 Found 21 hits with Last Name = 'begley' and Initial = 'dw'
Found 21 hits with Last Name = 'begley' and Initial = 'dw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795
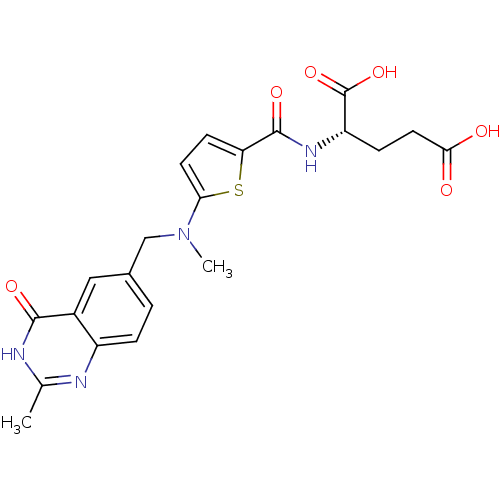
((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82167
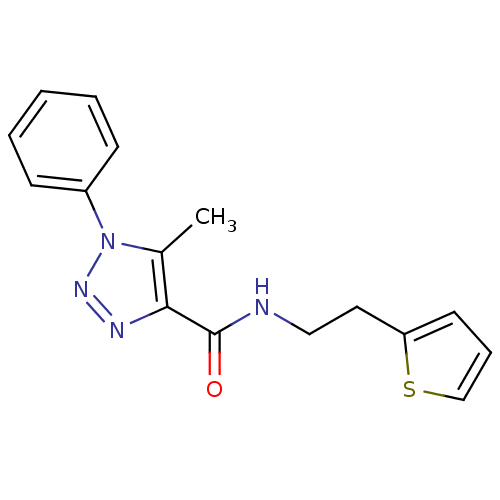
(MV1555)Show InChI InChI=1S/C16H16N4OS/c1-12-15(16(21)17-10-9-14-8-5-11-22-14)18-19-20(12)13-6-3-2-4-7-13/h2-8,11H,9-10H2,1H3,(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | 5.00E+3 | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82175

(MV1570)Show SMILES O=C(NCCc1c[nH]c2ccccc12)C(\NC(=O)c1cccs1)=C/c1cccs1 Show InChI InChI=1S/C22H19N3O2S2/c26-21(23-10-9-15-14-24-18-7-2-1-6-17(15)18)19(13-16-5-3-11-28-16)25-22(27)20-8-4-12-29-20/h1-8,11-14,24H,9-10H2,(H,23,26)(H,25,27)/b19-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+4 | 2.00E+3 | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82174
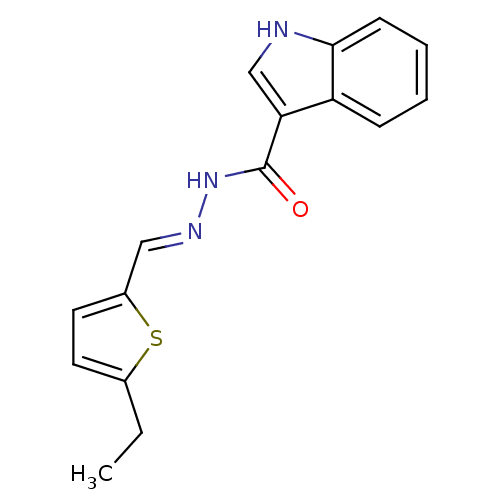
(MV1565)Show InChI InChI=1S/C16H15N3OS/c1-2-11-7-8-12(21-11)9-18-19-16(20)14-10-17-15-6-4-3-5-13(14)15/h3-10,17H,2H2,1H3,(H,19,20)/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+4 | 1.00E+3 | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82169
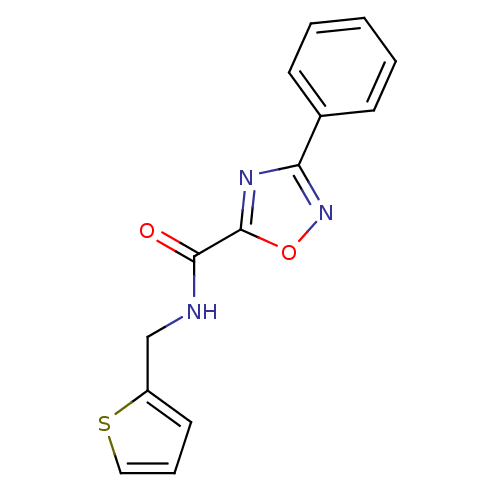
(MV1586)Show InChI InChI=1S/C14H11N3O2S/c18-13(15-9-11-7-4-8-20-11)14-16-12(17-19-14)10-5-2-1-3-6-10/h1-8H,9H2,(H,15,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82170

(MV1588)Show InChI InChI=1S/C15H16N4O2S2/c1-9-6-12(10(2)19(9)7-11-4-3-5-21-11)13(20)8-22-15-18-17-14(16)23-15/h3-6H,7-8H2,1-2H3,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82176

(MV1576)Show InChI InChI=1S/C19H18N2O4/c1-2-24-19(23)16-11-17-15(8-10-25-17)21(16)12-18(22)20-9-7-13-5-3-4-6-14(13)20/h3-6,8,10-11H,2,7,9,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82171
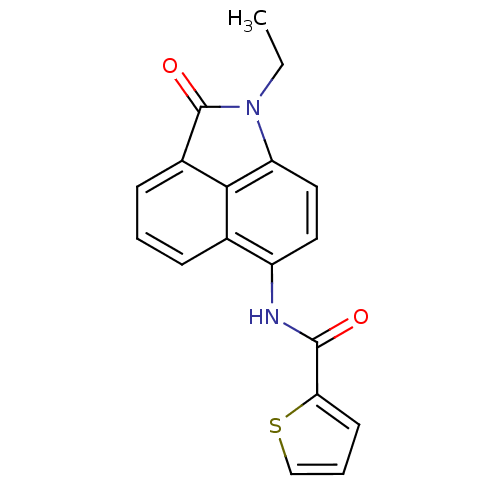
(MV1554)Show InChI InChI=1S/C18H14N2O2S/c1-2-20-14-9-8-13(19-17(21)15-7-4-10-23-15)11-5-3-6-12(16(11)14)18(20)22/h3-10H,2H2,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82173
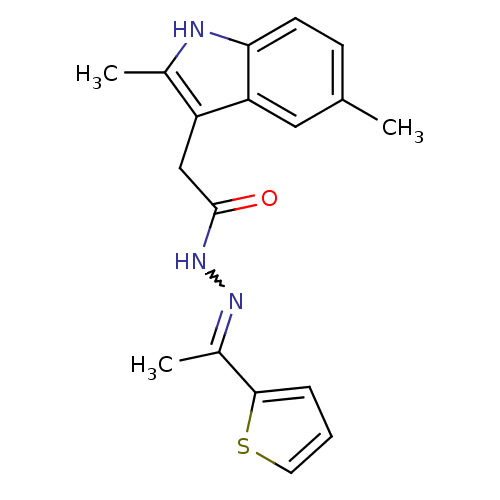
(MV1561)Show SMILES CC(=NNC(=O)Cc1c(C)[nH]c2ccc(C)cc12)c1cccs1 |w:2.2| Show InChI InChI=1S/C18H19N3OS/c1-11-6-7-16-15(9-11)14(12(2)19-16)10-18(22)21-20-13(3)17-5-4-8-23-17/h4-9,19H,10H2,1-3H3,(H,21,22)/b20-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82166
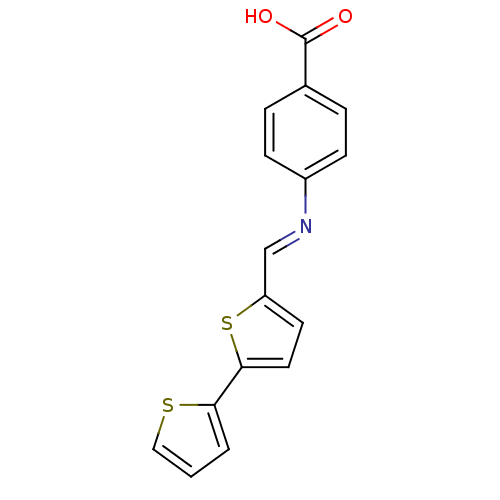
(MV1556)Show InChI InChI=1S/C16H11NO2S2/c18-16(19)11-3-5-12(6-4-11)17-10-13-7-8-15(21-13)14-2-1-9-20-14/h1-10H,(H,18,19)/b17-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82172
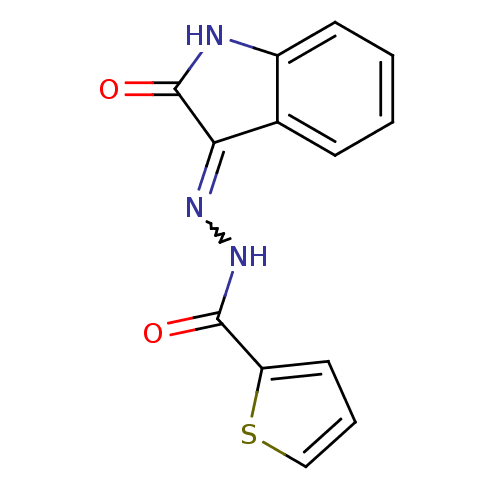
(MV1557)Show InChI InChI=1S/C13H9N3O2S/c17-12(10-6-3-7-19-10)16-15-11-8-4-1-2-5-9(8)14-13(11)18/h1-7H,(H,16,17)(H,14,15,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82177

(MV1574)Show InChI InChI=1S/C16H16N2O4S/c1-10(15(19)17-9-11-4-3-7-23-11)22-16(20)13-8-14-12(18(13)2)5-6-21-14/h3-8,10H,9H2,1-2H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82168
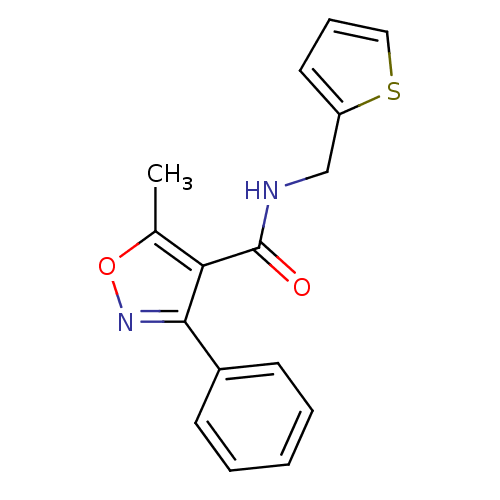
(MV1581)Show InChI InChI=1S/C16H14N2O2S/c1-11-14(16(19)17-10-13-8-5-9-21-13)15(18-20-11)12-6-3-2-4-7-12/h2-9H,10H2,1H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM82178
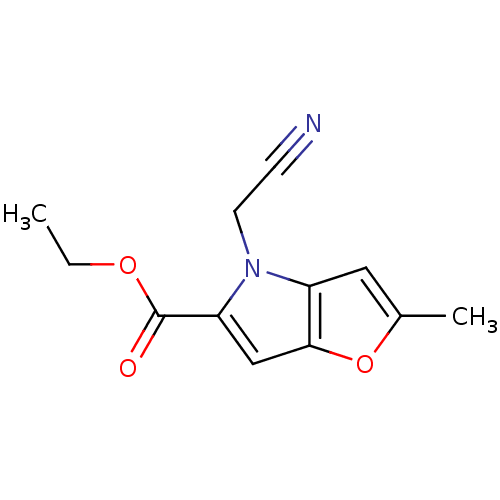
(MV1578)Show InChI InChI=1S/C12H12N2O3/c1-3-16-12(15)10-7-11-9(6-8(2)17-11)14(10)5-4-13/h6-7H,3,5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
University of Washington
| Assay Description
A tritium release assay was used to determin the in vitro inhibitory strengths of various compounds. The inhibitory potency of human TS was determin... |
Chem Biol Drug Des 76: 218-33 (2010)
Article DOI: 10.1111/j.1747-0285.2010.01010.x
BindingDB Entry DOI: 10.7270/Q2ZP44MF |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443773
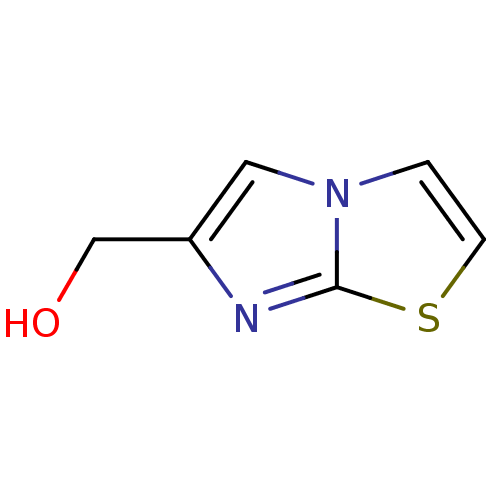
(CHEMBL1230528)Show InChI InChI=1S/C6H6N2OS/c9-4-5-3-8-1-2-10-6(8)7-5/h1-3,9H,4H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443774
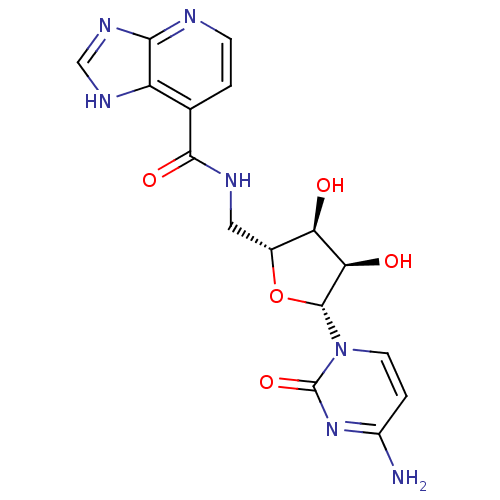
(CHEMBL3094106)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3ccnc4nc[nH]c34)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C16H17N7O5/c17-9-2-4-23(16(27)22-9)15-12(25)11(24)8(28-15)5-19-14(26)7-1-3-18-13-10(7)20-6-21-13/h1-4,6,8,11-12,15,24-25H,5H2,(H,19,26)(H2,17,22,27)(H,18,20,21)/t8-,11-,12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50194153

(5'-CDP | CDP | CHEMBL425252 | Cytidine | Cytidine ...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 Show InChI InChI=1S/C9H15N3O11P2/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(22-8)3-21-25(19,20)23-24(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443772

(CHEMBL1230597)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3ccncc3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C15H17N5O5/c16-10-3-6-20(15(24)19-10)14-12(22)11(21)9(25-14)7-18-13(23)8-1-4-17-5-2-8/h1-6,9,11-12,14,21-22H,7H2,(H,18,23)(H2,16,19,24)/t9-,11-,12-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443770

(CHEMBL3094104)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3cn4ccsc4n3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C15H16N6O5S/c16-9-1-2-21(14(25)19-9)13-11(23)10(22)8(26-13)5-17-12(24)7-6-20-3-4-27-15(20)18-7/h1-4,6,8,10-11,13,22-23H,5H2,(H,17,24)(H2,16,19,25)/t8-,10-,11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443775
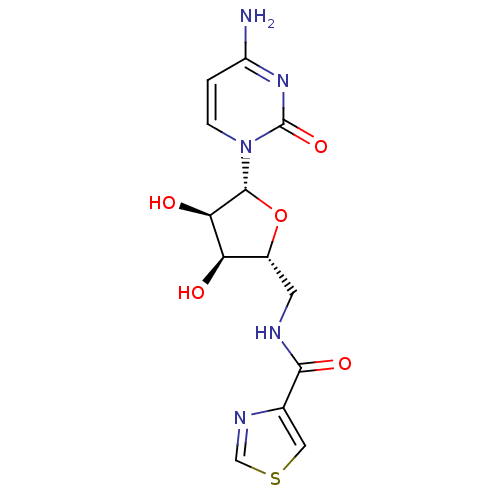
(CHEMBL3094105)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)c3cscn3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C13H15N5O5S/c14-8-1-2-18(13(22)17-8)12-10(20)9(19)7(23-12)3-15-11(21)6-4-24-5-16-6/h1-2,4-5,7,9-10,12,19-20H,3H2,(H,15,21)(H2,14,17,22)/t7-,9-,10-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
(Burkholderia pseudomallei (strain K96243)) | BDBM50443771
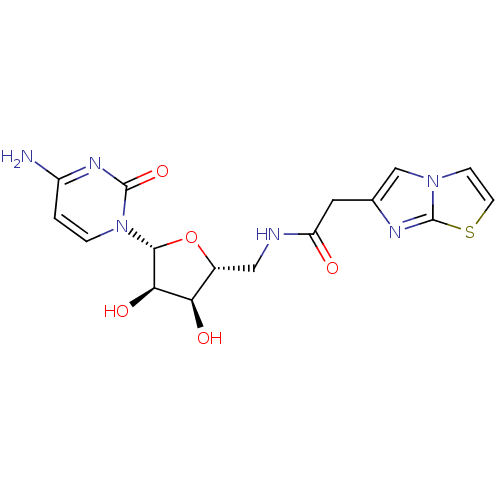
(CHEMBL3094103)Show SMILES Nc1ccn([C@@H]2O[C@H](CNC(=O)Cc3cn4ccsc4n3)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C16H18N6O5S/c17-10-1-2-22(15(26)20-10)14-13(25)12(24)9(27-14)6-18-11(23)5-8-7-21-3-4-28-16(21)19-8/h1-4,7,9,12-14,24-25H,5-6H2,(H,18,23)(H2,17,20,26)/t9-,12-,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University
Curated by ChEMBL
| Assay Description
Binding affinity to Burkolderia pseudomallei IspF by surface plasmon resonance method |
Bioorg Med Chem Lett 23: 6860-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.101
BindingDB Entry DOI: 10.7270/Q2NC62NX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data