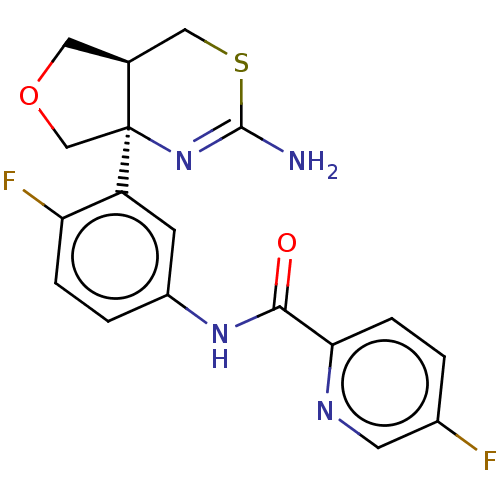
(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM150688
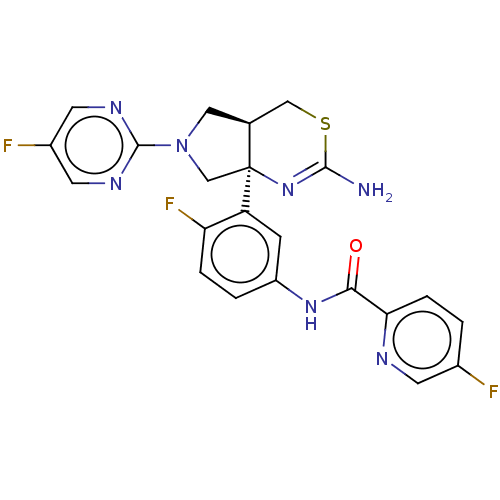
(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.388 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM150688

(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.481 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.555 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150688

(US8987254, 3 | US9999624, 3)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C22H18F3N7OS/c23-13-1-4-18(27-6-13)19(33)30-15-2-3-17(25)16(5-15)22-11-32(21-28-7-14(24)8-29-21)9-12(22)10-34-20(26)31-22/h1-8,12H,9-11H2,(H2,26,31)(H,30,33)/t12-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM400979

(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.615 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM150693

(US8987254, 8 | US9999624, 9)Show SMILES NC1=N[C@]2(CN(C[C@H]2CS1)c1ncc(F)cn1)c1cc(NC(=O)c2ncc(F)cc2F)ccc1F |r,t:1| Show InChI InChI=1S/C22H17F4N7OS/c23-12-3-17(26)18(28-5-12)19(34)31-14-1-2-16(25)15(4-14)22-10-33(21-29-6-13(24)7-30-21)8-11(22)9-35-20(27)32-22/h1-7,11H,8-10H2,(H2,27,32)(H,31,34)/t11-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM400979

(US9999624, Compound 4)Show SMILES COc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@]12CN(C[C@H]1CSC(N)=N2)c1ncc(F)cn1 |r,c:29| Show InChI InChI=1S/C22H20F2N8O2S/c1-34-18-8-26-17(7-27-18)19(33)30-14-2-3-16(24)15(4-14)22-11-32(21-28-5-13(23)6-29-21)9-12(22)10-35-20(25)31-22/h2-8,12H,9-11H2,1H3,(H2,25,31)(H,30,33)/t12-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50012647
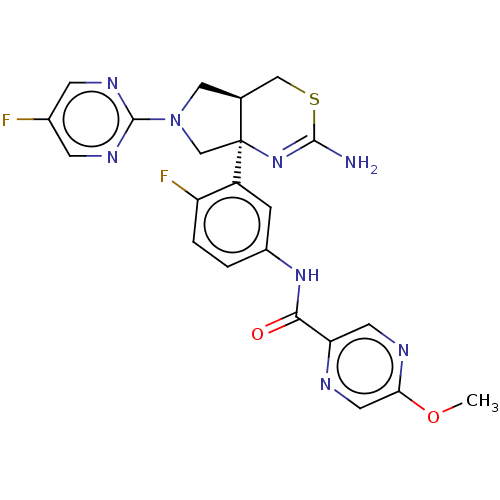
(CHEMBL2396989)Show SMILES [H][C@@]12COC[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16F2N4O2S/c19-11-1-4-15(22-6-11)16(25)23-12-2-3-14(20)13(5-12)18-9-26-7-10(18)8-27-17(21)24-18/h1-6,10H,7-9H2,(H2,21,24)(H,23,25)/t10-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50540172
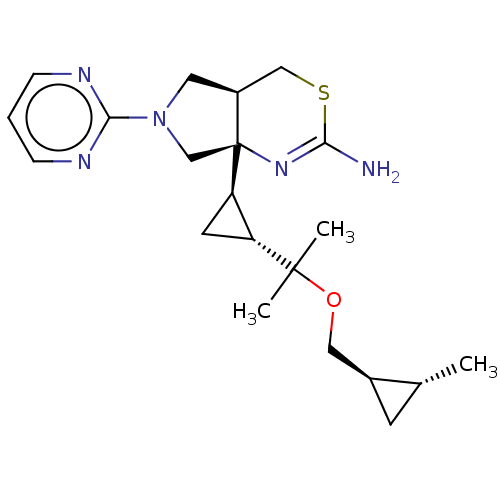
(CHEMBL4637426)Show SMILES [H][C@]1(C[C@H]1C(C)(C)OC[C@@H]1C[C@H]1C)[C@]12CN(C[C@@]1([H])CSC(N)=N2)c1ncccn1 |r,c:25| Show InChI InChI=1S/C21H31N5OS/c1-13-7-14(13)10-27-20(2,3)16-8-17(16)21-12-26(19-23-5-4-6-24-19)9-15(21)11-28-18(22)25-21/h4-6,13-17H,7-12H2,1-3H3,(H2,22,25)/t13-,14+,15+,16-,17-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158202
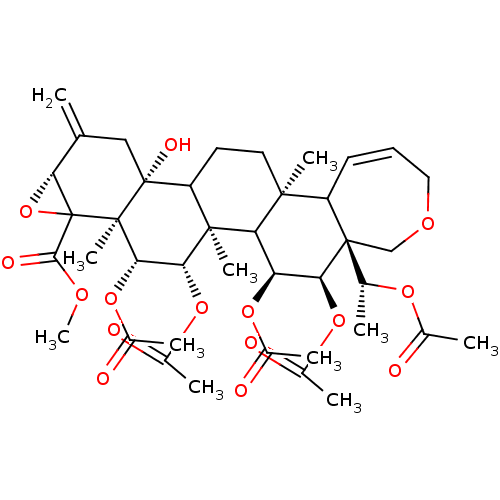
(CHEMBL434526 | Correloid derivative)Show SMILES COC(=O)C12O[C@@H]1C(=C)C[C@@]1(O)C3CC[C@@]4(C)C5C=CCOC[C@]5([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C4[C@@]3(C)[C@H](OC(C)=O)[C@H](OC(C)=O)[C@@]21C |c:19| Show InChI InChI=1S/C40H54O15/c1-19-17-39(47)27-14-15-35(8)26-13-12-16-49-18-38(26,20(2)50-21(3)41)31(52-23(5)43)28(51-22(4)42)29(35)36(27,9)32(53-24(6)44)33(54-25(7)45)37(39,10)40(30(19)55-40)34(46)48-11/h12-13,20,26-33,47H,1,14-18H2,2-11H3/t20-,26?,27?,28+,29?,30-,31+,32-,33+,35+,36+,37-,38-,39-,40?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Mus musculus (Mouse)) | BDBM50012647

(CHEMBL2396989)Show SMILES [H][C@@]12COC[C@@]1(N=C(N)SC2)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,t:7| Show InChI InChI=1S/C18H16F2N4O2S/c19-11-1-4-15(22-6-11)16(25)23-12-2-3-14(20)13(5-12)18-9-26-7-10(18)8-27-17(21)24-18/h1-6,10H,7-9H2,(H2,21,24)(H,23,25)/t10-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50371580

(CHEMBL1162175)Show SMILES CCNC(=O)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@H]2O[C@@H](O[C@@H]12)\C=C\c1ccccc1 |r| Show InChI InChI=1S/C22H25N6O8P/c1-2-23-22(29)27-19-16-20(25-11-24-19)28(12-26-16)21-18-17(14(34-21)10-33-37(30,31)32)35-15(36-18)9-8-13-6-4-3-5-7-13/h3-9,11-12,14-15,17-18,21H,2,10H2,1H3,(H2,30,31,32)(H2,23,24,25,27,29)/b9-8+/t14-,15+,17-,18-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay |
J Med Chem 51: 1007-25 (2008)
Article DOI: 10.1021/jm701348d
BindingDB Entry DOI: 10.7270/Q2P55PB6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158200

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES COc1ccccc1C(=C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:20,53| Show InChI InChI=1S/C41H54O11/c1-23(30-14-11-12-15-31(30)47-10)21-41(46)24(2)20-34(50-27(5)43)39(9)33(41)17-18-38(8)32-16-13-19-48-22-40(32,25(3)49-26(4)42)37(52-29(7)45)35(36(38)39)51-28(6)44/h11-16,20,25,32-37,46H,1,17-19,21-22H2,2-10H3/t25-,32?,33?,34-,35+,36?,37+,38+,39-,40-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158213
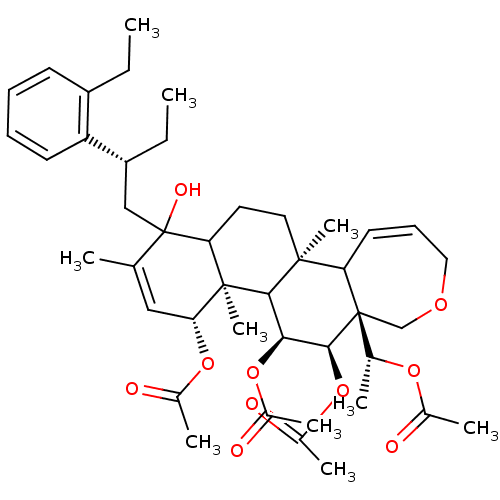
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CC[C@H](CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C)c1ccccc1CC |c:12,45| Show InChI InChI=1S/C43H60O10/c1-11-31-16-13-14-17-33(31)32(12-2)23-43(48)25(3)22-36(51-28(6)45)41(10)35(43)19-20-40(9)34-18-15-21-49-24-42(34,26(4)50-27(5)44)39(53-30(8)47)37(38(40)41)52-29(7)46/h13-18,22,26,32,34-39,48H,11-12,19-21,23-24H2,1-10H3/t26-,32-,34?,35?,36-,37+,38?,39+,40+,41-,42-,43?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158214
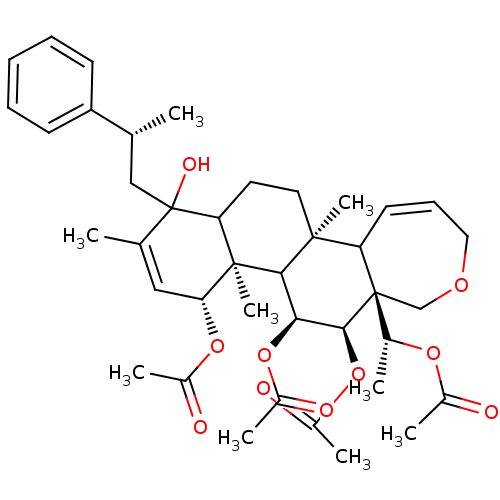
(Aceticacid(R)-1-[(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES C[C@H](CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C)c1ccccc1 |c:11,44| Show InChI InChI=1S/C40H54O10/c1-23(30-14-11-10-12-15-30)21-40(45)24(2)20-33(48-27(5)42)38(9)32(40)17-18-37(8)31-16-13-19-46-22-39(31,25(3)47-26(4)41)36(50-29(7)44)34(35(37)38)49-28(6)43/h10-16,20,23,25,31-36,45H,17-19,21-22H2,1-9H3/t23-,25-,31?,32?,33-,34+,35?,36+,37+,38-,39-,40?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158218
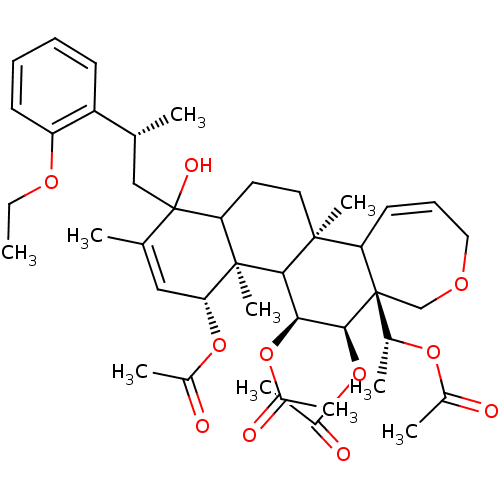
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CCOc1ccccc1[C@H](C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:21,54| Show InChI InChI=1S/C42H58O11/c1-11-49-32-16-13-12-15-31(32)24(2)22-42(47)25(3)21-35(51-28(6)44)40(10)34(42)18-19-39(9)33-17-14-20-48-23-41(33,26(4)50-27(5)43)38(53-30(8)46)36(37(39)40)52-29(7)45/h12-17,21,24,26,33-38,47H,11,18-20,22-23H2,1-10H3/t24-,26-,33?,34?,35-,36+,37?,38+,39+,40-,41-,42?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 of human T cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50371581
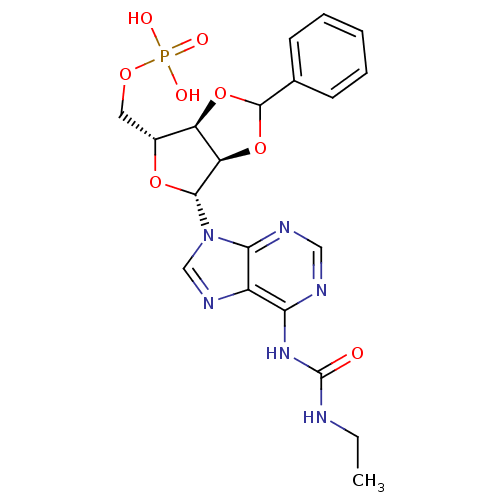
(CHEMBL1162179)Show SMILES CCNC(=O)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@H]2OC(O[C@@H]12)c1ccccc1 |r| Show InChI InChI=1S/C20H23N6O8P/c1-2-21-20(27)25-16-13-17(23-9-22-16)26(10-24-13)18-15-14(12(32-18)8-31-35(28,29)30)33-19(34-15)11-6-4-3-5-7-11/h3-7,9-10,12,14-15,18-19H,2,8H2,1H3,(H2,28,29,30)(H2,21,22,23,25,27)/t12-,14-,15-,18-,19?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay |
J Med Chem 51: 1007-25 (2008)
Article DOI: 10.1021/jm701348d
BindingDB Entry DOI: 10.7270/Q2P55PB6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158224

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES COc1ccccc1CCC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:19,52| Show InChI InChI=1S/C40H54O11/c1-23-21-33(49-26(4)42)38(8)32(40(23,45)19-16-29-13-10-11-14-30(29)46-9)17-18-37(7)31-15-12-20-47-22-39(31,24(2)48-25(3)41)36(51-28(6)44)34(35(37)38)50-27(5)43/h10-15,21,24,31-36,45H,16-20,22H2,1-9H3/t24-,31?,32?,33-,34+,35?,36+,37+,38-,39-,40?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158233
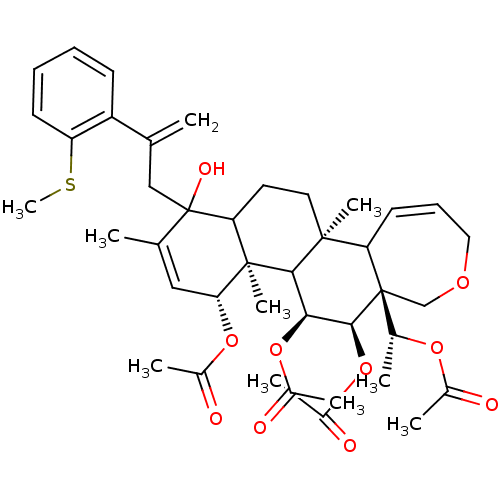
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CSc1ccccc1C(=C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:20,53| Show InChI InChI=1S/C41H54O10S/c1-23(30-14-11-12-15-31(30)52-10)21-41(46)24(2)20-34(49-27(5)43)39(9)33(41)17-18-38(8)32-16-13-19-47-22-40(32,25(3)48-26(4)42)37(51-29(7)45)35(36(38)39)50-28(6)44/h11-16,20,25,32-37,46H,1,17-19,21-22H2,2-10H3/t25-,32?,33?,34-,35+,36?,37+,38+,39-,40-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158233

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CSc1ccccc1C(=C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:20,53| Show InChI InChI=1S/C41H54O10S/c1-23(30-14-11-12-15-31(30)52-10)21-41(46)24(2)20-34(49-27(5)43)39(9)33(41)17-18-38(8)32-16-13-19-47-22-40(32,25(3)48-26(4)42)37(51-29(7)45)35(36(38)39)50-28(6)44/h11-16,20,25,32-37,46H,1,17-19,21-22H2,2-10H3/t25-,32?,33?,34-,35+,36?,37+,38+,39-,40-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 of human T cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50371580

(CHEMBL1162175)Show SMILES CCNC(=O)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@H]2O[C@@H](O[C@@H]12)\C=C\c1ccccc1 |r| Show InChI InChI=1S/C22H25N6O8P/c1-2-23-22(29)27-19-16-20(25-11-24-19)28(12-26-16)21-18-17(14(34-21)10-33-37(30,31)32)35-15(36-18)9-8-13-6-4-3-5-7-13/h3-9,11-12,14-15,17-18,21H,2,10H2,1H3,(H2,30,31,32)(H2,23,24,25,27,29)/b9-8+/t14-,15+,17-,18-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay |
J Med Chem 51: 1007-25 (2008)
Article DOI: 10.1021/jm701348d
BindingDB Entry DOI: 10.7270/Q2P55PB6 |
More data for this
Ligand-Target Pair | |
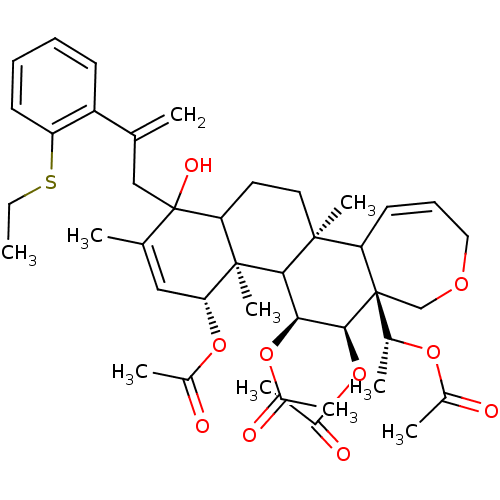
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158228
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CCSc1ccccc1C(=C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:21,54| Show InChI InChI=1S/C42H56O10S/c1-11-53-32-16-13-12-15-31(32)24(2)22-42(47)25(3)21-35(50-28(6)44)40(10)34(42)18-19-39(9)33-17-14-20-48-23-41(33,26(4)49-27(5)43)38(52-30(8)46)36(37(39)40)51-29(7)45/h12-17,21,26,33-38,47H,2,11,18-20,22-23H2,1,3-10H3/t26-,33?,34?,35-,36+,37?,38+,39+,40-,41-,42?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158227

(Aceticacid1-[4,5,6-triacetoxy-1-hydroxy-2,4a,11b-t...)Show SMILES C[C@@H](OC(C)=O)[C@]12COCC=CC1[C@]1(C)CCC3[C@](O)(CC(=C)c4ccccc4)C(C)=C[C@@H](OC(C)=O)[C@]3(C)C1[C@H](OC(C)=O)[C@@H]2OC(C)=O |c:10,32| Show InChI InChI=1S/C40H52O10/c1-23(30-14-11-10-12-15-30)21-40(45)24(2)20-33(48-27(5)42)38(9)32(40)17-18-37(8)31-16-13-19-46-22-39(31,25(3)47-26(4)41)36(50-29(7)44)34(35(37)38)49-28(6)43/h10-16,20,25,31-36,45H,1,17-19,21-22H2,2-9H3/t25-,31?,32?,33-,34+,35?,36+,37+,38-,39-,40+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158195
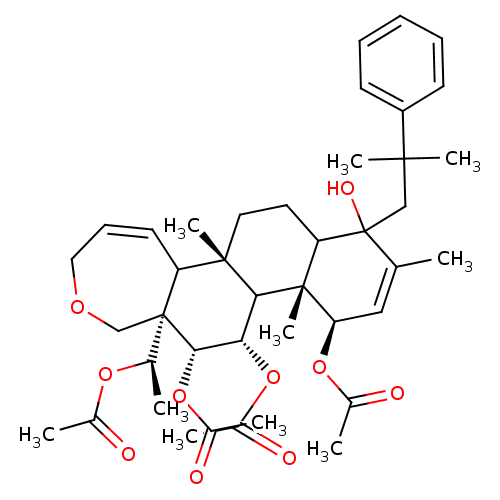
(Aceticacid(R)-1-[(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES C[C@@H](OC(C)=O)[C@]12COCC=CC1[C@]1(C)CCC3C(O)(CC(C)(C)c4ccccc4)C(C)=C[C@@H](OC(C)=O)[C@]3(C)C1[C@H](OC(C)=O)[C@@H]2OC(C)=O |c:10,33| Show InChI InChI=1S/C41H56O10/c1-24-21-33(49-27(4)43)39(10)32(41(24,46)22-37(7,8)30-15-12-11-13-16-30)18-19-38(9)31-17-14-20-47-23-40(31,25(2)48-26(3)42)36(51-29(6)45)34(35(38)39)50-28(5)44/h11-17,21,25,31-36,46H,18-20,22-23H2,1-10H3/t25-,31?,32?,33-,34+,35?,36+,38+,39-,40-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
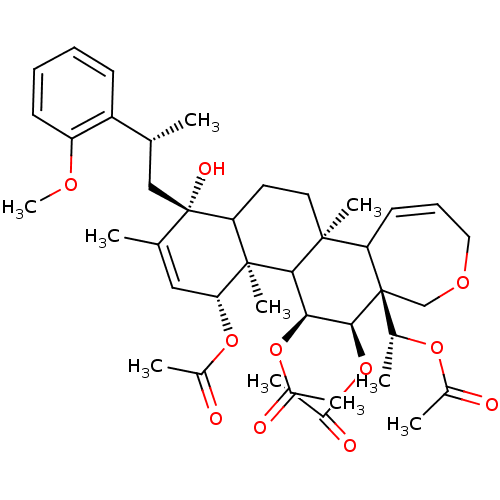
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158210
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES COc1ccccc1[C@H](C)C[C@@]1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:20,53| Show InChI InChI=1S/C41H56O11/c1-23(30-14-11-12-15-31(30)47-10)21-41(46)24(2)20-34(50-27(5)43)39(9)33(41)17-18-38(8)32-16-13-19-48-22-40(32,25(3)49-26(4)42)37(52-29(7)45)35(36(38)39)51-28(6)44/h11-16,20,23,25,32-37,46H,17-19,21-22H2,1-10H3/t23-,25-,32?,33?,34-,35+,36?,37+,38+,39-,40-,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158226
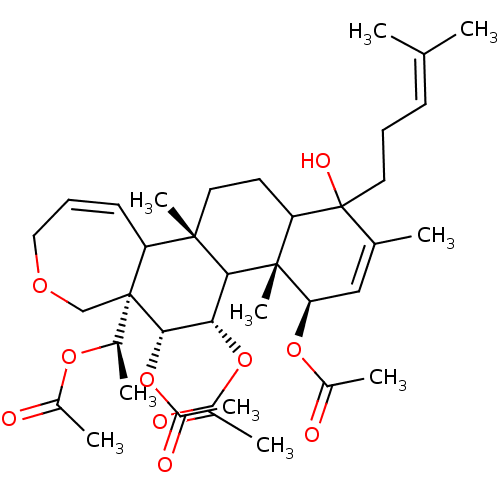
(Aceticacid(R)-1-[(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES [#6]-[#6@@H](-[#8]-[#6](-[#6])=O)[C@]12[#6]-[#8]-[#6]-[#6]=[#6]-[#6]1[C@]1([#6])[#6]-[#6]-[#6]3C([#8])([#6]-[#6]\[#6]=[#6](\[#6])-[#6])[#6](-[#6])=[#6]-[#6@@H](-[#8]-[#6](-[#6])=O)[C@]3([#6])[#6]1-[#6@H](-[#8]-[#6](-[#6])=O)-[#6@@H]2-[#8]-[#6](-[#6])=O |c:10,28| Show InChI InChI=1S/C37H54O10/c1-21(2)13-11-16-37(42)22(3)19-30(45-25(6)39)35(10)29(37)15-17-34(9)28-14-12-18-43-20-36(28,23(4)44-24(5)38)33(47-27(8)41)31(32(34)35)46-26(7)40/h12-14,19,23,28-33,42H,11,15-18,20H2,1-10H3/t23-,28?,29?,30-,31+,32?,33+,34+,35-,36-,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158209

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES COc1ccccc1[C@H](CC=C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:22,55| Show InChI InChI=1S/C43H58O11/c1-11-15-31(32-16-12-13-17-33(32)49-10)23-43(48)25(2)22-36(52-28(5)45)41(9)35(43)19-20-40(8)34-18-14-21-50-24-42(34,26(3)51-27(4)44)39(54-30(7)47)37(38(40)41)53-29(6)46/h11-14,16-18,22,26,31,34-39,48H,1,15,19-21,23-24H2,2-10H3/t26-,31-,34?,35?,36-,37+,38?,39+,40+,41-,42-,43?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50586355
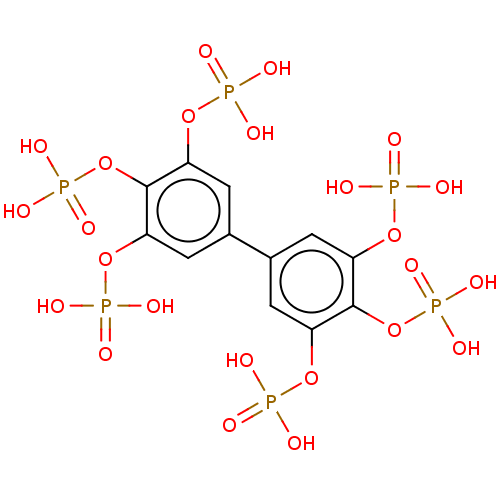
(CHEMBL5087243)Show SMILES OP(O)(=O)Oc1cc(cc(OP(O)(O)=O)c1OP(O)(O)=O)-c1cc(OP(O)(O)=O)c(OP(O)(O)=O)c(OP(O)(O)=O)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158197
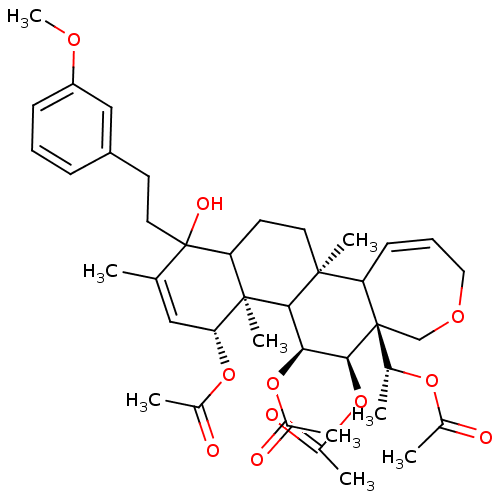
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES COc1cccc(CCC2(O)C3CC[C@@]4(C)C5C=CCOC[C@]5([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C4[C@@]3(C)[C@H](OC(C)=O)C=C2C)c1 |c:17,50| Show InChI InChI=1S/C40H54O11/c1-23-20-33(49-26(4)42)38(8)32(40(23,45)18-15-29-12-10-13-30(21-29)46-9)16-17-37(7)31-14-11-19-47-22-39(31,24(2)48-25(3)41)36(51-28(6)44)34(35(37)38)50-27(5)43/h10-14,20-21,24,31-36,45H,15-19,22H2,1-9H3/t24-,31?,32?,33-,34+,35?,36+,37+,38-,39-,40?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
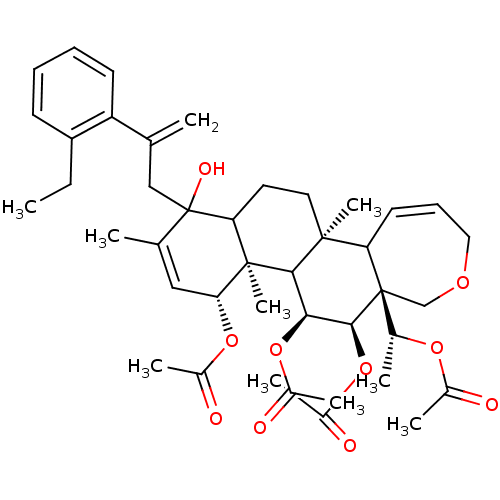
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158217
(Aceticacid1-{(R)-4,5,6-triacetoxy-1-[2-(2-ethyl-ph...)Show SMILES CCc1ccccc1C(=C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:20,53| Show InChI InChI=1S/C42H56O10/c1-11-31-15-12-13-16-32(31)24(2)22-42(47)25(3)21-35(50-28(6)44)40(10)34(42)18-19-39(9)33-17-14-20-48-23-41(33,26(4)49-27(5)43)38(52-30(8)46)36(37(39)40)51-29(7)45/h12-17,21,26,33-38,47H,2,11,18-20,22-23H2,1,3-10H3/t26-,33?,34?,35-,36+,37?,38+,39+,40-,41-,42?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 of human T cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158210

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES COc1ccccc1[C@H](C)C[C@@]1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:20,53| Show InChI InChI=1S/C41H56O11/c1-23(30-14-11-12-15-31(30)47-10)21-41(46)24(2)20-34(50-27(5)43)39(9)33(41)17-18-38(8)32-16-13-19-48-22-40(32,25(3)49-26(4)42)37(52-29(7)45)35(36(38)39)51-28(6)44/h11-16,20,23,25,32-37,46H,17-19,21-22H2,1-10H3/t23-,25-,32?,33?,34-,35+,36?,37+,38+,39-,40-,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 of human T cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158211
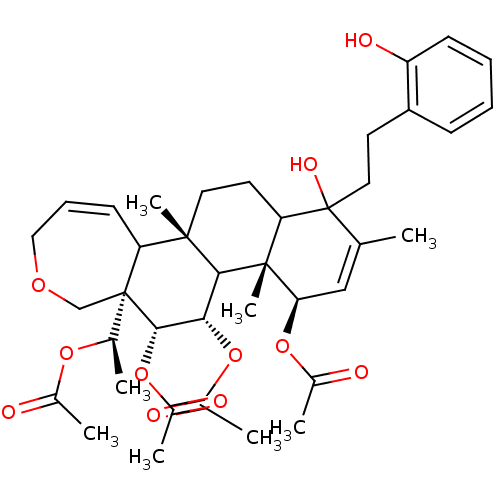
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES C[C@@H](OC(C)=O)[C@]12COCC=CC1[C@]1(C)CCC3C(O)(CCc4ccccc4O)C(C)=C[C@@H](OC(C)=O)[C@]3(C)C1[C@H](OC(C)=O)[C@@H]2OC(C)=O |c:10,32| Show InChI InChI=1S/C39H52O11/c1-22-20-32(48-25(4)41)37(8)31(39(22,45)18-15-28-12-9-10-13-29(28)44)16-17-36(7)30-14-11-19-46-21-38(30,23(2)47-24(3)40)35(50-27(6)43)33(34(36)37)49-26(5)42/h9-14,20,23,30-35,44-45H,15-19,21H2,1-8H3/t23-,30?,31?,32-,33+,34?,35+,36+,37-,38-,39?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158215
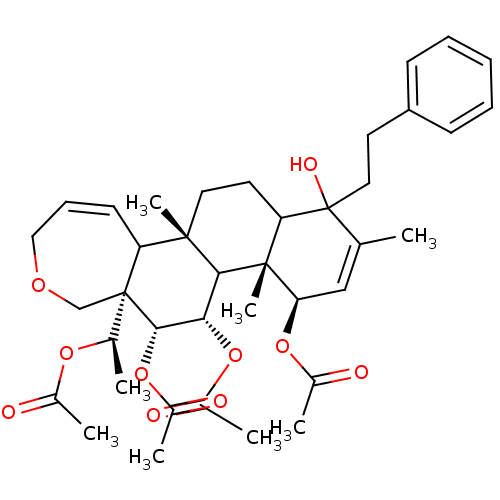
(Aceticacid(R)-1-((4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES C[C@@H](OC(C)=O)[C@]12COCC=CC1[C@]1(C)CCC3C(O)(CCc4ccccc4)C(C)=C[C@@H](OC(C)=O)[C@]3(C)C1[C@H](OC(C)=O)[C@@H]2OC(C)=O |c:10,31| Show InChI InChI=1S/C39H52O10/c1-23-21-32(47-26(4)41)37(8)31(39(23,44)19-16-29-13-10-9-11-14-29)17-18-36(7)30-15-12-20-45-22-38(30,24(2)46-25(3)40)35(49-28(6)43)33(34(36)37)48-27(5)42/h9-15,21,24,30-35,44H,16-20,22H2,1-8H3/t24-,30?,31?,32-,33+,34?,35+,36+,37-,38-,39?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50586354
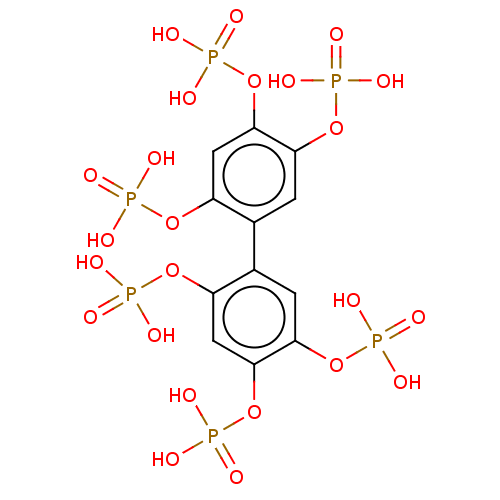
(CHEMBL5078323)Show SMILES OP(O)(=O)Oc1cc(OP(O)(O)=O)c(cc1OP(O)(O)=O)-c1cc(OP(O)(O)=O)c(OP(O)(O)=O)cc1OP(O)(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50540171
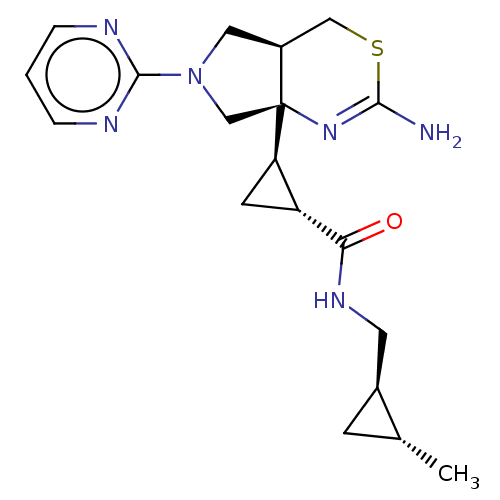
(CHEMBL4643727)Show SMILES [H][C@]1(C[C@H]1C(=O)NC[C@@H]1C[C@H]1C)[C@]12CN(C[C@@]1([H])CSC(N)=N2)c1ncccn1 |r,c:24| Show InChI InChI=1S/C19H26N6OS/c1-11-5-12(11)7-23-16(26)14-6-15(14)19-10-25(18-21-3-2-4-22-18)8-13(19)9-27-17(20)24-19/h2-4,11-15H,5-10H2,1H3,(H2,20,24)(H,23,26)/t11-,12+,13+,14-,15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00489
BindingDB Entry DOI: 10.7270/Q29W0KBW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158206
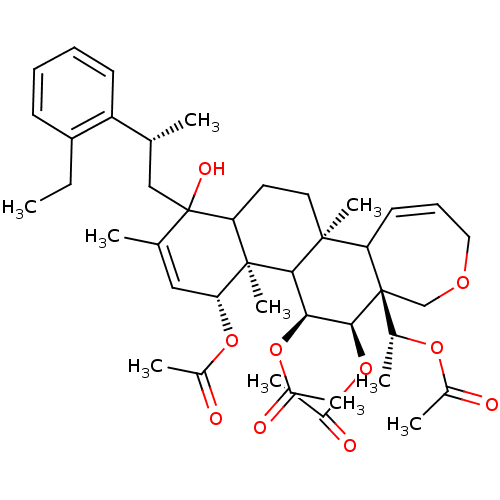
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CCc1ccccc1[C@H](C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:20,53| Show InChI InChI=1S/C42H58O10/c1-11-31-15-12-13-16-32(31)24(2)22-42(47)25(3)21-35(50-28(6)44)40(10)34(42)18-19-39(9)33-17-14-20-48-23-41(33,26(4)49-27(5)43)38(52-30(8)46)36(37(39)40)51-29(7)45/h12-17,21,24,26,33-38,47H,11,18-20,22-23H2,1-10H3/t24-,26-,33?,34?,35-,36+,37?,38+,39+,40-,41-,42?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158216
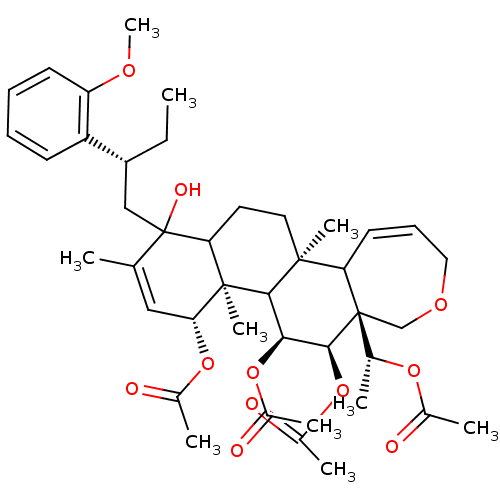
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CC[C@H](CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C)c1ccccc1OC |c:12,45| Show InChI InChI=1S/C42H58O11/c1-11-30(31-15-12-13-16-32(31)48-10)22-42(47)24(2)21-35(51-27(5)44)40(9)34(42)18-19-39(8)33-17-14-20-49-23-41(33,25(3)50-26(4)43)38(53-29(7)46)36(37(39)40)52-28(6)45/h12-17,21,25,30,33-38,47H,11,18-20,22-23H2,1-10H3/t25-,30-,33?,34?,35-,36+,37?,38+,39+,40-,41-,42?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50586353

(CHEMBL5092991)Show SMILES OP(O)(=O)Oc1cc(OP(O)(O)=O)c(c(OP(O)(O)=O)c1)-c1cc(OP(O)(O)=O)cc(OP(O)(O)=O)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158216

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CC[C@H](CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C)c1ccccc1OC |c:12,45| Show InChI InChI=1S/C42H58O11/c1-11-30(31-15-12-13-16-32(31)48-10)22-42(47)24(2)21-35(51-27(5)44)40(9)34(42)18-19-39(8)33-17-14-20-49-23-41(33,25(3)50-26(4)43)38(53-29(7)46)36(37(39)40)52-28(6)45/h12-17,21,25,30,33-38,47H,11,18-20,22-23H2,1-10H3/t25-,30-,33?,34?,35-,36+,37?,38+,39+,40-,41-,42?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 of human T cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158223

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES C[C@@H](OC(C)=O)[C@]12COCC=CC1[C@]1(C)CCC3C(O)(CCc4ccccc4OC(C)=O)C(C)=C[C@@H](OC(C)=O)[C@]3(C)C1[C@H](OC(C)=O)[C@@H]2OC(C)=O |c:10,35| Show InChI InChI=1S/C41H54O12/c1-23-21-34(51-27(5)44)39(9)33(41(23,47)19-16-30-13-10-11-14-31(30)50-26(4)43)17-18-38(8)32-15-12-20-48-22-40(32,24(2)49-25(3)42)37(53-29(7)46)35(36(38)39)52-28(6)45/h10-15,21,24,32-37,47H,16-20,22H2,1-9H3/t24-,32?,33?,34-,35+,36?,37+,38+,39-,40-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50586357

(CHEMBL5080660)Show SMILES OP(O)(=O)Oc1cc(OP(O)(O)=O)c(OP(O)(O)=O)cc1OCCOc1cc(OP(O)(O)=O)c(OP(O)(O)=O)cc1OP(O)(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158228

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CCSc1ccccc1C(=C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:21,54| Show InChI InChI=1S/C42H56O10S/c1-11-53-32-16-13-12-15-31(32)24(2)22-42(47)25(3)21-35(50-28(6)44)40(10)34(42)18-19-39(9)33-17-14-20-48-23-41(33,26(4)49-27(5)43)38(52-30(8)46)36(37(39)40)51-29(7)45/h12-17,21,26,33-38,47H,2,11,18-20,22-23H2,1,3-10H3/t26-,33?,34?,35-,36+,37?,38+,39+,40-,41-,42?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 of human T cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158209

(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES COc1ccccc1[C@H](CC=C)CC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:22,55| Show InChI InChI=1S/C43H58O11/c1-11-15-31(32-16-12-13-17-33(32)49-10)23-43(48)25(2)22-36(52-28(5)45)41(9)35(43)19-20-40(8)34-18-14-21-50-24-42(34,26(3)51-27(4)44)39(54-30(7)47)37(38(40)41)53-29(6)46/h11-14,16-18,22,26,31,34-39,48H,1,15,19-21,23-24H2,2-10H3/t26-,31-,34?,35?,36-,37+,38?,39+,40+,41-,42-,43?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 of human T cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158207
(Aceticacid(R)-1-{(4R,4aR,5S,6R,6aR,11bS)-4,5,6-tri...)Show SMILES CCOc1ccccc1CCC1(O)C2CC[C@@]3(C)C4C=CCOC[C@]4([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C3[C@@]2(C)[C@H](OC(C)=O)C=C1C |c:20,53| Show InChI InChI=1S/C41H56O11/c1-10-48-31-15-12-11-14-30(31)17-20-41(46)24(2)22-34(50-27(5)43)39(9)33(41)18-19-38(8)32-16-13-21-47-23-40(32,25(3)49-26(4)42)37(52-29(7)45)35(36(38)39)51-28(6)44/h11-16,22,25,32-37,46H,10,17-21,23H2,1-9H3/t25-,32?,33?,34-,35+,36?,37+,38+,39-,40-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated potassium channel subunit Kv1.3 in chinese hamster ovary cells |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50158230
(CHEMBL362199 | Correloid derivative)Show SMILES COC(=O)C12O[C@@H]1C(=O)CC1(O)C3CC[C@@]4(C)C5C=CCOC[C@]5([C@@H](C)OC(C)=O)[C@@H](OC(C)=O)[C@@H](OC(C)=O)C4[C@@]3(C)[C@H](OC(C)=O)[C@H](OC(C)=O)[C@@]21C |c:19| Show InChI InChI=1S/C39H52O16/c1-18(50-19(2)40)37-17-49-15-11-12-25(37)34(7)14-13-26-35(8,28(34)27(51-20(3)41)30(37)52-21(4)42)31(53-22(5)43)32(54-23(6)44)36(9)38(26,47)16-24(45)29-39(36,55-29)33(46)48-10/h11-12,18,25-32,47H,13-17H2,1-10H3/t18-,25?,26?,27+,28?,29-,30+,31-,32+,34+,35+,36-,37-,38?,39?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against voltage-gated potassium channel subunit Kv1.3 |
Bioorg Med Chem Lett 15: 447-51 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.058
BindingDB Entry DOI: 10.7270/Q2BP0281 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data