 Found 3374 hits with Last Name = 'bell' and Initial = 'l'
Found 3374 hits with Last Name = 'bell' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475465
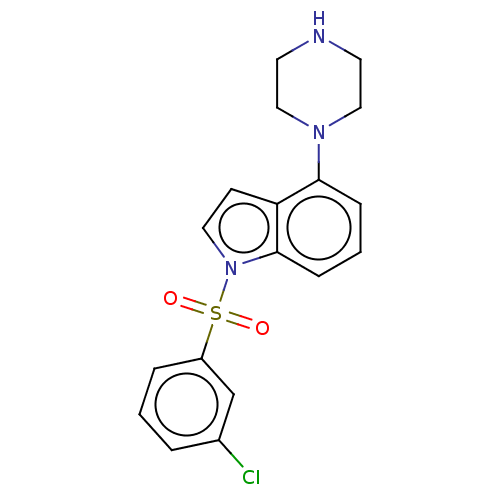
(CHEMBL196410)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-3-1-4-15(13-14)25(23,24)22-10-7-16-17(5-2-6-18(16)22)21-11-8-20-9-12-21/h1-7,10,13,20H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475467

(CHEMBL425015)Show SMILES Clc1cccc(c1)S(=O)(=O)c1c[nH]c2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-13-3-1-4-14(11-13)25(23,24)17-12-21-18-15(17)5-2-6-16(18)22-9-7-20-8-10-22/h1-6,11-12,20-21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475480
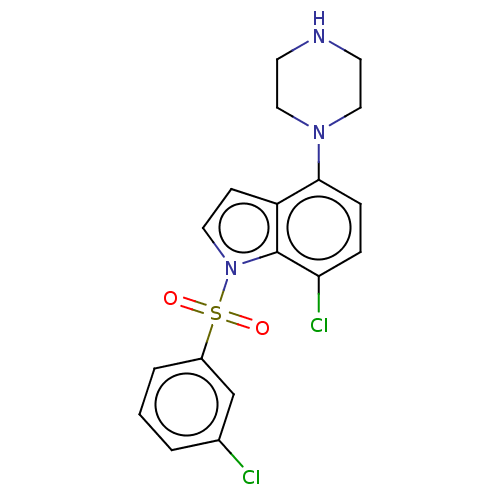
(CHEMBL193629)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(ccc(Cl)c12)N1CCNCC1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(5-4-16(20)18(15)23)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475462
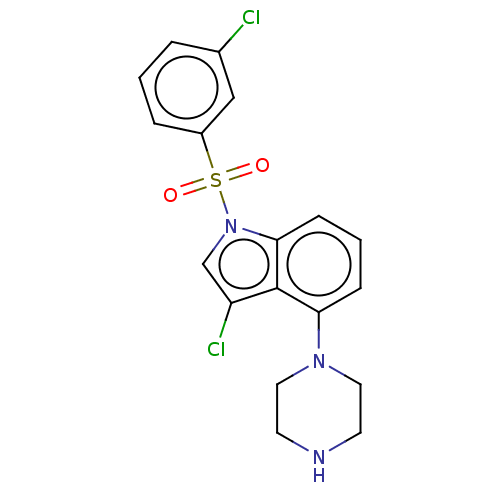
(CHEMBL371375)Show SMILES Clc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-3-1-4-14(11-13)26(24,25)23-12-15(20)18-16(5-2-6-17(18)23)22-9-7-21-8-10-22/h1-6,11-12,21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475475
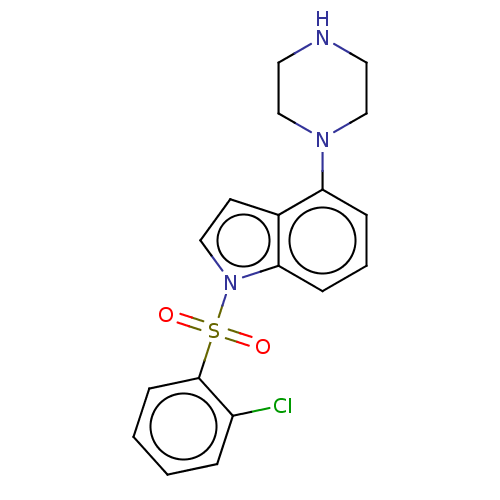
(CHEMBL372513)Show InChI InChI=1S/C18H18ClN3O2S/c19-15-4-1-2-7-18(15)25(23,24)22-11-8-14-16(5-3-6-17(14)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475477
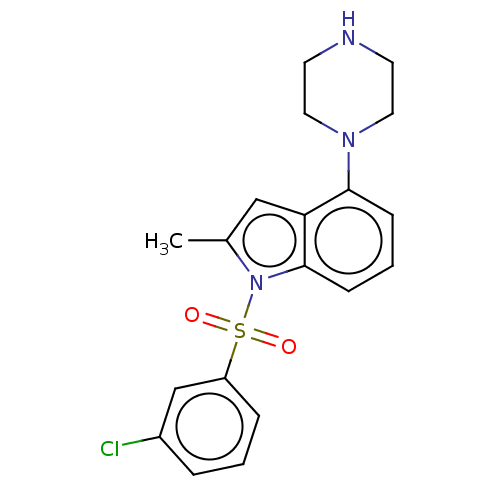
(CHEMBL372929)Show SMILES Cc1cc2c(cccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-12-17-18(22-10-8-21-9-11-22)6-3-7-19(17)23(14)26(24,25)16-5-2-4-15(20)13-16/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222900
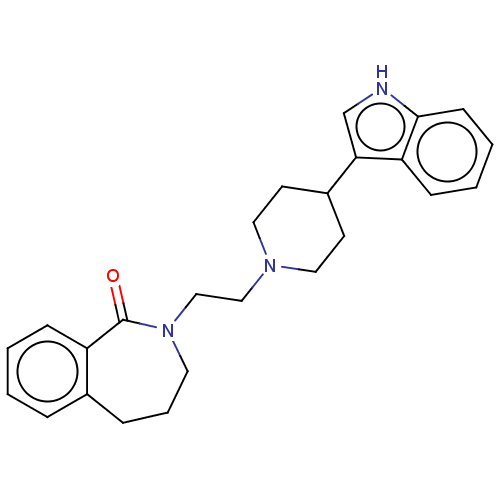
(CHEMBL267062)Show SMILES O=C1N(CCN2CCC(CC2)c2c[nH]c3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C25H29N3O/c29-25-21-8-2-1-6-19(21)7-5-13-28(25)17-16-27-14-11-20(12-15-27)23-18-26-24-10-4-3-9-22(23)24/h1-4,6,8-10,18,20,26H,5,7,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410477

(CHEMBL54719)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(OCc4ccccn4)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O2/c1-14-10-15-7-9-29(19(15)11-18(14)22(23,24)25)21(30)28-16-5-6-20(27-12-16)31-13-17-4-2-3-8-26-17/h2-6,8,10-12H,7,9,13H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044607
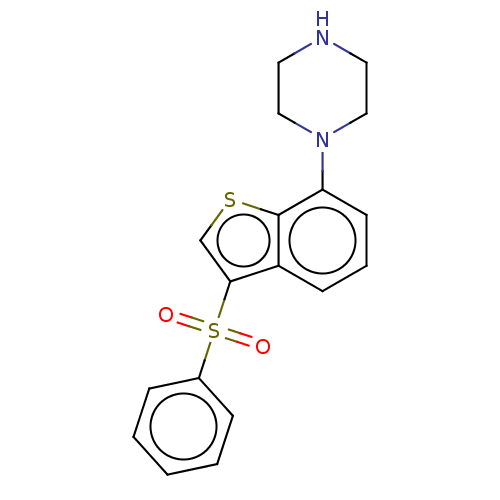
(CHEMBL372537)Show InChI InChI=1S/C18H18N2O2S2/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475463
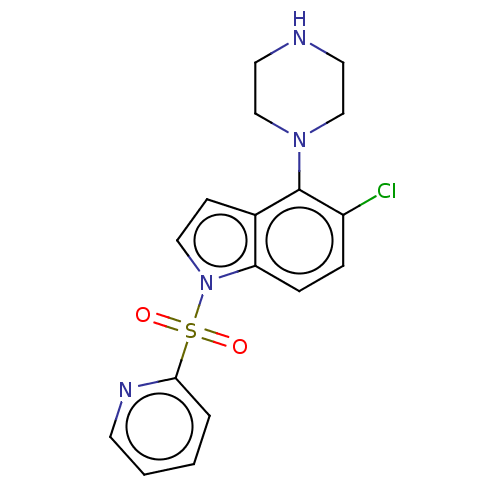
(CHEMBL194915)Show InChI InChI=1S/C17H17ClN4O2S/c18-14-4-5-15-13(17(14)21-11-8-19-9-12-21)6-10-22(15)25(23,24)16-3-1-2-7-20-16/h1-7,10,19H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222781
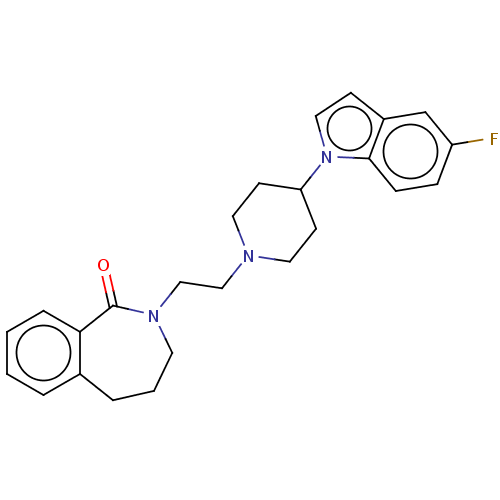
(CHEMBL9951)Show SMILES Fc1ccc2n(ccc2c1)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C25H28FN3O/c26-21-7-8-24-20(18-21)9-15-29(24)22-10-13-27(14-11-22)16-17-28-12-3-5-19-4-1-2-6-23(19)25(28)30/h1-2,4,6-9,15,18,22H,3,5,10-14,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475473
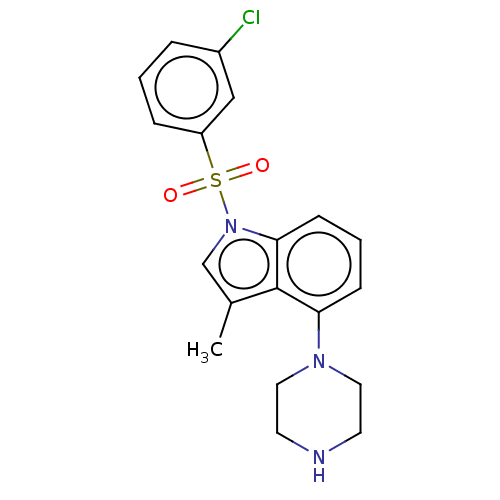
(CHEMBL194039)Show SMILES Cc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-13-23(26(24,25)16-5-2-4-15(20)12-16)18-7-3-6-17(19(14)18)22-10-8-21-9-11-22/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410476
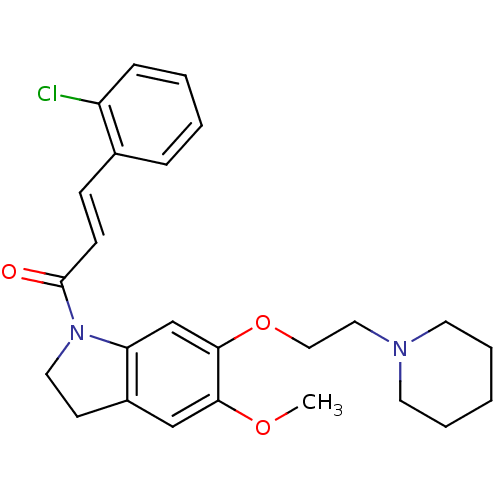
(CHEMBL197630)Show SMILES COc1cc2CCN(C(=O)\C=C\c3ccccc3Cl)c2cc1OCCN1CCCCC1 Show InChI InChI=1S/C25H29ClN2O3/c1-30-23-17-20-11-14-28(25(29)10-9-19-7-3-4-8-21(19)26)22(20)18-24(23)31-16-15-27-12-5-2-6-13-27/h3-4,7-10,17-18H,2,5-6,11-16H2,1H3/b10-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410480
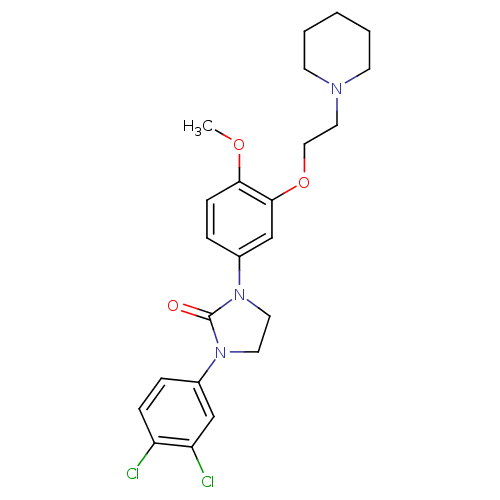
(CHEMBL197807)Show SMILES COc1ccc(cc1OCCN1CCCCC1)N1CCN(C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C23H27Cl2N3O3/c1-30-21-8-6-18(16-22(21)31-14-13-26-9-3-2-4-10-26)28-12-11-27(23(28)29)17-5-7-19(24)20(25)15-17/h5-8,15-16H,2-4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410475
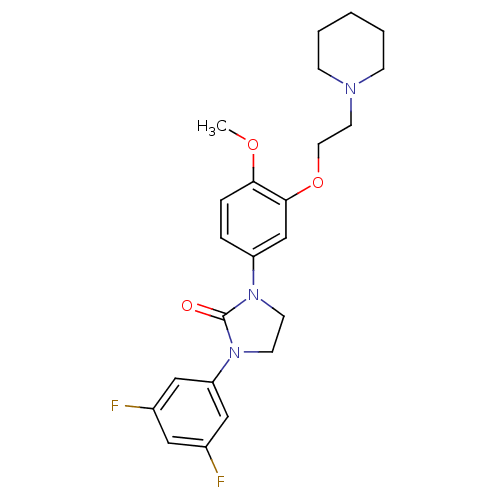
(CHEMBL372339)Show SMILES COc1ccc(cc1OCCN1CCCCC1)N1CCN(C1=O)c1cc(F)cc(F)c1 Show InChI InChI=1S/C23H27F2N3O3/c1-30-21-6-5-19(16-22(21)31-12-11-26-7-3-2-4-8-26)27-9-10-28(23(27)29)20-14-17(24)13-18(25)15-20/h5-6,13-16H,2-4,7-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475470

(CHEMBL370209)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,17-6-1-2-8-19-17)21-11-7-14-15(4-3-5-16(14)21)20-12-9-18-10-13-20/h1-8,11,18H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475479
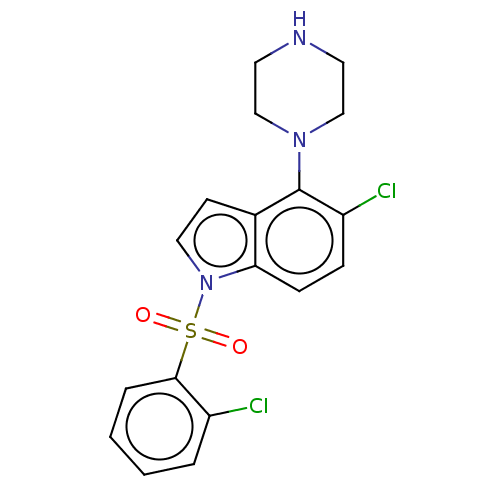
(CHEMBL371176)Show SMILES Clc1ccccc1S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-14-3-1-2-4-17(14)26(24,25)23-10-7-13-16(23)6-5-15(20)18(13)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85097
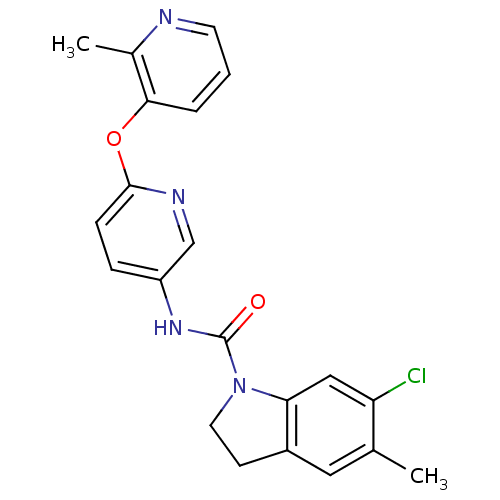
(CAS_181632-25-7 | CHEMBL14563 | SB 242084)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1Cl Show InChI InChI=1S/C21H19ClN4O2/c1-13-10-15-7-9-26(18(15)11-17(13)22)21(27)25-16-5-6-20(24-12-16)28-19-4-3-8-23-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086065

(5-Methyl-6-trifluoromethyl-2,3-dihydro-indole-1-ca...)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O2/c1-13-10-15-7-9-29(18(15)11-17(13)22(23,24)25)21(30)28-16-5-6-20(27-12-16)31-19-4-3-8-26-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410478
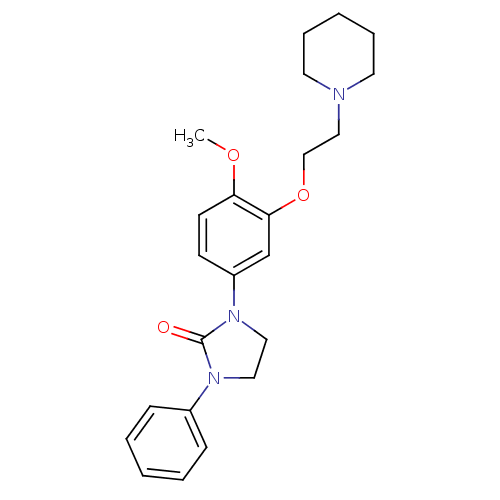
(CHEMBL383800)Show InChI InChI=1S/C23H29N3O3/c1-28-21-11-10-20(18-22(21)29-17-16-24-12-6-3-7-13-24)26-15-14-25(23(26)27)19-8-4-2-5-9-19/h2,4-5,8-11,18H,3,6-7,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410467
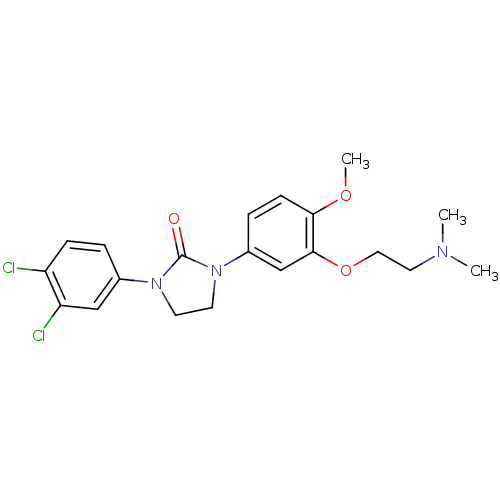
(CHEMBL196953)Show SMILES COc1ccc(cc1OCCN(C)C)N1CCN(C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H23Cl2N3O3/c1-23(2)10-11-28-19-13-15(5-7-18(19)27-3)25-9-8-24(20(25)26)14-4-6-16(21)17(22)12-14/h4-7,12-13H,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
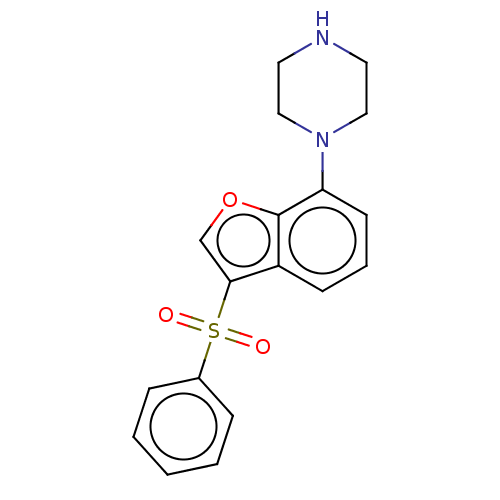
(Homo sapiens (Human)) | BDBM50475464
(CHEMBL197574)Show InChI InChI=1S/C18H18N2O3S/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
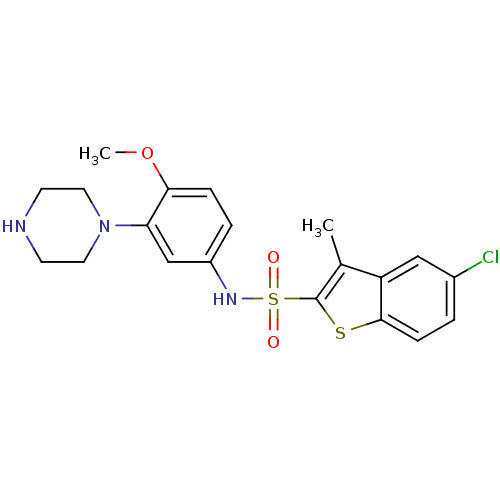
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410471
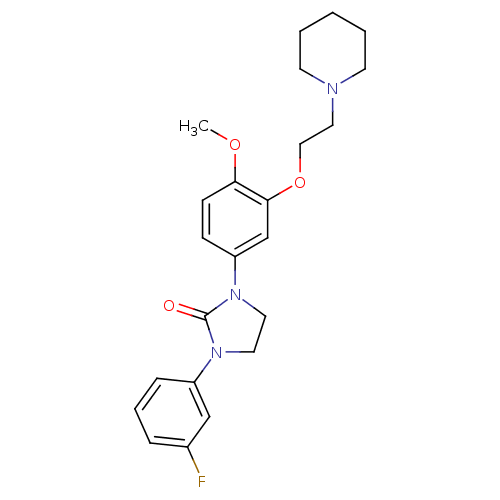
(CHEMBL198330)Show SMILES COc1ccc(cc1OCCN1CCCCC1)N1CCN(C1=O)c1cccc(F)c1 Show InChI InChI=1S/C23H28FN3O3/c1-29-21-9-8-20(17-22(21)30-15-14-25-10-3-2-4-11-25)27-13-12-26(23(27)28)19-7-5-6-18(24)16-19/h5-9,16-17H,2-4,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475466
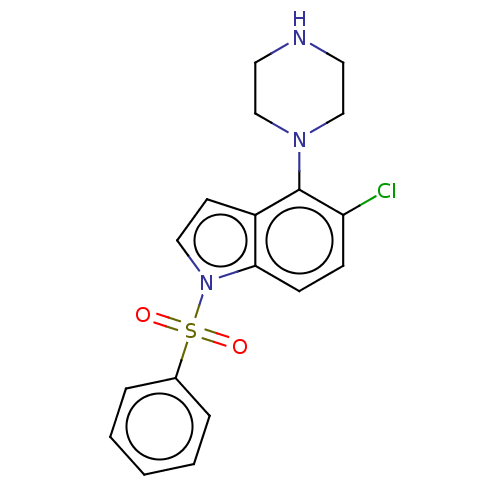
(CHEMBL193665)Show InChI InChI=1S/C18H18ClN3O2S/c19-16-6-7-17-15(18(16)21-12-9-20-10-13-21)8-11-22(17)25(23,24)14-4-2-1-3-5-14/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372
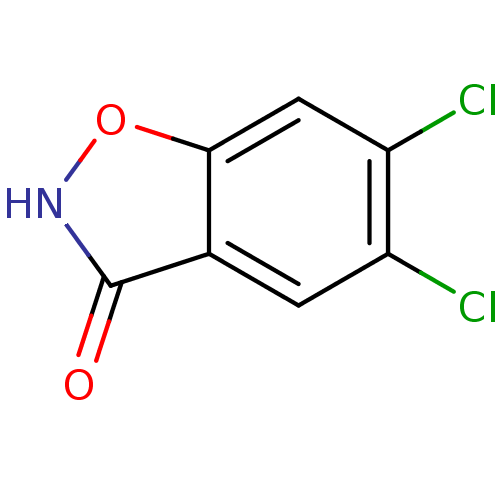
(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222777
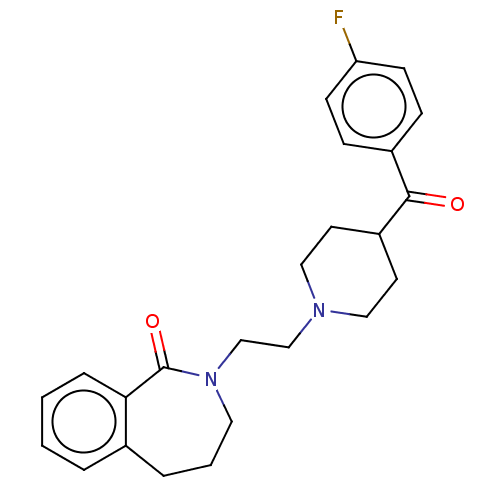
(CHEMBL9616)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C24H27FN2O2/c25-21-9-7-19(8-10-21)23(28)20-11-14-26(15-12-20)16-17-27-13-3-5-18-4-1-2-6-22(18)24(27)29/h1-2,4,6-10,20H,3,5,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50222900

(CHEMBL267062)Show SMILES O=C1N(CCN2CCC(CC2)c2c[nH]c3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C25H29N3O/c29-25-21-8-2-1-6-19(21)7-5-13-28(25)17-16-27-14-11-20(12-15-27)23-18-26-24-10-4-3-9-22(23)24/h1-4,6,8-10,18,20,26H,5,7,11-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 2A receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475471
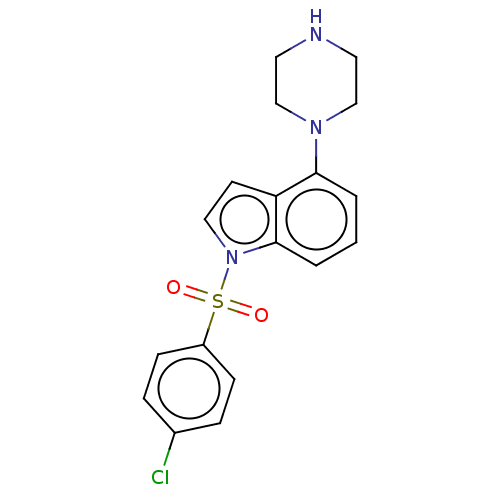
(CHEMBL371876)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-4-6-15(7-5-14)25(23,24)22-11-8-16-17(2-1-3-18(16)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410473
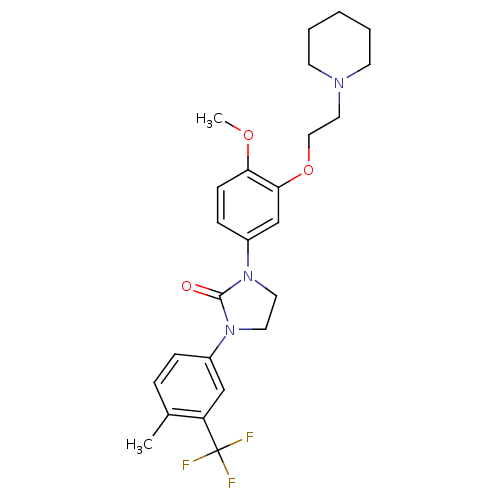
(CHEMBL198171)Show SMILES COc1ccc(cc1OCCN1CCCCC1)N1CCN(C1=O)c1ccc(C)c(c1)C(F)(F)F Show InChI InChI=1S/C25H30F3N3O3/c1-18-6-7-19(16-21(18)25(26,27)28)30-12-13-31(24(30)32)20-8-9-22(33-2)23(17-20)34-15-14-29-10-4-3-5-11-29/h6-9,16-17H,3-5,10-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222899
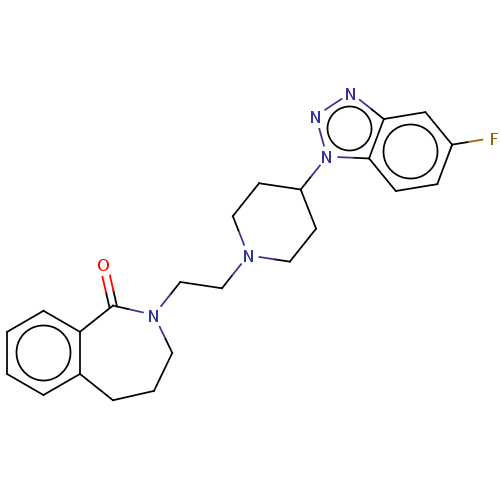
(CHEMBL9347)Show SMILES Fc1ccc2n(nnc2c1)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H26FN5O/c24-18-7-8-22-21(16-18)25-26-29(22)19-9-12-27(13-10-19)14-15-28-11-3-5-17-4-1-2-6-20(17)23(28)30/h1-2,4,6-8,16,19H,3,5,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50125260

(2-{2-[4-(5-Fluoro-2-oxo-2,3-dihydro-benzoimidazol-...)Show SMILES Fc1ccc2n(C3CCN(CCN4CCCc5ccccc5C4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C24H27FN4O2/c25-18-7-8-22-21(16-18)26-24(31)29(22)19-9-12-27(13-10-19)14-15-28-11-3-5-17-4-1-2-6-20(17)23(28)30/h1-2,4,6-8,16,19H,3,5,9-15H2,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222780
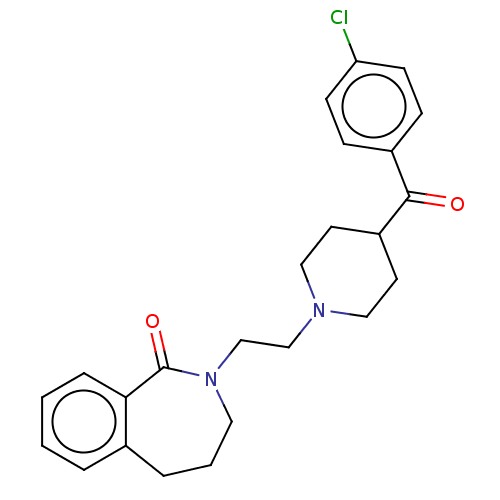
(CHEMBL275343)Show SMILES Clc1ccc(cc1)C(=O)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C24H27ClN2O2/c25-21-9-7-19(8-10-21)23(28)20-11-14-26(15-12-20)16-17-27-13-3-5-18-4-1-2-6-22(18)24(27)29/h1-2,4,6-10,20H,3,5,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475481

(CHEMBL197297)Show SMILES Cn1cc(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-22-13-18(26(24,25)15-5-2-4-14(20)12-15)16-6-3-7-17(19(16)22)23-10-8-21-9-11-23/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222894
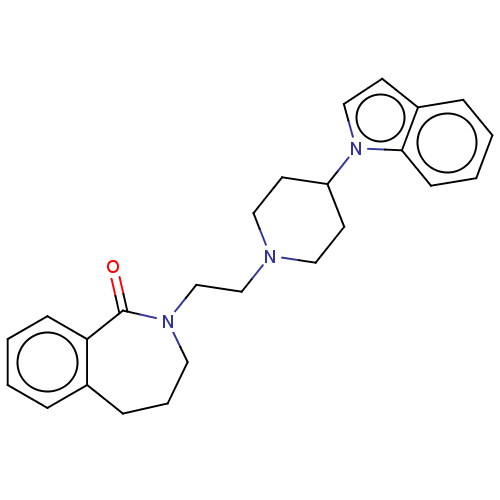
(CHEMBL9952)Show SMILES O=C1N(CCN2CCC(CC2)n2ccc3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C25H29N3O/c29-25-23-9-3-1-6-20(23)8-5-14-27(25)19-18-26-15-12-22(13-16-26)28-17-11-21-7-2-4-10-24(21)28/h1-4,6-7,9-11,17,22H,5,8,12-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM17290
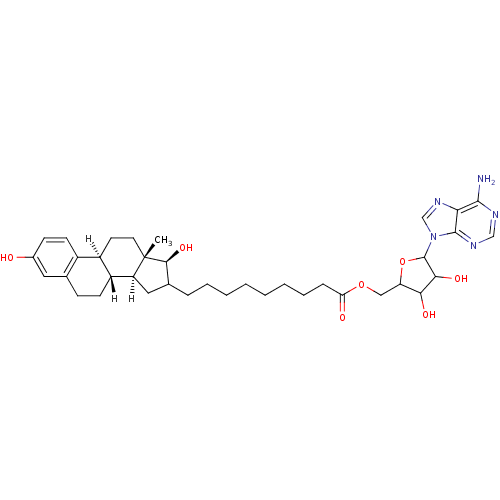
(E2-adenosine hybrid compound, 8 | EM-1745 | EM1745...)Show SMILES [H][C@@]12CC(CCCCCCCCC(=O)OCC3OC(C(O)C3O)n3cnc4c(N)ncnc34)[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C37H51N5O7/c1-37-15-14-25-24-13-11-23(43)16-21(24)10-12-26(25)27(37)17-22(33(37)47)8-6-4-2-3-5-7-9-29(44)48-18-28-31(45)32(46)36(49-28)42-20-41-30-34(38)39-19-40-35(30)42/h11,13,16,19-20,22,25-28,31-33,36,43,45-47H,2-10,12,14-15,17-18H2,1H3,(H2,38,39,40)/t22?,25-,26-,27+,28?,31?,32?,33+,36?,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CHUL
| Assay Description
For steady-state kinetic study of hybrid inhibitors, a Fluorolog 3 instrument was used to monitor the fluorescent signal of NADPH formed during estra... |
FASEB J 16: 1829-31 (2002)
Article DOI: 10.1096/fj.02-0026fje
BindingDB Entry DOI: 10.7270/Q23T9FGW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410470
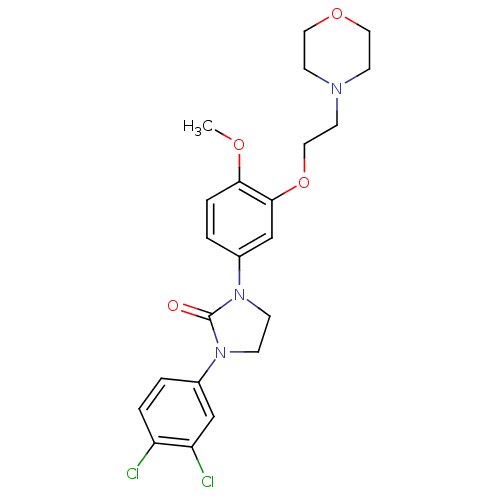
(CHEMBL198007)Show SMILES COc1ccc(cc1OCCN1CCOCC1)N1CCN(C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H25Cl2N3O4/c1-29-20-5-3-17(15-21(20)31-13-10-25-8-11-30-12-9-25)27-7-6-26(22(27)28)16-2-4-18(23)19(24)14-16/h2-5,14-15H,6-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475478
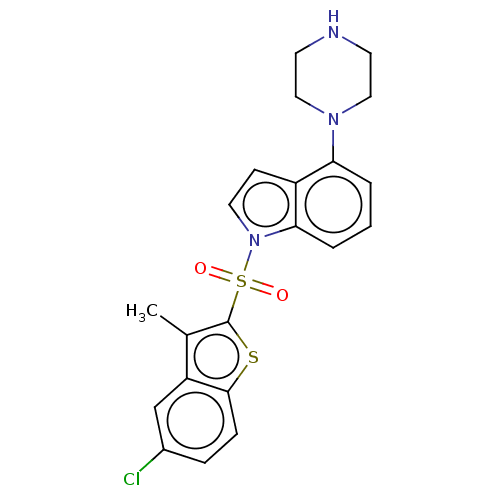
(CHEMBL196644)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2S2/c1-14-17-13-15(22)5-6-20(17)28-21(14)29(26,27)25-10-7-16-18(3-2-4-19(16)25)24-11-8-23-9-12-24/h2-7,10,13,23H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725
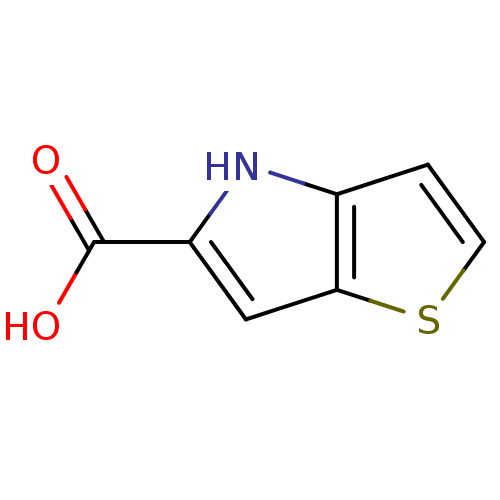
(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410472
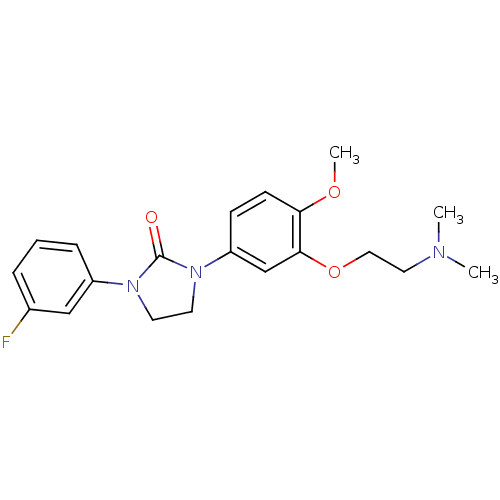
(CHEMBL197170)Show InChI InChI=1S/C20H24FN3O3/c1-22(2)11-12-27-19-14-17(7-8-18(19)26-3)24-10-9-23(20(24)25)16-6-4-5-15(21)13-16/h4-8,13-14H,9-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex |
J Med Chem 52: 840-51 (2009)
Article DOI: 10.1021/jm801330n
BindingDB Entry DOI: 10.7270/Q22V2JXB |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
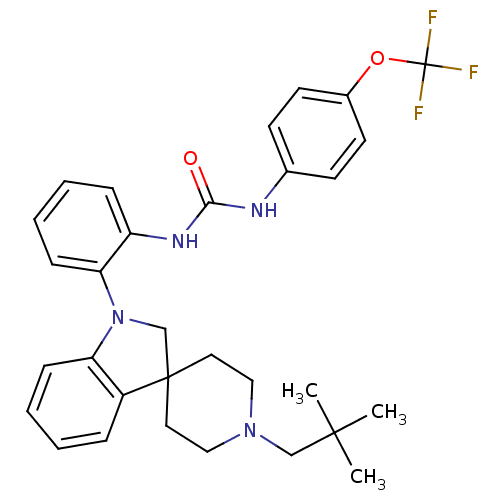
(Homo sapiens (Human)) | BDBM50445214
(CHEMBL3104636)Show SMILES CC(C)(C)CN1CCC2(CN(c3ccccc23)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C31H35F3N4O2/c1-29(2,3)20-37-18-16-30(17-19-37)21-38(26-10-6-4-8-24(26)30)27-11-7-5-9-25(27)36-28(39)35-22-12-14-23(15-13-22)40-31(32,33)34/h4-15H,16-21H2,1-3H3,(H2,35,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
J Med Chem 56: 9275-95 (2013)
Article DOI: 10.1021/jm4013906
BindingDB Entry DOI: 10.7270/Q2KW5HHP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
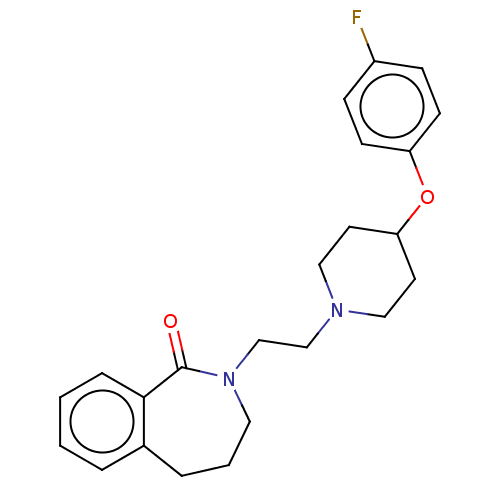
(Homo sapiens (Human)) | BDBM50222778
(CHEMBL275451)Show InChI InChI=1S/C23H27FN2O2/c24-19-7-9-20(10-8-19)28-21-11-14-25(15-12-21)16-17-26-13-3-5-18-4-1-2-6-22(18)23(26)27/h1-2,4,6-10,21H,3,5,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
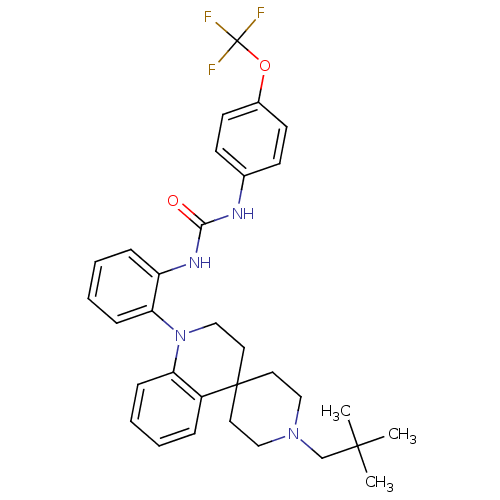
(Homo sapiens (Human)) | BDBM50445208
(CHEMBL3105199)Show SMILES CC(C)(C)CN1CCC2(CC1)CCN(c1ccccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1)c1ccccc21 Show InChI InChI=1S/C32H37F3N4O2/c1-30(2,3)22-38-19-16-31(17-20-38)18-21-39(27-10-6-4-8-25(27)31)28-11-7-5-9-26(28)37-29(40)36-23-12-14-24(15-13-23)41-32(33,34)35/h4-15H,16-22H2,1-3H3,(H2,36,37,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
J Med Chem 56: 9275-95 (2013)
Article DOI: 10.1021/jm4013906
BindingDB Entry DOI: 10.7270/Q2KW5HHP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475482

(CHEMBL193379)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(ccc12)C#N Show InChI InChI=1S/C19H17ClN4O2S/c20-15-2-1-3-16(12-15)27(25,26)24-9-6-17-18(24)5-4-14(13-21)19(17)23-10-7-22-8-11-23/h1-6,9,12,22H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50445201
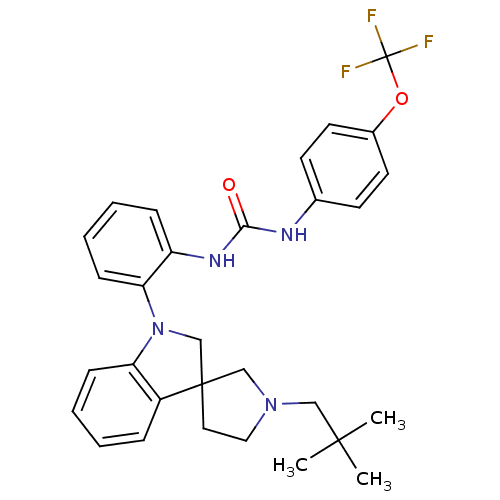
(CHEMBL3105201)Show SMILES CC(C)(C)CN1CCC2(C1)CN(c1ccccc21)c1ccccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C30H33F3N4O2/c1-28(2,3)18-36-17-16-29(19-36)20-37(25-10-6-4-8-23(25)29)26-11-7-5-9-24(26)35-27(38)34-21-12-14-22(15-13-21)39-30(31,32)33/h4-15H,16-20H2,1-3H3,(H2,34,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis |
J Med Chem 56: 9275-95 (2013)
Article DOI: 10.1021/jm4013906
BindingDB Entry DOI: 10.7270/Q2KW5HHP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data