

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1C (Gallus gallus) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470867 (CHEMBL318179) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470869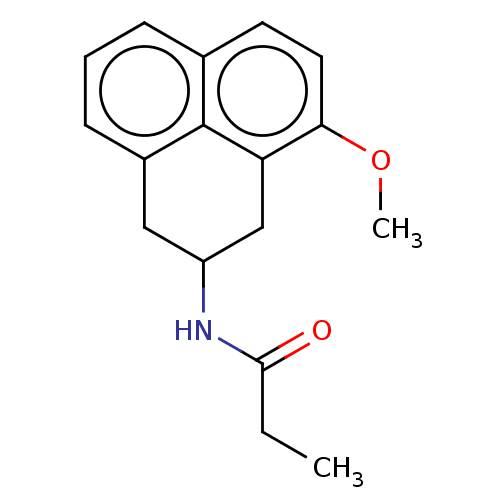 (CHEMBL12477) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470868 (CHEMBL12795) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470866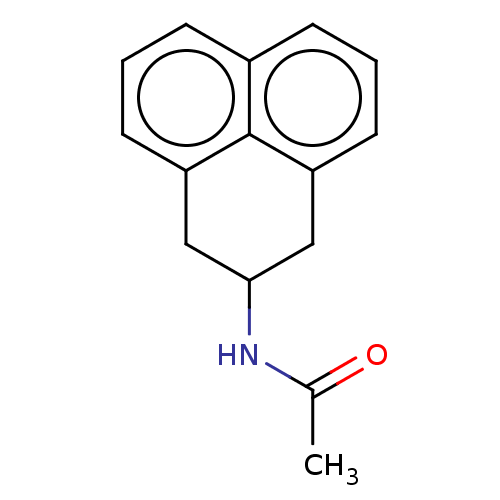 (CHEMBL320810) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470871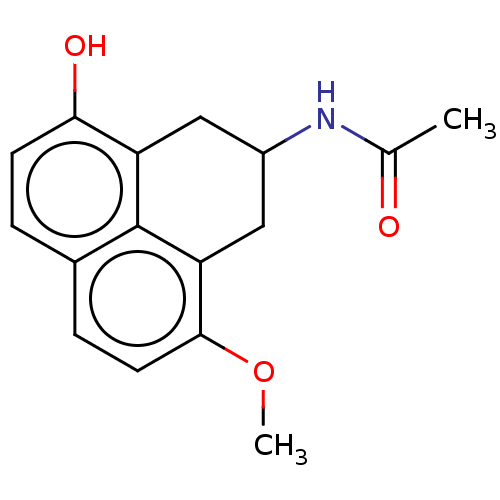 (CHEMBL106276) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50035176 (8-M-ADOT | CHEMBL53015 | N-(8-Methoxy-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470870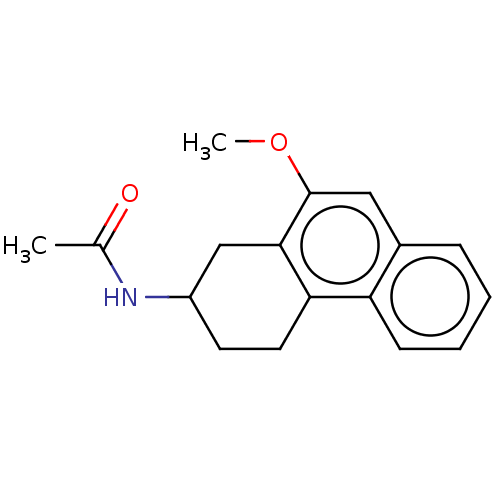 (CHEMBL104997) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470866 (CHEMBL320810) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470865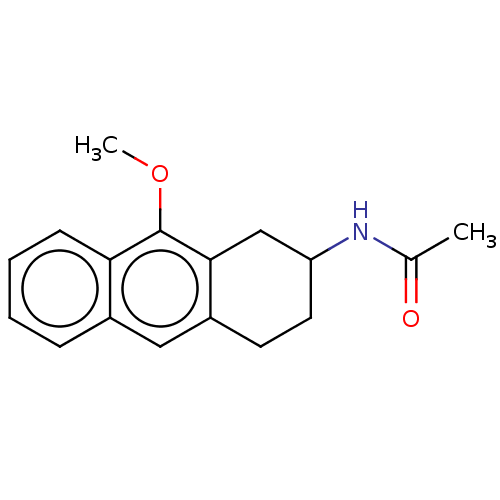 (CHEMBL317669) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM29612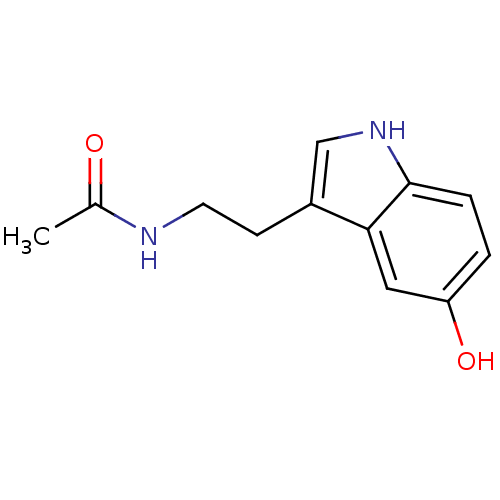 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-BIOCIS Curated by ChEMBL | Assay Description In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes | J Med Chem 39: 3089-95 (1996) Article DOI: 10.1021/jm960219h BindingDB Entry DOI: 10.7270/Q2736TNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074687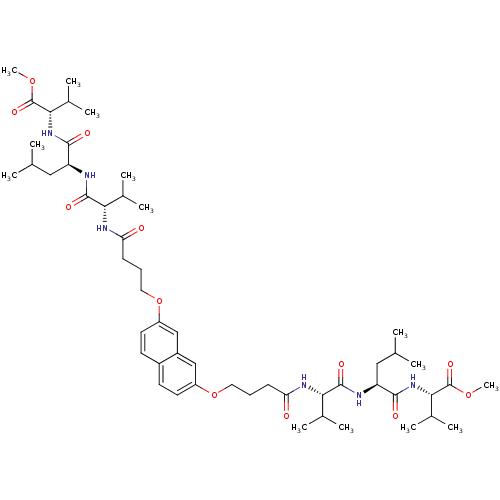 (2-[2-(2-{4-[7-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074689 (2-[2-(2-{4-[6-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074688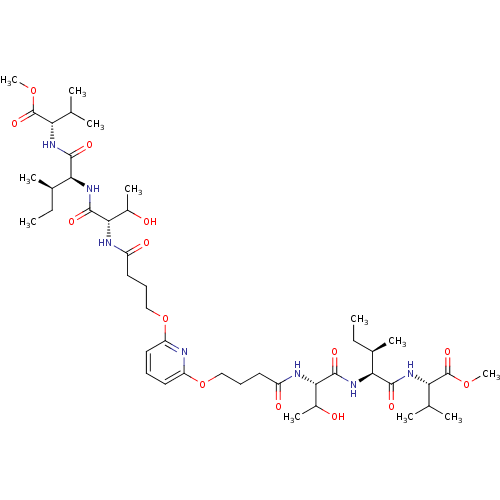 (2-[2-(3-Hydroxy-2-{4-[6-(3-{2-hydroxy-1-[1-(1-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074686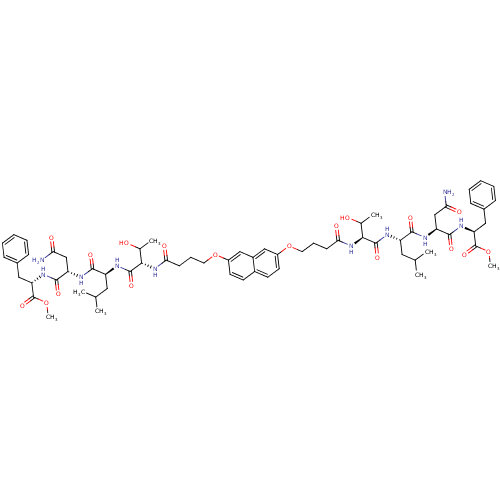 (4-[7,2-bis{(4-Oxo-butoxy)-thr-leu-asp-phe-OMe]-nap...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074684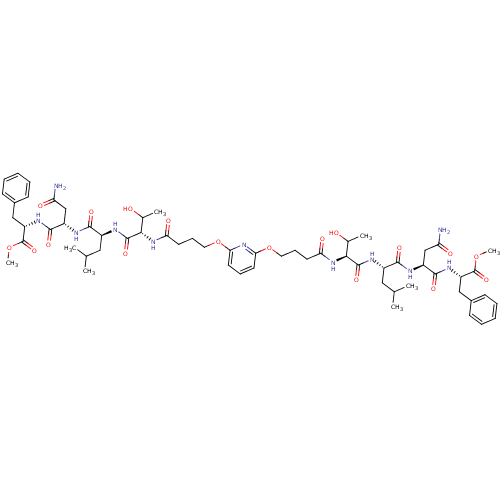 (CHEMBL442133 | [7,2-bis{(4-Oxo-butoxy)-thr-leu-asp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074682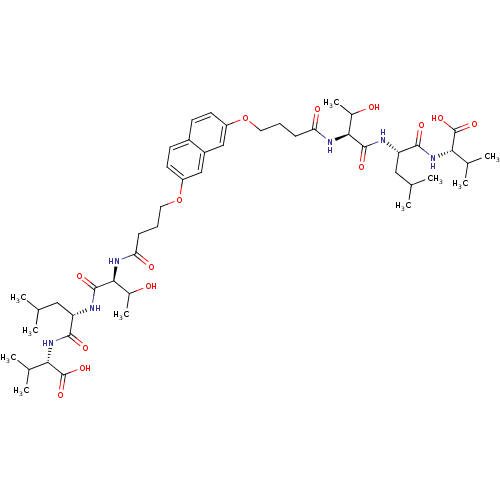 ((S)-2-[(S)-2-((S)-2-{4-[7-(3-{(S)-1-[(S)-1-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074683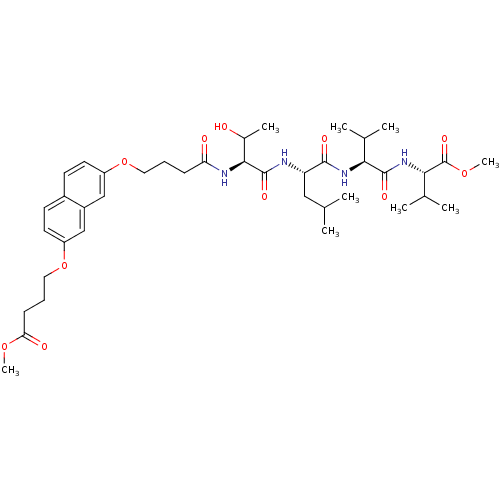 ((S)-2-{(S)-2-[(S)-2-((S)-3-Hydroxy-2-{4-[7-(3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074685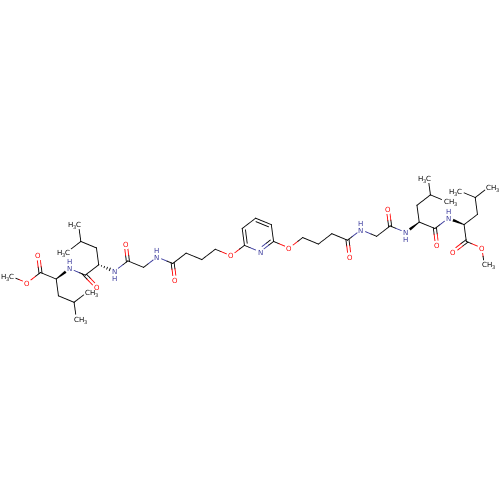 ((S)-2-{(S)-2-[2-(4-{6-[3-({[(S)-1-((S)-1-Methoxyca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074690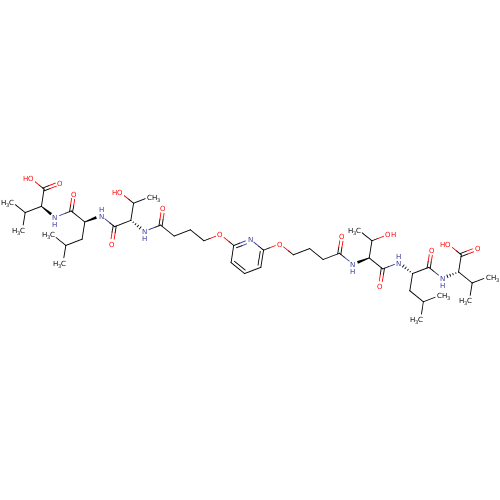 ((S)-2-[(S)-2-((S)-2-{4-[6-(3-{(S)-1-[(S)-1-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-sud Curated by ChEMBL | Assay Description The compound was tested for HIV-1 protease inhibitory activity against plasmid pET9c-PR expressing HIV-1 protease. | J Med Chem 42: 957-62 (1999) Article DOI: 10.1021/jm9803976 BindingDB Entry DOI: 10.7270/Q2DJ5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||