 Found 5626 hits with Last Name = 'bane' and Initial = 's'
Found 5626 hits with Last Name = 'bane' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50592891

(CHEMBL5191016)Show SMILES NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298756

((5S,8S,11S,14S,17R)-11-(4-aminobutyl)-5,8-bis(hydr...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)N(C)C(=O)OCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C32H55BN6O10/c1-20(2)15-24(29(43)38-27(33(47)48)16-21(3)4)36-28(42)23(13-9-10-14-34)35-30(44)25(17-40)37-31(45)26(18-41)39(5)32(46)49-19-22-11-7-6-8-12-22/h6-8,11-12,20-21,23-27,40-41,47-48H,9-10,13-19,34H2,1-5H3,(H,35,44)(H,36,42)(H,37,45)(H,38,43)/t23-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11870

(4-{[4-(4-Chlorophenoxy)phenyl]sulfonyl}-N-hydroxy ...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H18ClNO6S/c19-13-1-3-14(4-2-13)26-15-5-7-16(8-6-15)27(23,24)18(17(21)20-22)9-11-25-12-10-18/h1-8,22H,9-12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11870

(4-{[4-(4-Chlorophenoxy)phenyl]sulfonyl}-N-hydroxy ...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H18ClNO6S/c19-13-1-3-14(4-2-13)26-15-5-7-16(8-6-15)27(23,24)18(17(21)20-22)9-11-25-12-10-18/h1-8,22H,9-12H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11870

(4-{[4-(4-Chlorophenoxy)phenyl]sulfonyl}-N-hydroxy ...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H18ClNO6S/c19-13-1-3-14(4-2-13)26-15-5-7-16(8-6-15)27(23,24)18(17(21)20-22)9-11-25-12-10-18/h1-8,22H,9-12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM13126
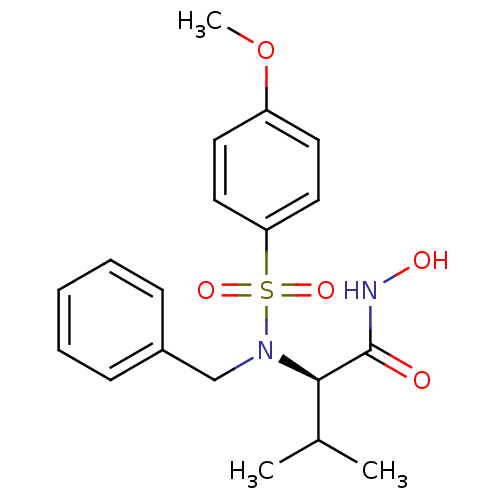
((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298754
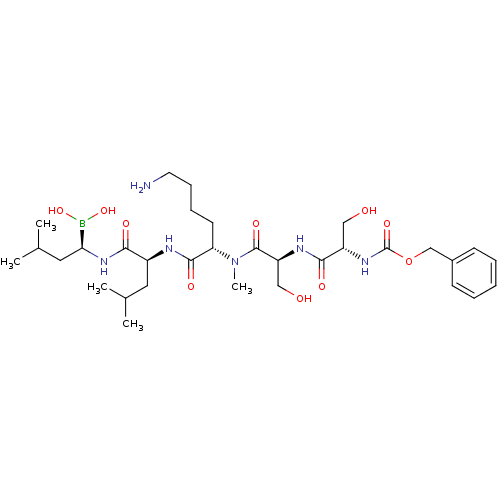
((5S,8S,11S,14S,17R)-11-(4-aminobutyl)-5,8-bis(hydr...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)N(C)C(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C32H55BN6O10/c1-20(2)15-23(28(42)38-27(33(47)48)16-21(3)4)35-30(44)26(13-9-10-14-34)39(5)31(45)25(18-41)36-29(43)24(17-40)37-32(46)49-19-22-11-7-6-8-12-22/h6-8,11-12,20-21,23-27,40-41,47-48H,9-10,13-19,34H2,1-5H3,(H,35,44)(H,36,43)(H,37,46)(H,38,42)/t23-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50592900
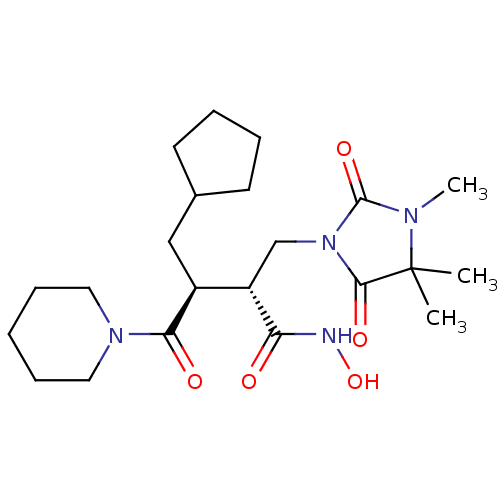
(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50592895
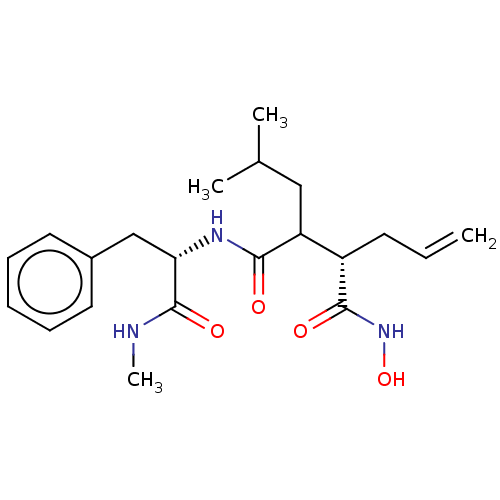
(CHEMBL5187653)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)C(CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190196
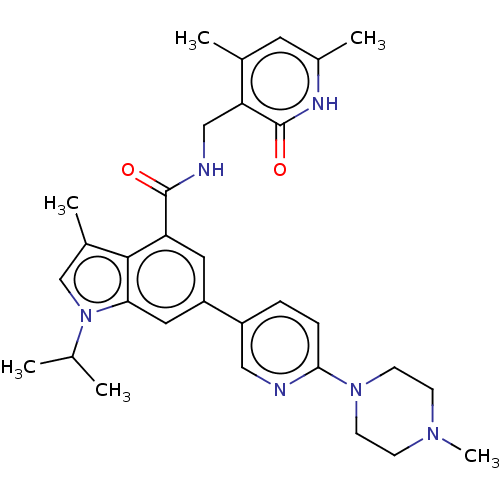
(EPZ008277 | US9175331, 25)Show SMILES CC(C)n1cc(C)c2c(cc(cc12)-c1ccc(nc1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C31H38N6O2/c1-19(2)37-18-21(4)29-25(30(38)33-17-26-20(3)13-22(5)34-31(26)39)14-24(15-27(29)37)23-7-8-28(32-16-23)36-11-9-35(6)10-12-36/h7-8,13-16,18-19H,9-12,17H2,1-6H3,(H,33,38)(H,34,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114419
BindingDB Entry DOI: 10.7270/Q2PR80Z0 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190196

(EPZ008277 | US9175331, 25)Show SMILES CC(C)n1cc(C)c2c(cc(cc12)-c1ccc(nc1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C31H38N6O2/c1-19(2)37-18-21(4)29-25(30(38)33-17-26-20(3)13-22(5)34-31(26)39)14-24(15-27(29)37)23-7-8-28(32-16-23)36-11-9-35(6)10-12-36/h7-8,13-16,18-19H,9-12,17H2,1-6H3,(H,33,38)(H,34,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114419
BindingDB Entry DOI: 10.7270/Q2PR80Z0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50592895

(CHEMBL5187653)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)C(CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50592900

(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298760

(CHEMBL574766 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...)Show SMILES CCCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC(C)C)C=O |r| Show InChI InChI=1S/C32H52N6O9/c1-4-5-13-24(28(42)34-23(17-39)16-21(2)3)35-29(43)25(14-9-10-15-33)36-30(44)26(18-40)37-31(45)27(19-41)38-32(46)47-20-22-11-7-6-8-12-22/h6-8,11-12,17,21,23-27,40-41H,4-5,9-10,13-16,18-20,33H2,1-3H3,(H,34,42)(H,35,43)(H,36,44)(H,37,45)(H,38,46)/t23-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11548

(CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(CN2C(CCS2(=O)=O)C(=O)NO)cc1 Show InChI InChI=1S/C18H20N2O5S/c1-25-16-8-6-15(7-9-16)14-4-2-13(3-5-14)12-20-17(18(21)19-22)10-11-26(20,23)24/h2-9,17,22H,10-12H2,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298738

(CHEMBL583783 | benzyl(2S,5S,8S,11S,14S)-8-(3-amino...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C31H48N6O10/c1-18(2)12-21(14-38)33-28(43)23(13-19(3)4)35-27(42)22(10-11-26(32)41)34-29(44)24(15-39)36-30(45)25(16-40)37-31(46)47-17-20-8-6-5-7-9-20/h5-9,14,18-19,21-25,39-40H,10-13,15-17H2,1-4H3,(H2,32,41)(H,33,43)(H,34,44)(H,35,42)(H,36,45)(H,37,46)/t21-,22-,23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50077789
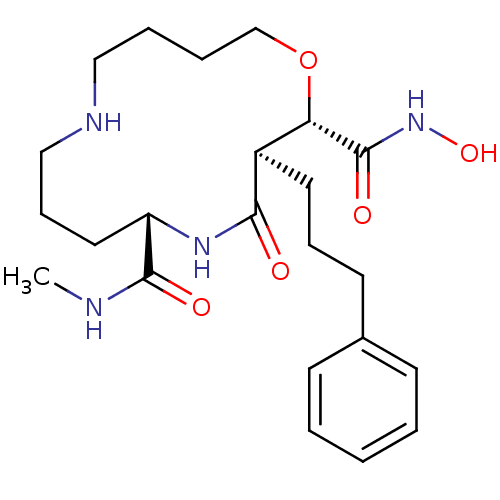
((2S,3R,6S)-4-Oxo-3-(3-phenyl-propyl)-1-oxa-5,10-di...)Show SMILES CNC(=O)[C@@H]1CCCNCCCCO[C@@H]([C@@H](CCCc2ccccc2)C(=O)N1)C(=O)NO Show InChI InChI=1S/C23H36N4O5/c1-24-22(29)19-13-8-15-25-14-5-6-16-32-20(23(30)27-31)18(21(28)26-19)12-7-11-17-9-3-2-4-10-17/h2-4,9-10,18-20,25,31H,5-8,11-16H2,1H3,(H,24,29)(H,26,28)(H,27,30)/t18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50592895

(CHEMBL5187653)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)C(CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50592900

(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298761
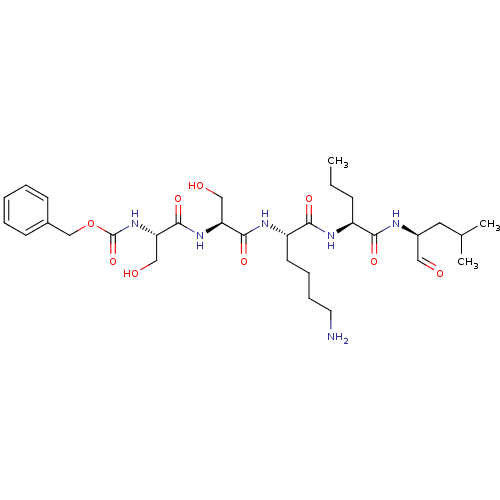
(CHEMBL574788 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...)Show SMILES CCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC(C)C)C=O |r| Show InChI InChI=1S/C31H50N6O9/c1-4-10-23(27(41)33-22(16-38)15-20(2)3)34-28(42)24(13-8-9-14-32)35-29(43)25(17-39)36-30(44)26(18-40)37-31(45)46-19-21-11-6-5-7-12-21/h5-7,11-12,16,20,22-26,39-40H,4,8-10,13-15,17-19,32H2,1-3H3,(H,33,41)(H,34,42)(H,35,43)(H,36,44)(H,37,45)/t22-,23-,24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM207629

(US9265734, R01 | US9796664, Compound R01)Show InChI InChI=1S/C20H25N3O2/c1-15-10-12-16(13-11-15)20(25)22-14-6-2-3-9-19(24)23-18-8-5-4-7-17(18)21/h4-5,7-8,10-13H,2-3,6,9,14,21H2,1H3,(H,22,25)(H,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50592895

(CHEMBL5187653)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)C(CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298757
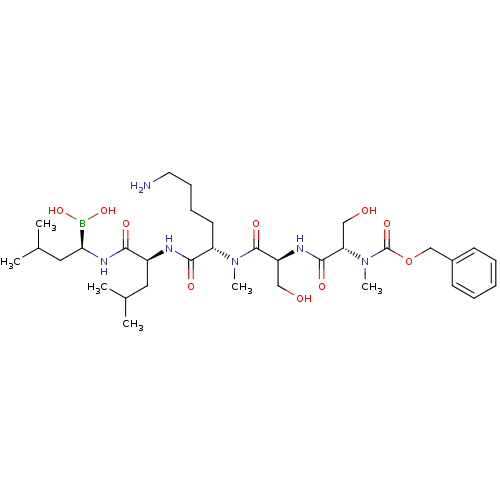
((5S,8S,11S,14S,17R)-11-(4-aminobutyl)-5,8-bis(hydr...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)N(C)C(=O)[C@H](CO)NC(=O)[C@H](CO)N(C)C(=O)OCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C33H57BN6O10/c1-21(2)16-24(29(43)38-28(34(48)49)17-22(3)4)36-30(44)26(14-10-11-15-35)39(5)32(46)25(18-41)37-31(45)27(19-42)40(6)33(47)50-20-23-12-8-7-9-13-23/h7-9,12-13,21-22,24-28,41-42,48-49H,10-11,14-20,35H2,1-6H3,(H,36,44)(H,37,45)(H,38,43)/t24-,25-,26-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298762
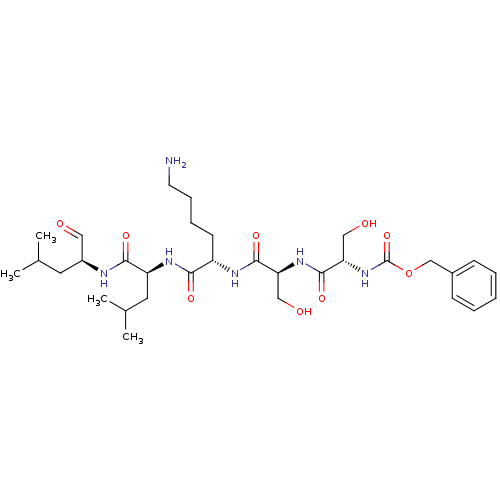
(CHEMBL574924 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C32H52N6O9/c1-20(2)14-23(16-39)34-29(43)25(15-21(3)4)36-28(42)24(12-8-9-13-33)35-30(44)26(17-40)37-31(45)27(18-41)38-32(46)47-19-22-10-6-5-7-11-22/h5-7,10-11,16,20-21,23-27,40-41H,8-9,12-15,17-19,33H2,1-4H3,(H,34,43)(H,35,44)(H,36,42)(H,37,45)(H,38,46)/t23-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50592895

(CHEMBL5187653)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)C(CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298739

(CHEMBL583784 | benzyl(2S,5S,8S,11S,14S)-14-formyl-...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C=O |r| Show InChI InChI=1S/C31H49N5O9S/c1-19(2)13-22(15-37)32-28(41)24(14-20(3)4)34-27(40)23(11-12-46-5)33-29(42)25(16-38)35-30(43)26(17-39)36-31(44)45-18-21-9-7-6-8-10-21/h6-10,15,19-20,22-26,38-39H,11-14,16-18H2,1-5H3,(H,32,41)(H,33,42)(H,34,40)(H,35,43)(H,36,44)/t22-,23-,24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50592895

(CHEMBL5187653)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)C(CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM120158
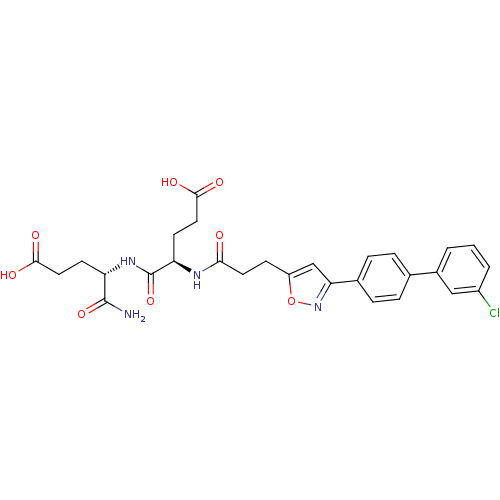
(US8691753, 6)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)CCc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H29ClN4O8/c29-19-3-1-2-18(14-19)16-4-6-17(7-5-16)23-15-20(41-33-23)8-11-24(34)31-22(10-13-26(37)38)28(40)32-21(27(30)39)9-12-25(35)36/h1-7,14-15,21-22H,8-13H2,(H2,30,39)(H,31,34)(H,32,40)(H,35,36)(H,37,38)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298740
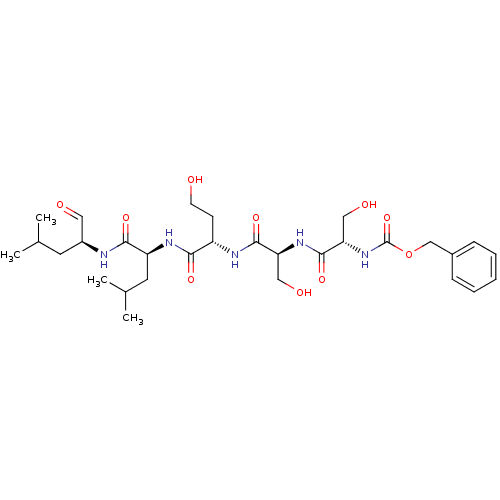
(CHEMBL574324 | benzyl(2S,5S,8S,11S,14S)-14-formyl-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCO)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C30H47N5O10/c1-18(2)12-21(14-37)31-27(41)23(13-19(3)4)33-26(40)22(10-11-36)32-28(42)24(15-38)34-29(43)25(16-39)35-30(44)45-17-20-8-6-5-7-9-20/h5-9,14,18-19,21-25,36,38-39H,10-13,15-17H2,1-4H3,(H,31,41)(H,32,42)(H,33,40)(H,34,43)(H,35,44)/t21-,22-,23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11870

(4-{[4-(4-Chlorophenoxy)phenyl]sulfonyl}-N-hydroxy ...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H18ClNO6S/c19-13-1-3-14(4-2-13)26-15-5-7-16(8-6-15)27(23,24)18(17(21)20-22)9-11-25-12-10-18/h1-8,22H,9-12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50592895

(CHEMBL5187653)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)C(CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50592897

(CHEMBL74539)Show SMILES OC(=O)[C@H](CSc1ccccc1)CC(=O)c1ccc(cc1)-c1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298714
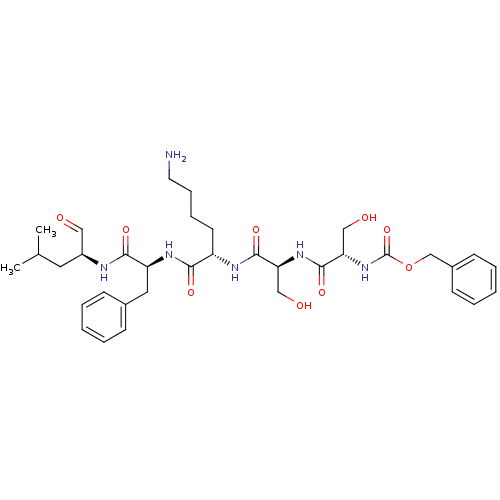
(CHEMBL574925 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C35H50N6O9/c1-23(2)17-26(19-42)37-32(46)28(18-24-11-5-3-6-12-24)39-31(45)27(15-9-10-16-36)38-33(47)29(20-43)40-34(48)30(21-44)41-35(49)50-22-25-13-7-4-8-14-25/h3-8,11-14,19,23,26-30,43-44H,9-10,15-18,20-22,36H2,1-2H3,(H,37,46)(H,38,47)(H,39,45)(H,40,48)(H,41,49)/t26-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298715

(CHEMBL575847 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCS(C)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C31H50N6O10S/c1-20(2)15-22(16-38)33-27(41)24(12-14-48(3)46)35-28(42)23(11-7-8-13-32)34-29(43)25(17-39)36-30(44)26(18-40)37-31(45)47-19-21-9-5-4-6-10-21/h4-6,9-10,16,20,22-26,39-40H,7-8,11-15,17-19,32H2,1-3H3,(H,33,41)(H,34,43)(H,35,42)(H,36,44)(H,37,45)/t22-,23-,24-,25-,26-,48?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298741

(CHEMBL574931 | benzyl(2S,5S,8S,11S,14S)-7-cyclohex...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)N(C1CCCCC1)C(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C35H55N5O9/c1-22(2)16-26(18-41)36-32(45)28(17-23(3)4)37-31(44)24(5)40(27-14-10-7-11-15-27)34(47)30(20-43)38-33(46)29(19-42)39-35(48)49-21-25-12-8-6-9-13-25/h6,8-9,12-13,18,22-24,26-30,42-43H,7,10-11,14-17,19-21H2,1-5H3,(H,36,45)(H,37,44)(H,38,46)(H,39,48)/t24-,26-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298716

(CHEMBL574792 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C35H50N6O10/c1-22(2)16-25(18-42)37-32(47)28(17-23-11-13-26(45)14-12-23)39-31(46)27(10-6-7-15-36)38-33(48)29(19-43)40-34(49)30(20-44)41-35(50)51-21-24-8-4-3-5-9-24/h3-5,8-9,11-14,18,22,25,27-30,43-45H,6-7,10,15-17,19-21,36H2,1-2H3,(H,37,47)(H,38,48)(H,39,46)(H,40,49)(H,41,50)/t25-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298717
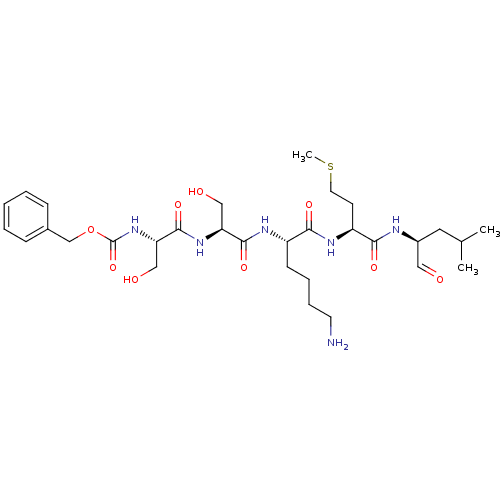
(CHEMBL574845 | benzyl(2S,5S,8S,11S,14S)-8-(4-amino...)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC(C)C)C=O |r| Show InChI InChI=1S/C31H50N6O9S/c1-20(2)15-22(16-38)33-27(41)24(12-14-47-3)35-28(42)23(11-7-8-13-32)34-29(43)25(17-39)36-30(44)26(18-40)37-31(45)46-19-21-9-5-4-6-10-21/h4-6,9-10,16,20,22-26,39-40H,7-8,11-15,17-19,32H2,1-3H3,(H,33,41)(H,34,43)(H,35,42)(H,36,44)(H,37,45)/t22-,23-,24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264810

(CHEMBL445971 | N-(4-(4-(thiiran-2-ylmethylsulfonyl...)Show SMILES CS(=O)(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H17NO5S3/c1-24(18,19)17-12-2-4-13(5-3-12)22-14-6-8-16(9-7-14)25(20,21)11-15-10-23-15/h2-9,15,17H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50064344

((3S,5R,14S)-14-(methylcarbamoyl)-2,8-dioxo-3-phene...)Show SMILES CNC(=O)[C@@H]1CCCCNC(=O)CC[C@@H](N[C@@H](CCc2ccccc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C22H32N4O5/c1-23-20(28)16-9-5-6-14-24-19(27)13-12-18(22(30)31)25-17(21(29)26-16)11-10-15-7-3-2-4-8-15/h2-4,7-8,16-18,25H,5-6,9-14H2,1H3,(H,23,28)(H,24,27)(H,26,29)(H,30,31)/t16-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298742
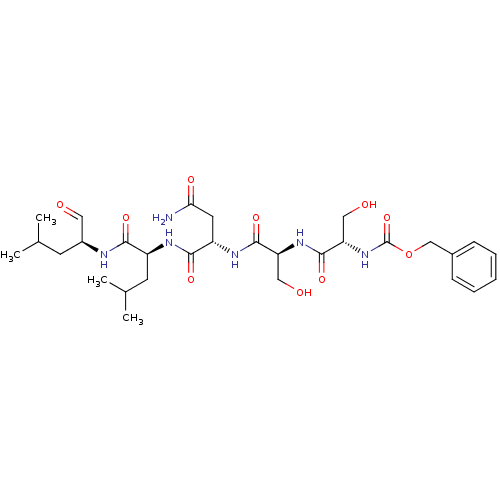
(CHEMBL583785 | benzyl(2S,5S,8S,11S,14S)-8-(2-amino...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C30H46N6O10/c1-17(2)10-20(13-37)32-26(41)21(11-18(3)4)33-27(42)22(12-25(31)40)34-28(43)23(14-38)35-29(44)24(15-39)36-30(45)46-16-19-8-6-5-7-9-19/h5-9,13,17-18,20-24,38-39H,10-12,14-16H2,1-4H3,(H2,31,40)(H,32,41)(H,33,42)(H,34,43)(H,35,44)(H,36,45)/t20-,21-,22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Prostate-specific antigen
(Homo sapiens (Human)) | BDBM50298718
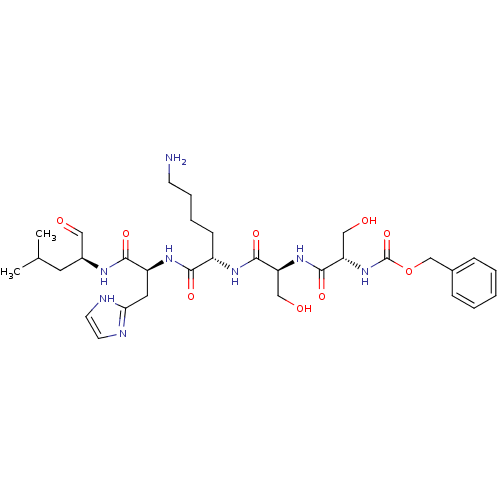
(CHEMBL574926 | benzyl(2S,5S,8S,11S,14S)-11-((1H-im...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ncc[nH]1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C32H48N8O9/c1-20(2)14-22(16-41)36-29(45)24(15-27-34-12-13-35-27)38-28(44)23(10-6-7-11-33)37-30(46)25(17-42)39-31(47)26(18-43)40-32(48)49-19-21-8-4-3-5-9-21/h3-5,8-9,12-13,16,20,22-26,42-43H,6-7,10-11,14-15,17-19,33H2,1-2H3,(H,34,35)(H,36,45)(H,37,46)(H,38,44)(H,39,47)(H,40,48)/t22-,23-,24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of prostate-specific antigen assessed as substrate hydrolysis by fluorescence assay |
Bioorg Med Chem 17: 4888-93 (2009)
Article DOI: 10.1016/j.bmc.2009.06.012
BindingDB Entry DOI: 10.7270/Q2T43T49 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50592892
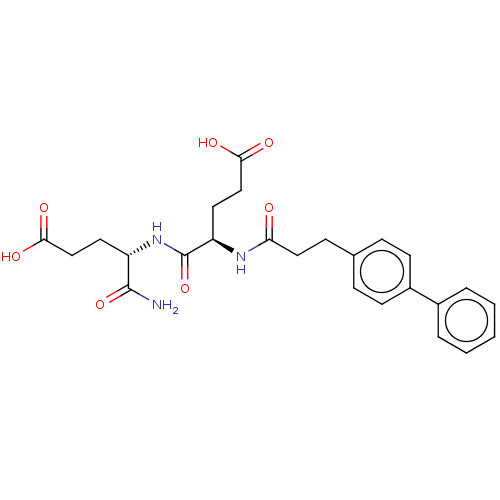
(CHEMBL5190158)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)CCc1ccc(cc1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data