

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160893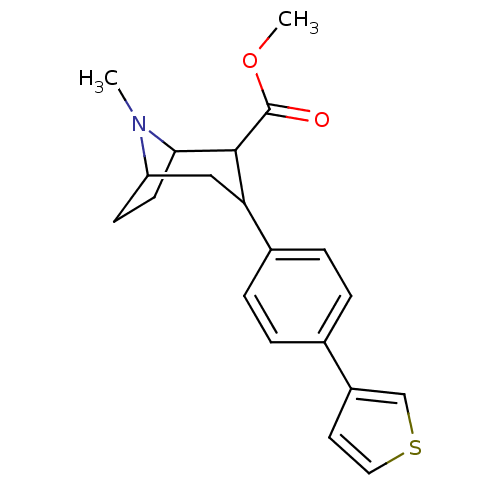 (8-Methyl-3-(4-thiophen-3-yl-phenyl)-8-aza-bicyclo[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 15: 1131-3 (2005) Article DOI: 10.1016/j.bmcl.2004.12.014 BindingDB Entry DOI: 10.7270/Q2PK0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160893 (8-Methyl-3-(4-thiophen-3-yl-phenyl)-8-aza-bicyclo[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 15: 1131-3 (2005) Article DOI: 10.1016/j.bmcl.2004.12.014 BindingDB Entry DOI: 10.7270/Q2PK0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50368145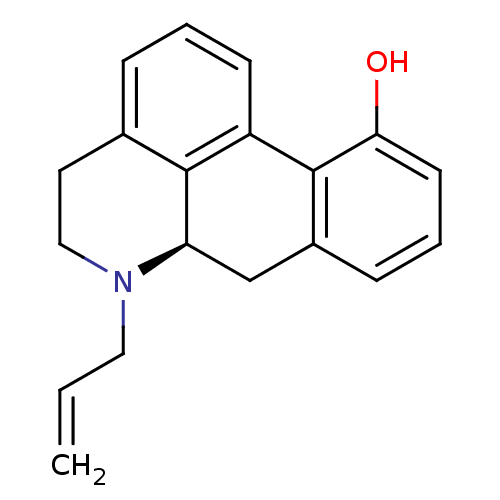 (CHEMBL1788212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Striatal Dopamine Receptor in rat brain through radioreceptor assay carried out with agonist ligand... | J Med Chem 34: 24-8 (1991) BindingDB Entry DOI: 10.7270/Q24Q7VKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50051007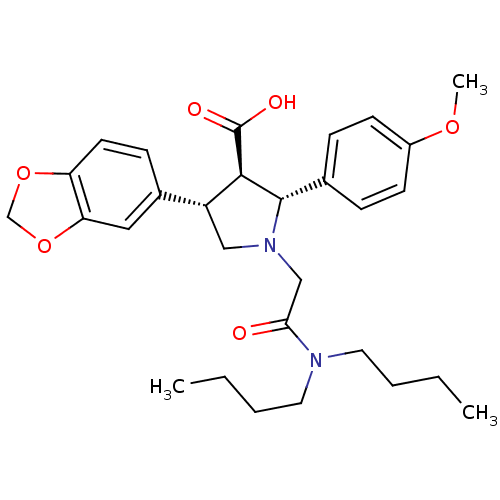 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464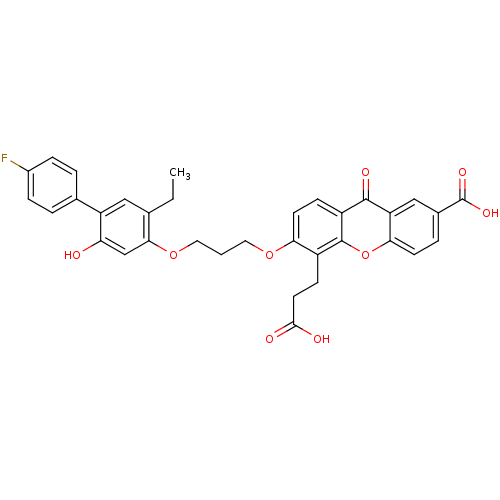 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM52987 ((6aR)-6-propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from rat dopamine D2 receptor | J Med Chem 50: 171-81 (2007) Article DOI: 10.1021/jm060959i BindingDB Entry DOI: 10.7270/Q22J6CPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM52987 ((6aR)-6-propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity at rat striatal Dopamine receptor D2 using [3H]- piperone radioligand | J Med Chem 33: 1800-5 (1990) BindingDB Entry DOI: 10.7270/Q2K35SMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50106857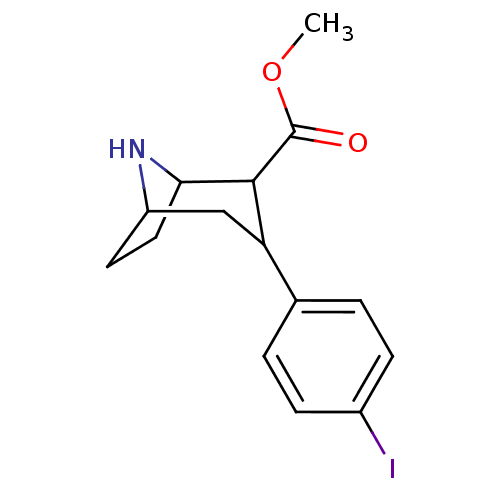 (3-(4-Iodo-phenyl)-8-aza-bicyclo[3.2.1]octane-2-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcohol and Drug Abuse Research Center Curated by ChEMBL | Assay Description Ability to displace [3H]- paroxetine from Serotonin transporter in rat cerebral cortical homogenate | Bioorg Med Chem Lett 11: 3049-53 (2001) BindingDB Entry DOI: 10.7270/Q23N22PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Norepinephrine transporter (RAT) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | J Pharmacol Exp Ther 301: 1097-102 (2002) Article DOI: 10.1124/jpet.301.3.1097 BindingDB Entry DOI: 10.7270/Q2ZW1JH0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165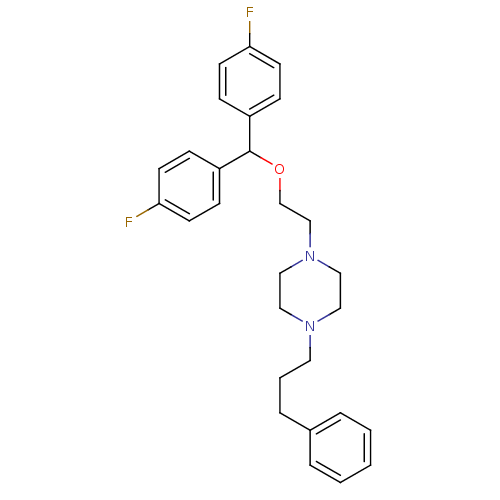 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine transporter in rat striatal membranes by [3H]GBR-12395 displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50106857 (3-(4-Iodo-phenyl)-8-aza-bicyclo[3.2.1]octane-2-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale School of Medicine Curated by ChEMBL | Assay Description Binding affinity to serotonin transporter (SERT) in rat forebrain tissue | Bioorg Med Chem Lett 14: 2117-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.043 BindingDB Entry DOI: 10.7270/Q2WS8SQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50106857 (3-(4-Iodo-phenyl)-8-aza-bicyclo[3.2.1]octane-2-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale School of Medicine Curated by ChEMBL | Assay Description Binding affinity to serotonin transporter (SERT) in rat forebrain tissue | Bioorg Med Chem Lett 14: 2117-20 (2004) Article DOI: 10.1016/j.bmcl.2004.02.043 BindingDB Entry DOI: 10.7270/Q2WS8SQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50050976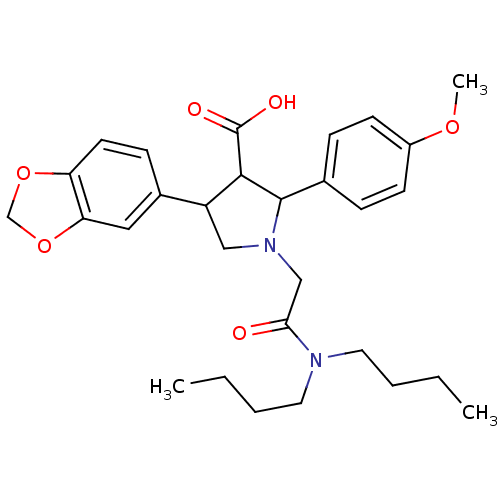 (4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbamoylmethyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345553 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-bromobenzoni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029477 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-2-hydroxy-biphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM86266 (MCL-204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | Eur J Pharmacol 474: 137-40 (2003) Article DOI: 10.1016/s0014-2999(03)02008-9 BindingDB Entry DOI: 10.7270/Q2DN43MQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048558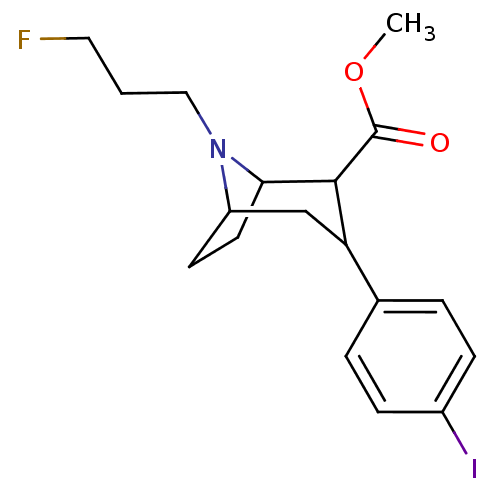 (8-(3-Fluoro-propyl)-3-(4-iodo-phenyl)-8-aza-bicycl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160892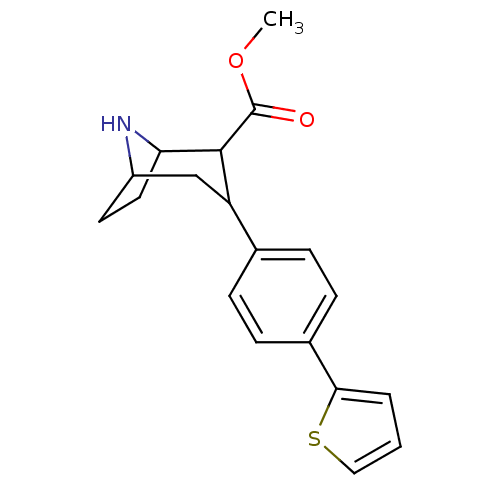 (3-(4-Thiophen-2-yl-phenyl)-8-aza-bicyclo[3.2.1]oct...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 15: 1131-3 (2005) Article DOI: 10.1016/j.bmcl.2004.12.014 BindingDB Entry DOI: 10.7270/Q2PK0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160892 (3-(4-Thiophen-2-yl-phenyl)-8-aza-bicyclo[3.2.1]oct...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 15: 1131-3 (2005) Article DOI: 10.1016/j.bmcl.2004.12.014 BindingDB Entry DOI: 10.7270/Q2PK0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM86180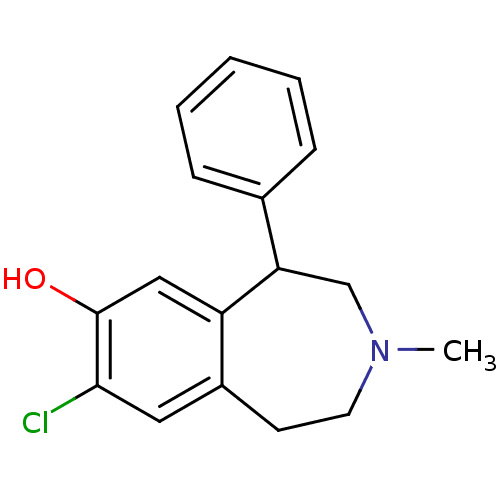 (CAS_87075-17-0 | NSC_5018 | SCH 23390 | SCH23390 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | Eur J Pharmacol 474: 137-40 (2003) Article DOI: 10.1016/s0014-2999(03)02008-9 BindingDB Entry DOI: 10.7270/Q2DN43MQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889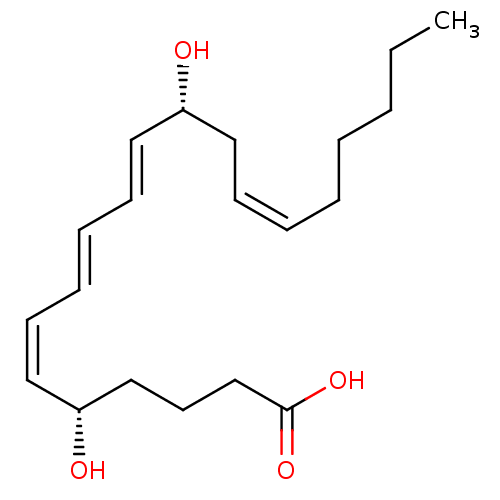 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Compound was evaluated for affinity towards dopamine D1-like receptor in homogenate of caudateputamen tissue from rat brain | Bioorg Med Chem Lett 10: 1113-5 (2000) BindingDB Entry DOI: 10.7270/Q21N81NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Compound was evaluated for affinity towards dopamine D1-like receptor in homogenate of caudateputamen tissue from rat brain | Bioorg Med Chem Lett 10: 1113-5 (2000) BindingDB Entry DOI: 10.7270/Q21N81NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345552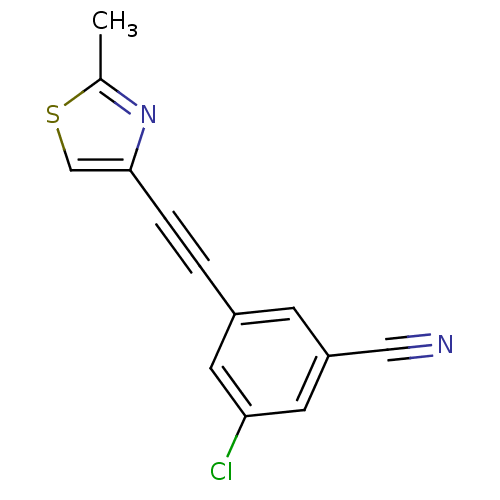 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-chlorobenzon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50050964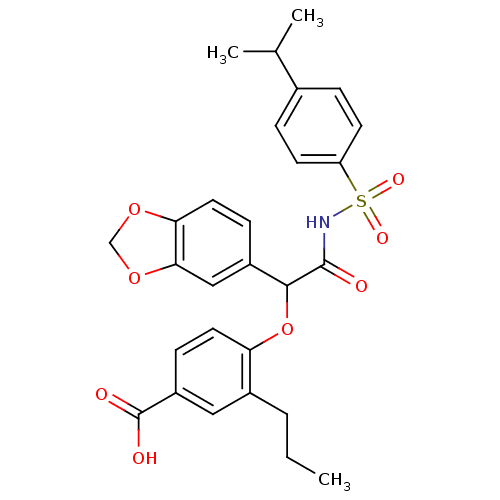 (4-[1-Benzo[1,3]dioxol-5-yl-2-(4-isopropyl-benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50048561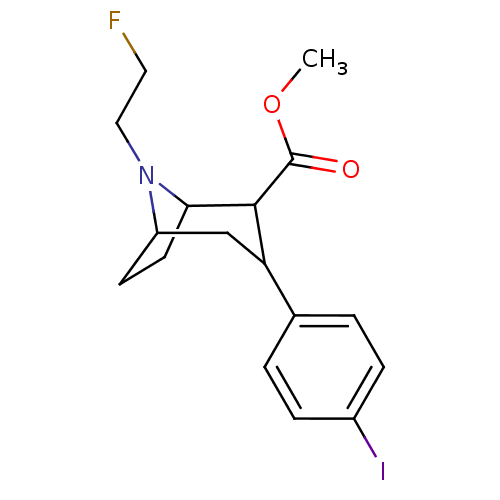 (8-(2-Fluoro-ethyl)-3-(4-iodo-phenyl)-8-aza-bicyclo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50175644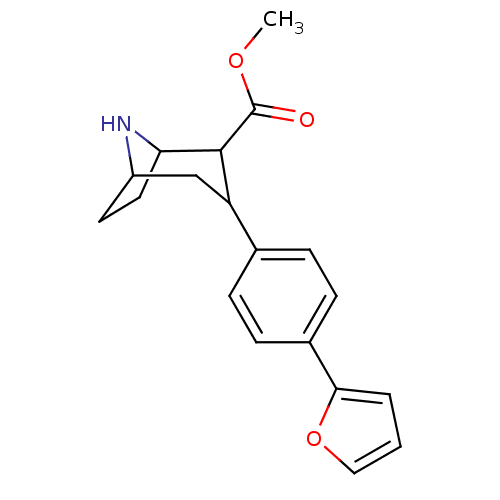 (CHEMBL200044 | methyl 3-(4-(furan-2-yl)phenyl)-8-a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in rat frontoparietal cerebral cortex | Bioorg Med Chem Lett 16: 217-20 (2005) Article DOI: 10.1016/j.bmcl.2005.09.016 BindingDB Entry DOI: 10.7270/Q2R210Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50175644 (CHEMBL200044 | methyl 3-(4-(furan-2-yl)phenyl)-8-a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in rat frontoparietal cerebral cortex | Bioorg Med Chem Lett 16: 217-20 (2005) Article DOI: 10.1016/j.bmcl.2005.09.016 BindingDB Entry DOI: 10.7270/Q2R210Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160894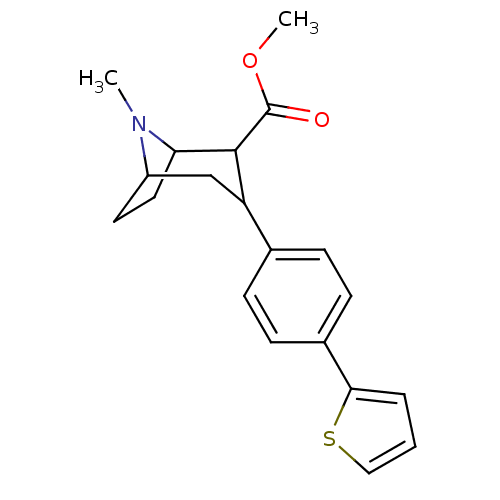 (8-Methyl-3-(4-thiophen-2-yl-phenyl)-8-aza-bicyclo[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 15: 1131-3 (2005) Article DOI: 10.1016/j.bmcl.2004.12.014 BindingDB Entry DOI: 10.7270/Q2PK0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160894 (8-Methyl-3-(4-thiophen-2-yl-phenyl)-8-aza-bicyclo[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 15: 1131-3 (2005) Article DOI: 10.1016/j.bmcl.2004.12.014 BindingDB Entry DOI: 10.7270/Q2PK0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | J Pharmacol Exp Ther 301: 1097-102 (2002) Article DOI: 10.1124/jpet.301.3.1097 BindingDB Entry DOI: 10.7270/Q2ZW1JH0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267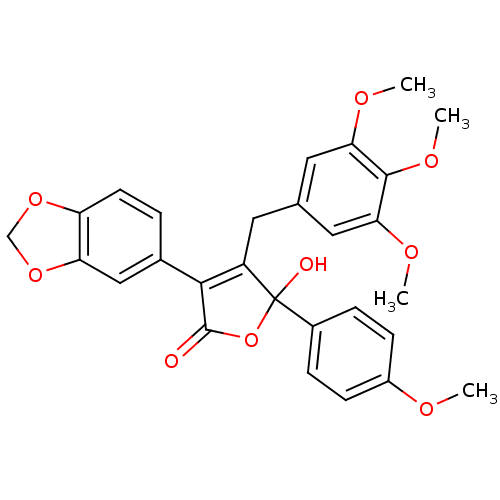 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010692 ((R)-(-)-2-methoxy-N-npropylnorapomorphine | (R)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from rat dopamine D2 receptor | J Med Chem 50: 171-81 (2007) Article DOI: 10.1021/jm060959i BindingDB Entry DOI: 10.7270/Q22J6CPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010692 ((R)-(-)-2-methoxy-N-npropylnorapomorphine | (R)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity at rat striatal Dopamine receptor D2 using [3H]- piperone radioligand | J Med Chem 33: 1800-5 (1990) BindingDB Entry DOI: 10.7270/Q2K35SMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50155461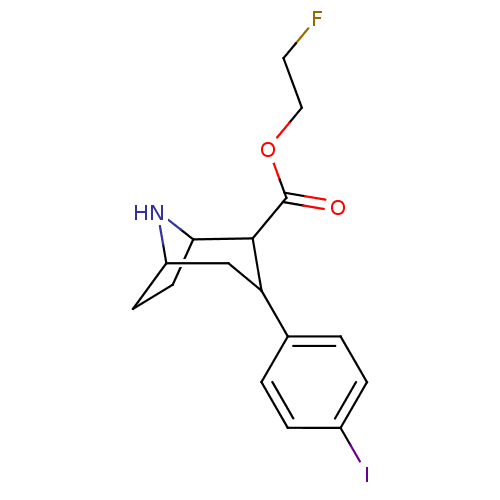 (3-(4-Iodo-phenyl)-8-aza-bicyclo[3.2.1]octane-2-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]-cyanoimiprimine from serotonin transporter of rat cerebral cortical homogenate | Bioorg Med Chem Lett 14: 5635-9 (2004) Article DOI: 10.1016/j.bmcl.2004.08.049 BindingDB Entry DOI: 10.7270/Q2RB743J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM86275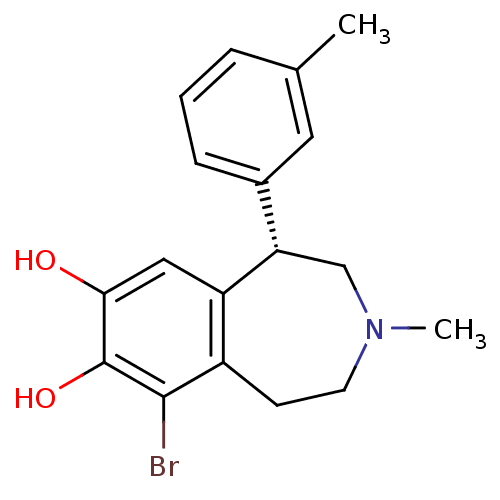 (MCL-203) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | Eur J Pharmacol 474: 137-40 (2003) Article DOI: 10.1016/s0014-2999(03)02008-9 BindingDB Entry DOI: 10.7270/Q2DN43MQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50291007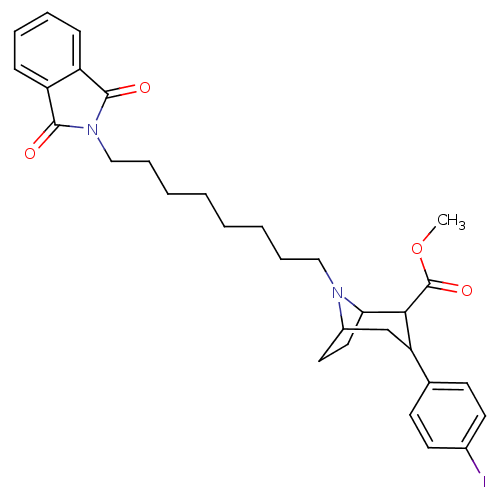 ((3S,5R)-methyl 3-(4-iodophenyl)-8-(8-(1,3-dioxoiso...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity of compound for Serotonin transporter with [3H]- paroxetine as radioligand in corpus striatum tissue from rat forebrain | Bioorg Med Chem Lett 7: 337-340 (1997) Article DOI: 10.1016/S0960-894X(97)00009-7 BindingDB Entry DOI: 10.7270/Q2T72HGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19854 (CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345572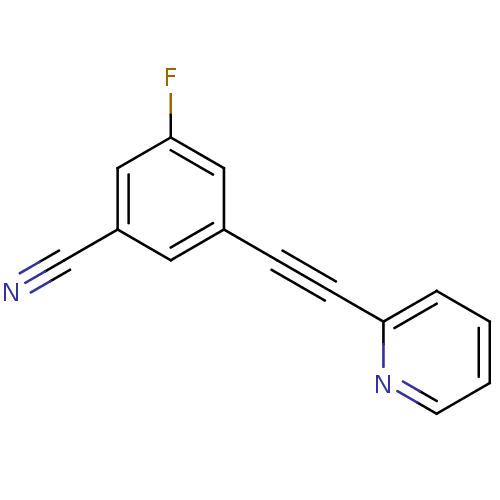 (3-(2-(pyridin-2-yl)ethynyl)-5-fluorobenzonitrile |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50314676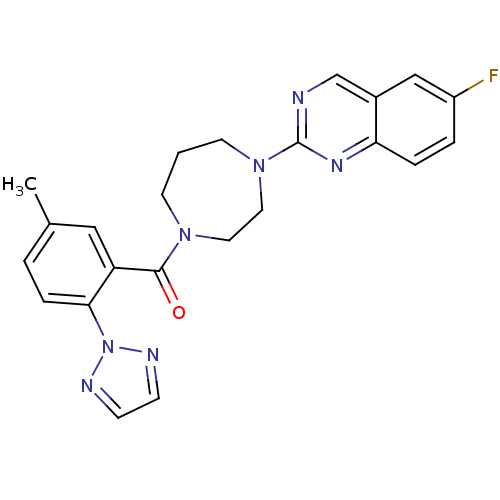 ((4-(6-fluoroquinazolin-2-yl)-1,4-diazepan-1-yl)(5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50291008 ((3S,5S)-methyl 3-(4-iodophenyl)-8-(4-(1,3-dioxoiso...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity of compound for Serotonin transporter with [3H]- paroxetine as radioligand in corpus striatum tissue from rat forebrain | Bioorg Med Chem Lett 7: 337-340 (1997) Article DOI: 10.1016/S0960-894X(97)00009-7 BindingDB Entry DOI: 10.7270/Q2T72HGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50012990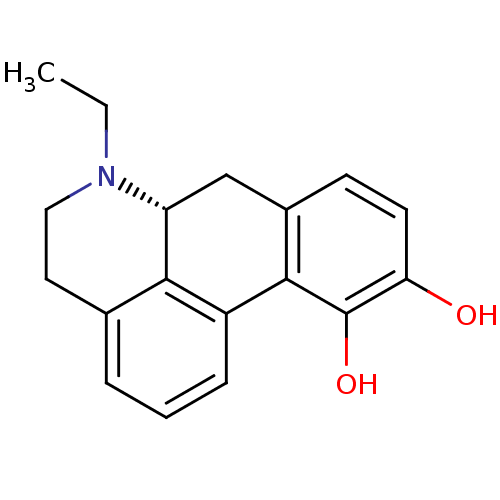 (6-Ethyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Dopamine receptor D2 affinity was tested in vitro against corpus striatum from rat brain membranes | J Med Chem 33: 39-44 (1990) BindingDB Entry DOI: 10.7270/Q2183732 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50004167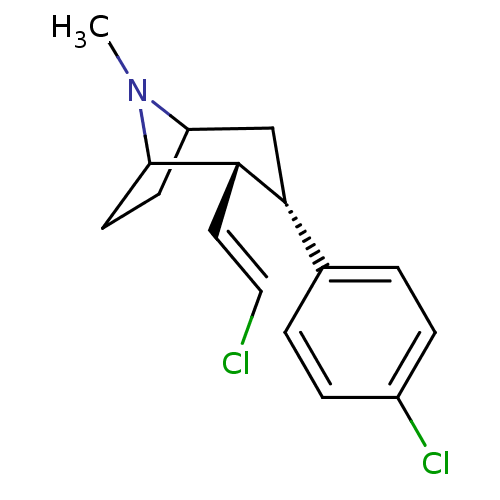 ((2R,3S)-3-(4-Chloro-phenyl)-2-((E)-2-chloro-vinyl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding constant for the ability to displace [3H]mazindol to dopamine receptor was calculated from the Cheng-Prusoff relationship | Bioorg Med Chem Lett 3: 1327-1332 (1993) Article DOI: 10.1016/S0960-894X(00)80341-8 BindingDB Entry DOI: 10.7270/Q2RJ4JZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50012990 (6-Ethyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Agonist activity was tested in vitro against Dopamine receptor from rat brain membranes with [3H]ADT-6,7-dihydroxy-2-aminotetralin] | J Med Chem 33: 39-44 (1990) BindingDB Entry DOI: 10.7270/Q2183732 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160898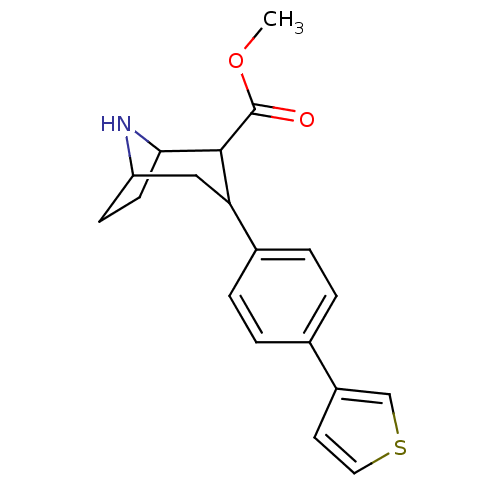 (3-(4-Thiophen-3-yl-phenyl)-8-aza-bicyclo[3.2.1]oct...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 15: 1131-3 (2005) Article DOI: 10.1016/j.bmcl.2004.12.014 BindingDB Entry DOI: 10.7270/Q2PK0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160898 (3-(4-Thiophen-3-yl-phenyl)-8-aza-bicyclo[3.2.1]oct...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 15: 1131-3 (2005) Article DOI: 10.1016/j.bmcl.2004.12.014 BindingDB Entry DOI: 10.7270/Q2PK0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50155459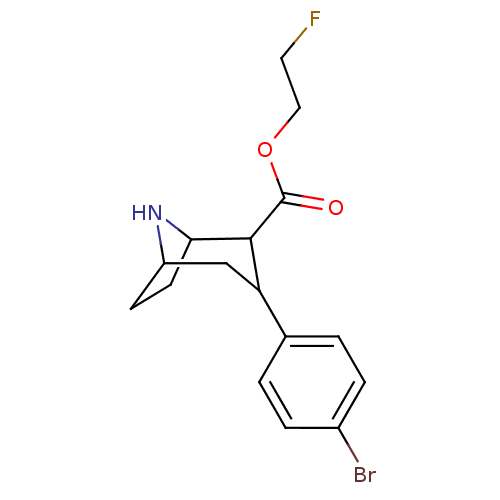 (3-(4-Bromo-phenyl)-8-aza-bicyclo[3.2.1]octane-2-ca...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]-cyanoimiprimine from serotonin transporter of rat cerebral cortical homogenate | Bioorg Med Chem Lett 14: 5635-9 (2004) Article DOI: 10.1016/j.bmcl.2004.08.049 BindingDB Entry DOI: 10.7270/Q2RB743J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7449 total ) | Next | Last >> |