

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (CALF) | BDBM85393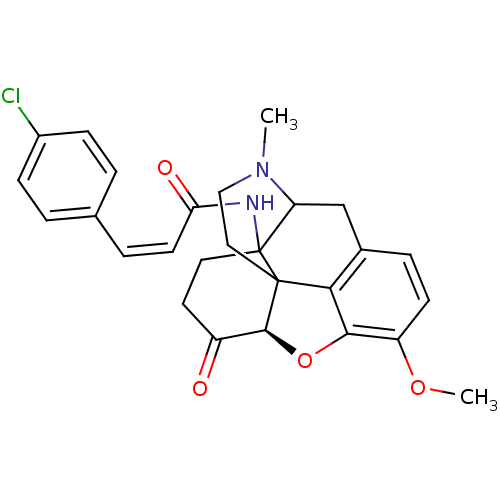 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075926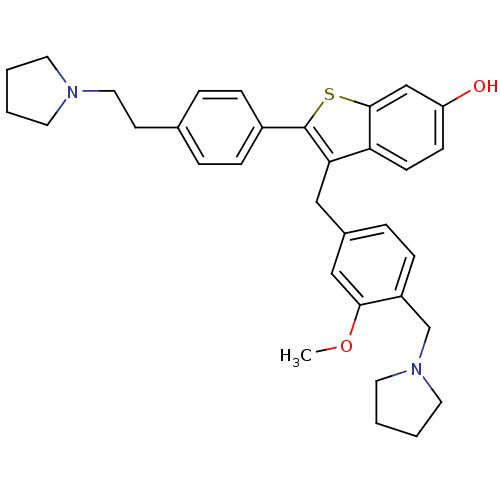 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443429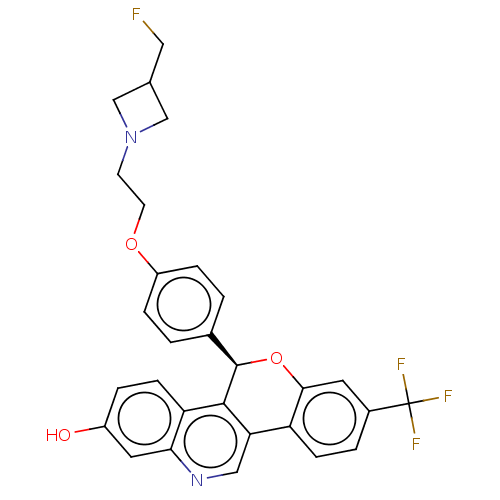 (US10654866, Example 1A | US11117902, Example 1B | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443430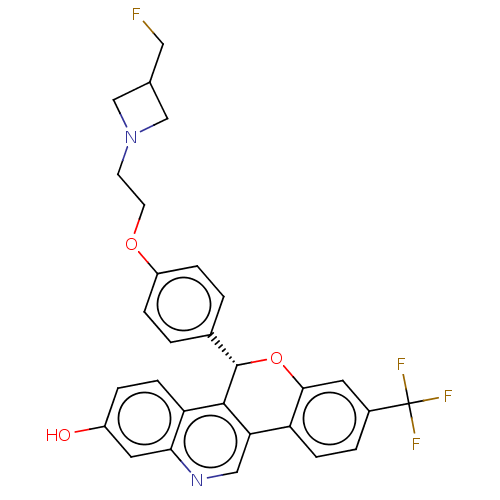 (US10654866, Example 1B | US11117902, Example 1A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443430 (US10654866, Example 1B | US11117902, Example 1A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443437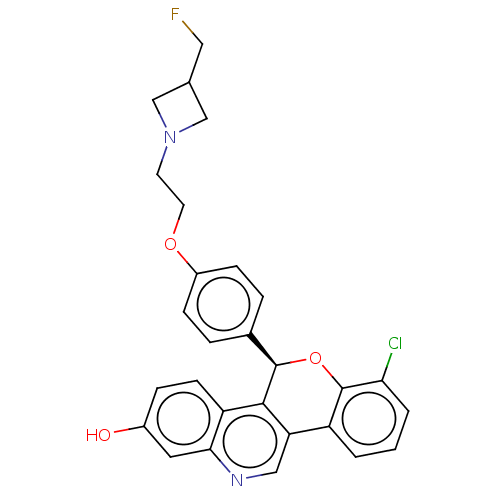 (US10654866, Example 4A | US11117902, Example 4B | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443444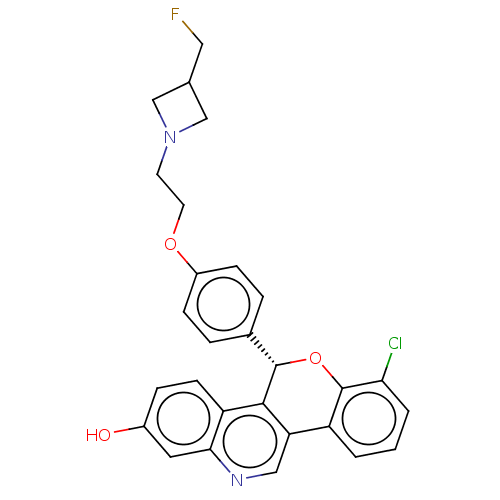 (US10654866, Example 4B | US11117902, Example 4A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443444 (US10654866, Example 4B | US11117902, Example 4A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075934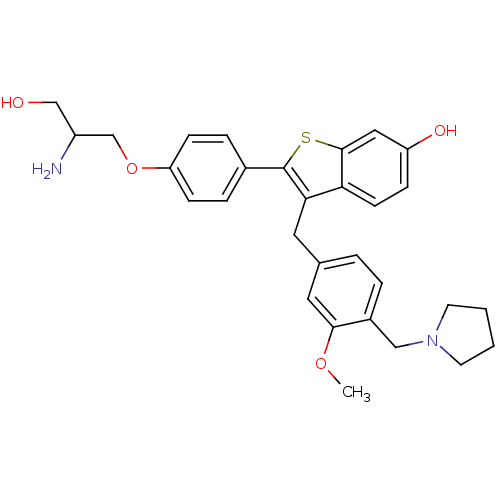 (2-[4-(2-Amino-3-hydroxy-propoxy)-phenyl]-3-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85396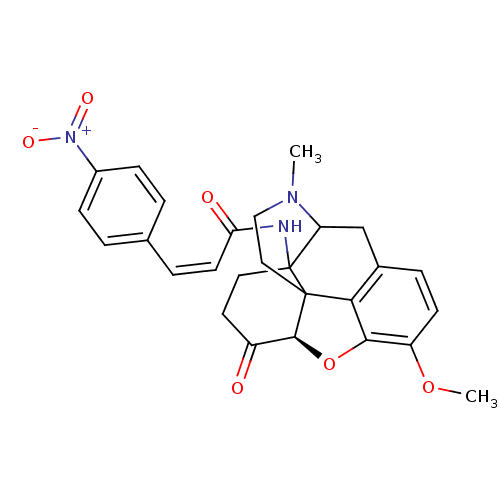 (CACO) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075928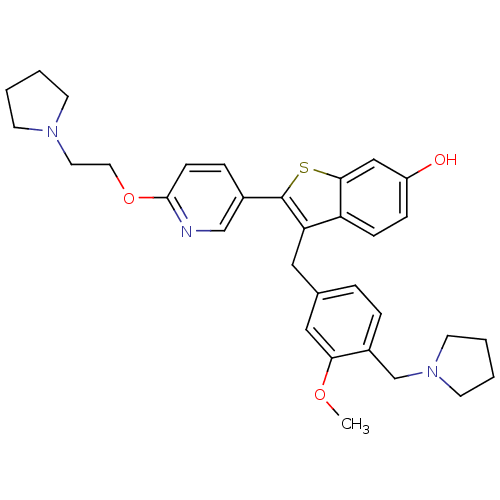 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075937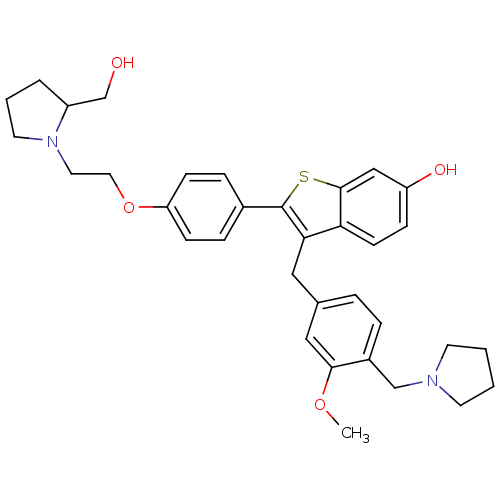 (2-{4-[2-(2-Hydroxymethyl-pyrrolidin-1-yl)-ethoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443449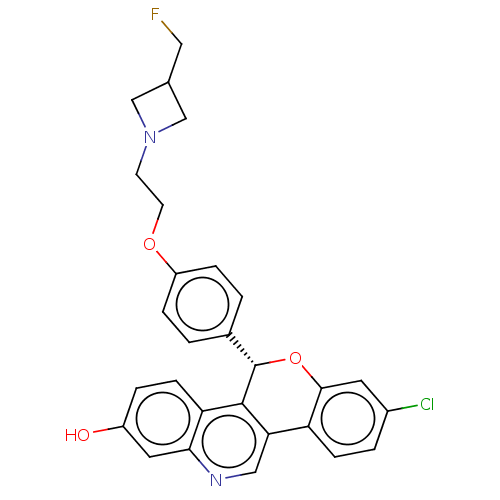 (US10654866, Example 3B | US11117902, Example 3A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443449 (US10654866, Example 3B | US11117902, Example 3A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM518821 (US11117902, Example 3B | US11634426, Example 3A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443450 (US10654866, Example 4 | US11117902, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443450 (US10654866, Example 4 | US11117902, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443450 (US10654866, Example 4 | US11117902, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443433 (US10654866, Example 2A | US11117902, Example 2B | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443434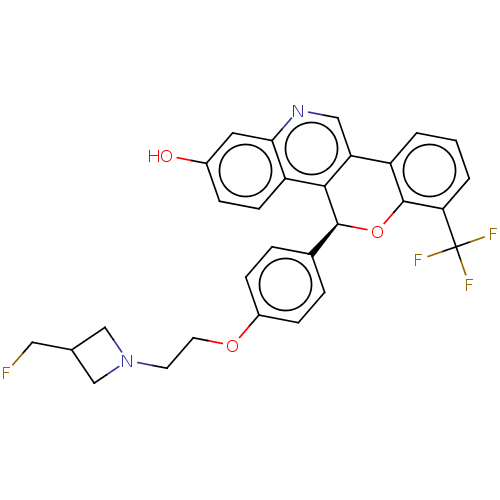 (US10654866, Example 2B | US11117902, Example 2A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443433 (US10654866, Example 2A | US11117902, Example 2B | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Bos taurus) | BDBM85393 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443428 (Racemic 5-(4-{2-[3-(Fluoromethyl)azetidin-1-yl]eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443428 (Racemic 5-(4-{2-[3-(Fluoromethyl)azetidin-1-yl]eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443428 (Racemic 5-(4-{2-[3-(Fluoromethyl)azetidin-1-yl]eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075938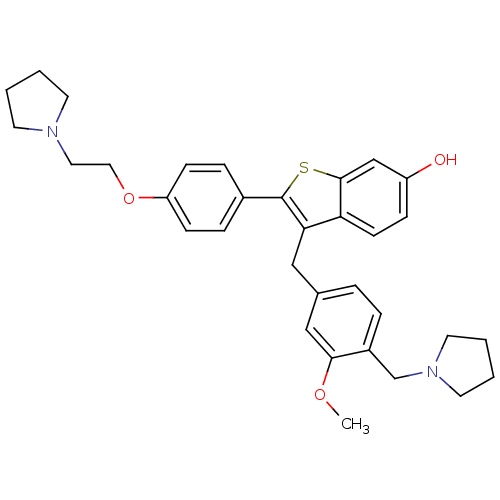 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075932 (2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-N-{4-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443442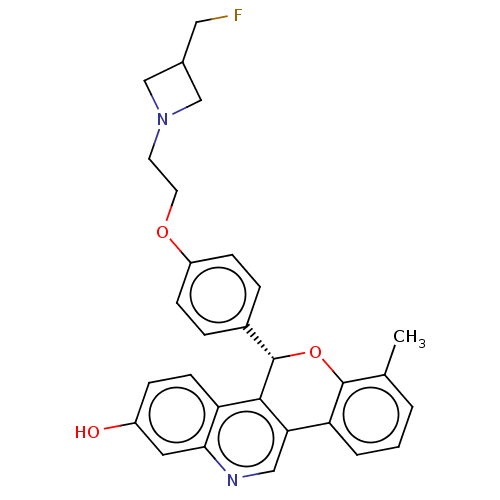 (US10654866, Example 8A | US11117902, Example 8A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443443 (US10654866, Example 8B | US11117902, Example 8B | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443443 (US10654866, Example 8B | US11117902, Example 8B | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (CALF) | BDBM85393 (14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85394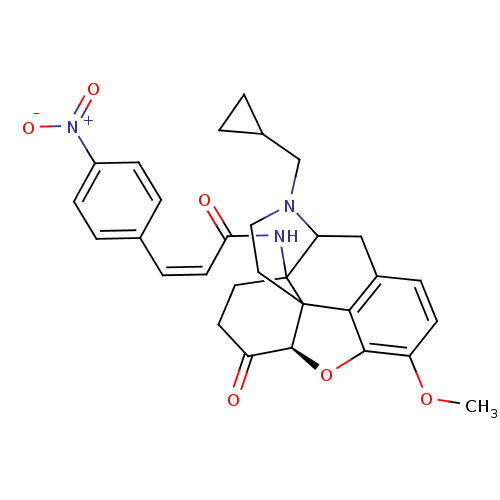 (N-CPM-CACO) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443448 (US10654866, Example 6B | US11117902, Example 6A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443448 (US10654866, Example 6B | US11117902, Example 6A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443447 (US10654866, Example 6A | US11117902, Example 6B | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443446 (US10654866, Example 5B | US11117902, Example 5A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443446 (US10654866, Example 5B | US11117902, Example 5A | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443445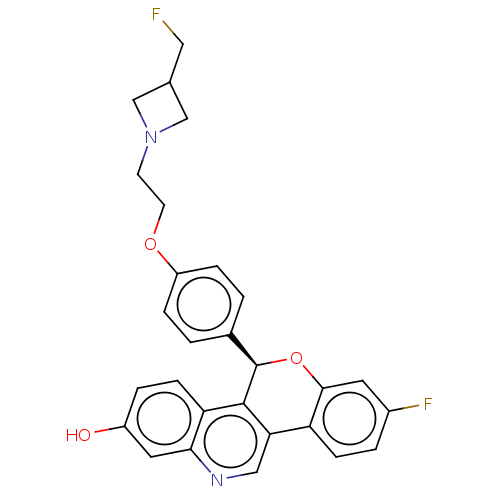 (US10654866, Example 5A | US11117902, Example 5B | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM85395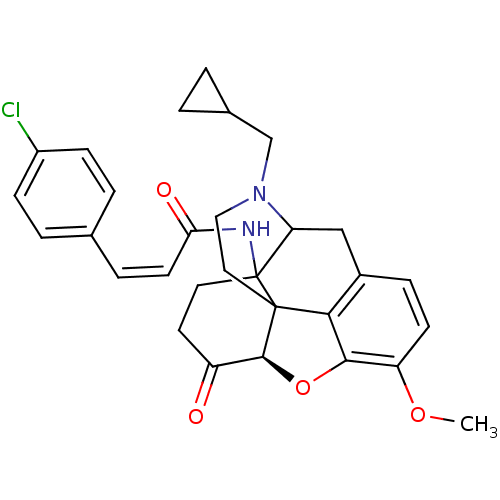 (MC-CAM) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 304-11 (1999) BindingDB Entry DOI: 10.7270/Q2MC8XJC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443440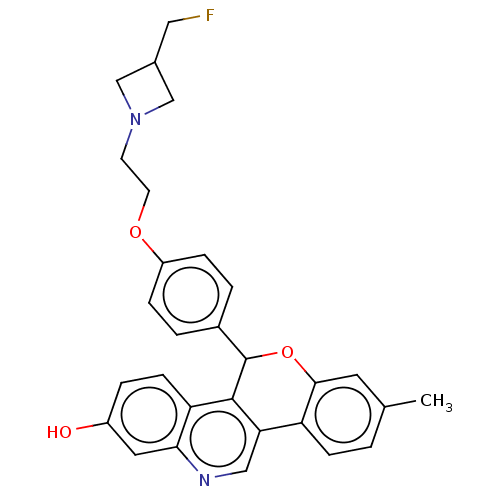 (US10654866, Example 7 | US11634426, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM518852 (US11117902, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443440 (US10654866, Example 7 | US11634426, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075935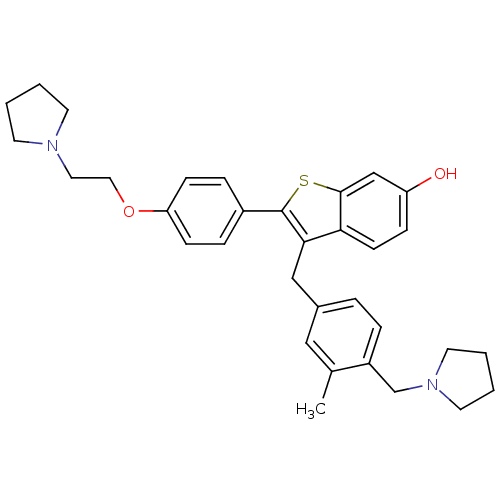 (3-(3-Methyl-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075931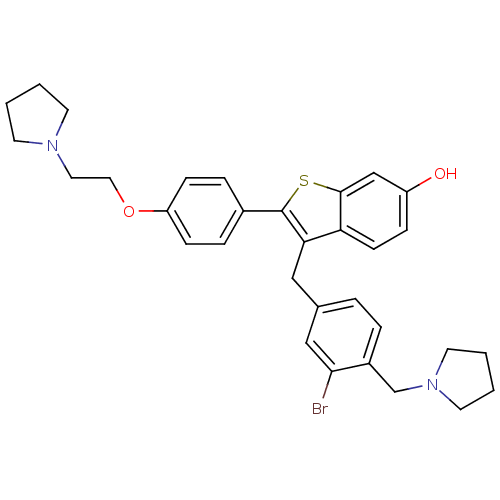 (3-(3-BROMO-4-PYRROLIDIN-1-YLMETHYL-BENZYL)-2-[4-PY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443432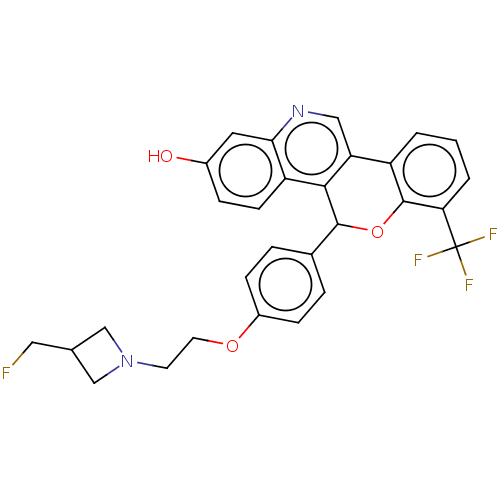 (Racemic 5-(4-{2- [3-(fluoromethyl) azetidin-1- yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2FKJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443432 (Racemic 5-(4-{2- [3-(fluoromethyl) azetidin-1- yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the following ER competition binding assays is to determine the affinity of a test compound against ERα (wild type), ERα (Y5... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM443432 (Racemic 5-(4-{2- [3-(fluoromethyl) azetidin-1- yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Run the competition binding assay in a buffer containing 50 mM HEPES, pH 7.5, 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mg/mL ovalbumin, and 5 mM DTT... | US Patent US10654866 (2020) BindingDB Entry DOI: 10.7270/Q2D50R05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050482 (10-Amino-9-methoxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (CALF) | BDBM50050482 (10-Amino-9-methoxymethyl-1-methyl-tricyclo[7.3.1.0...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Activity of the compound was evaluated by inhibition of the binding of 0.8 nM [3H]- DAMGO at Opioid receptor mu 1 binding site | J Med Chem 39: 1956-66 (1996) Article DOI: 10.1021/jm950817g BindingDB Entry DOI: 10.7270/Q2CC0ZR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 741 total ) | Next | Last >> |