

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235671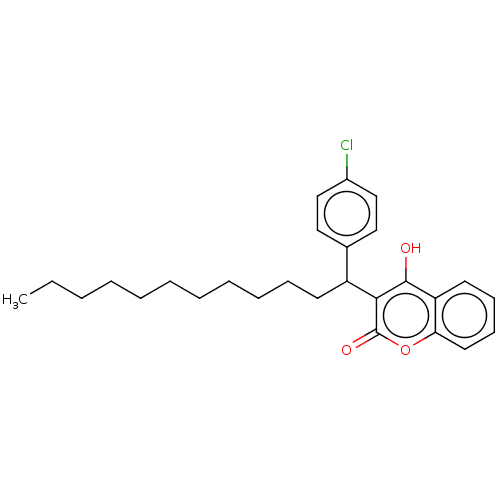 (CHEMBL4097212) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of endothelin-converting enzyme in human umbilical vein endothelial cells | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235661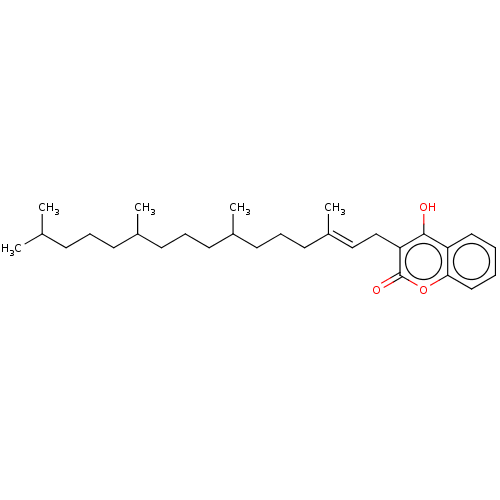 (CHEMBL4088796) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235667 (CHEMBL4080688) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of endothelin-converting enzyme in human umbilical vein endothelial cells | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235670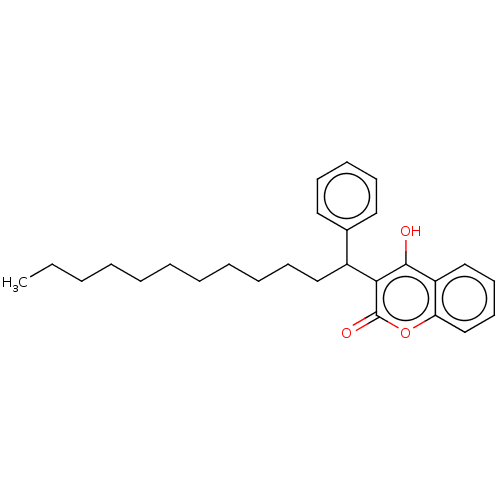 (CHEMBL4079988) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of VKORC1 in rat liver microsomes in presence of 0.003 to 0.2 mM vitamin K | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235673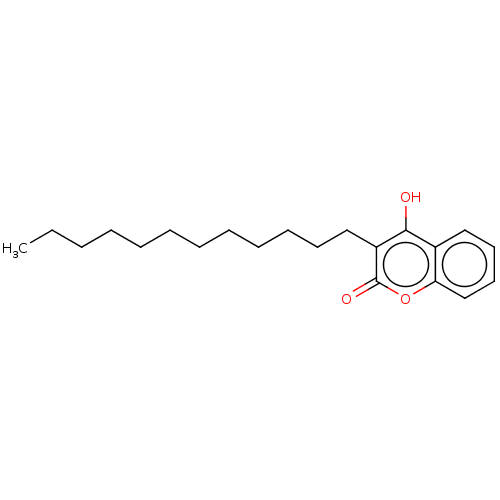 (CHEMBL4060572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of VKORC1 in rat liver microsomes in presence of 0.003 to 0.2 mM vitamin K | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235668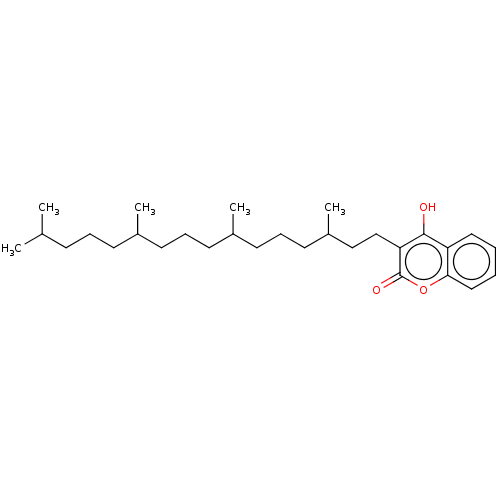 (CHEMBL4098946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235672 (CHEMBL4061606) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of VKORC1 in rat liver microsomes in presence of 0.003 to 0.2 mM vitamin K | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235674 (CHEMBL4070018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of VKORC1 in rat liver microsomes in presence of 0.003 to 0.2 mM vitamin K | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235664 (CHEMBL4083422) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of VKORC1 in rat liver microsomes in presence of 0.003 to 0.2 mM vitamin K | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235665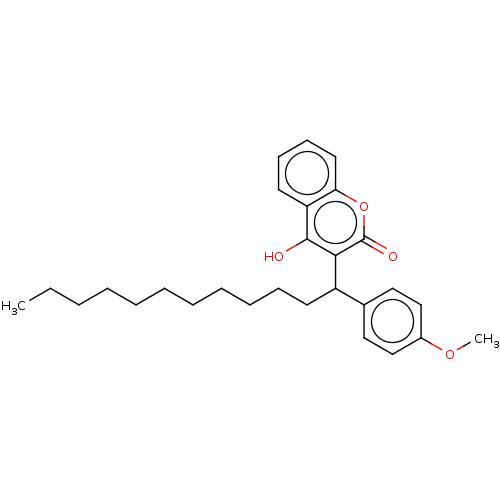 (CHEMBL4104227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme of guinea pig lung membrane | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235669 (CHEMBL4080910) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of VKORC1 in rat liver microsomes in presence of 0.003 to 0.2 mM vitamin K | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM768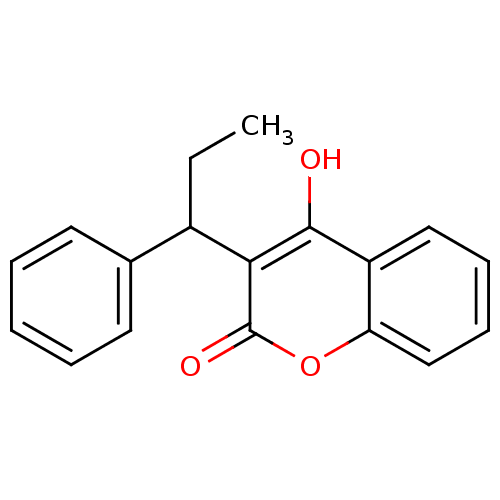 (4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235663 (CHEMBL4070271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50343352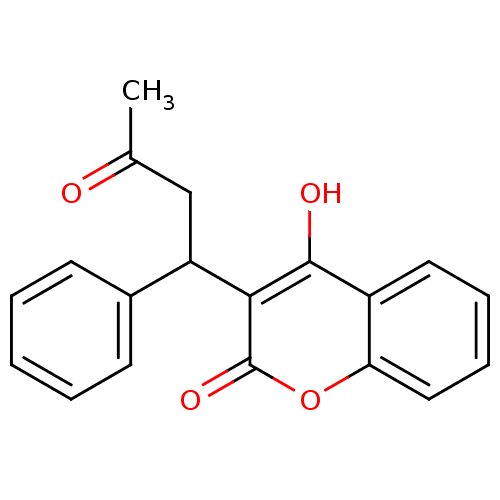 (2-hydroxy-3-(3-oxo-1-phenylbutyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 600 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Institut National de Recherche Agronomique (INRA)-Vetagro Sup, Veterinary School of Lyon | Assay Description Briefly, standard reactions were performed in 200 mM Hepes buffer, pH 7.4, containing 150 mM KCl, 1 mM dithiothreitol, 0.25 to 2 g liter-1... | J Biol Chem 288: 28733-42 (2013) Article DOI: 10.1074/jbc.M113.457119 BindingDB Entry DOI: 10.7270/Q27943HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Homo sapiens (Human)) | BDBM50343352 (2-hydroxy-3-(3-oxo-1-phenylbutyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.80E+3 | -34.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Institut National de Recherche Agronomique (INRA)-Vetagro Sup, Veterinary School of Lyon | Assay Description Briefly, standard reactions were performed in 200 mM Hepes buffer, pH 7.4, containing 150 mM KCl, 1 mM dithiothreitol, 0.25 to 2 g liter-1... | J Biol Chem 288: 28733-42 (2013) Article DOI: 10.1074/jbc.M113.457119 BindingDB Entry DOI: 10.7270/Q27943HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235662 (CHEMBL4091210) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1 (Rattus norvegicus (Rat)) | BDBM50235666 (CHEMBL4104918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1-like protein 1 (Rattus norvegicus (Rat)) | BDBM50343352 (2-hydroxy-3-(3-oxo-1-phenylbutyl)-4H-chromen-4-one...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 3.26E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Institut National de Recherche Agronomique (INRA)-Vetagro Sup, Veterinary School of Lyon | Assay Description Briefly, standard reactions were performed in 200 mM Hepes buffer, pH 7.4, containing 150 mM KCl, 1 mM dithiothreitol, 0.25 to 2 g liter-1... | J Biol Chem 288: 28733-42 (2013) Article DOI: 10.1074/jbc.M113.457119 BindingDB Entry DOI: 10.7270/Q27943HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K epoxide reductase complex subunit 1-like protein 1 (Homo sapiens (Human)) | BDBM50343352 (2-hydroxy-3-(3-oxo-1-phenylbutyl)-4H-chromen-4-one...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 5.20E+4 | -25.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Institut National de Recherche Agronomique (INRA)-Vetagro Sup, Veterinary School of Lyon | Assay Description Briefly, standard reactions were performed in 200 mM Hepes buffer, pH 7.4, containing 150 mM KCl, 1 mM dithiothreitol, 0.25 to 2 g liter-1... | J Biol Chem 288: 28733-42 (2013) Article DOI: 10.1074/jbc.M113.457119 BindingDB Entry DOI: 10.7270/Q27943HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377836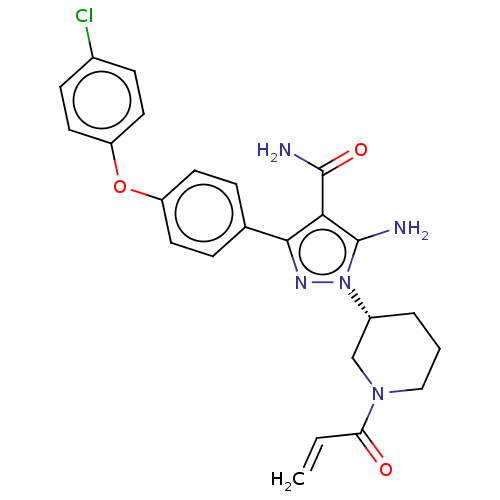 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521321 (CHEMBL4442732) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521322 (CHEMBL4436118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521317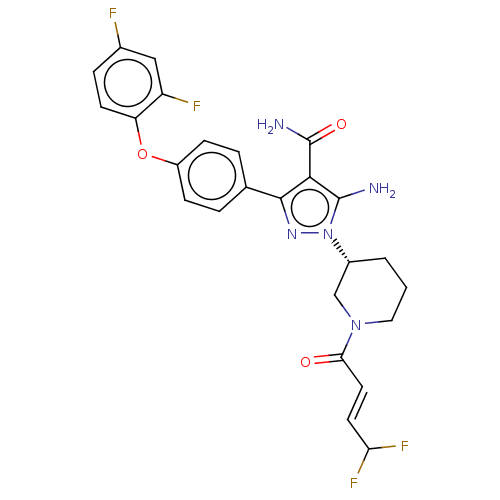 (CHEMBL4559065) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50512857 (CHEMBL4456283 | US10815213, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521316 (CHEMBL4560385) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521315 (CHEMBL4469663) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50512857 (CHEMBL4456283 | US10815213, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521318 (CHEMBL4440096 | US10815213, Example 151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521312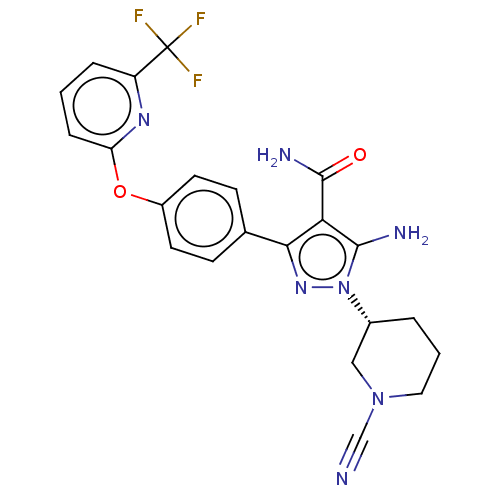 (CHEMBL4456974 | US10815213, Example 116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521314 (CHEMBL4556666 | US10815213, Example 145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521316 (CHEMBL4560385) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521322 (CHEMBL4436118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human C-terminal His6-tagged BTK C481S mutant expressed in Sf9 insect cells using FAM-Srctide peptide as substrate preincub... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human C-terminal His6-tagged BTK C481S mutant expressed in Sf9 insect cells using FAM-Srctide peptide as substrate preincub... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521313 (CHEMBL4572913) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50521322 (CHEMBL4436118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human C-terminal His-tagged SRC cytoplasmic domain expressed in baculovirus expression system using FAM-Srctide... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521311 (CHEMBL4591391 | US10815213, Example 150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521315 (CHEMBL4469663) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377824 ((R)-5-amino-3-(4-((5-chloro-3-fluoropyridin-2-yl)o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521312 (CHEMBL4456974 | US10815213, Example 116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM377836 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human EGFR cytoplasmic domain expressed in baculovirus expression system using FITC-C6-KKAEEEEYFELVAKK-NH2 as substrate preincubated fo... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521321 (CHEMBL4442732) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human C-terminal His6-tagged BTK C481S mutant expressed in Sf9 insect cells using FAM-Srctide peptide as substrate preincub... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human EGFR cytoplasmic domain expressed in baculovirus expression system using FITC-C6-KKAEEEEYFELVAKK-NH2 as substrate preincubated fo... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377824 ((R)-5-amino-3-(4-((5-chloro-3-fluoropyridin-2-yl)o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50512857 (CHEMBL4456283 | US10815213, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in human whole blood assessed as reduction in anti-human IgE antibody-stimulated histamine release pretreated for 2 hrs followed by... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50521321 (CHEMBL4442732) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human EGFR cytoplasmic domain expressed in baculovirus expression system using FITC-C6-KKAEEEEYFELVAKK-NH2 as substrate preincubated fo... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521318 (CHEMBL4440096 | US10815213, Example 151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human C-terminal His6-tagged BTK C481S mutant expressed in Sf9 insect cells using FAM-Srctide peptide as substrate preincub... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521312 (CHEMBL4456974 | US10815213, Example 116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of CD69 expression on B cells pretreated for 1 ... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521312 (CHEMBL4456974 | US10815213, Example 116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human C-terminal His6-tagged BTK C481S mutant expressed in Sf9 insect cells using FAM-Srctide peptide as substrate preincub... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human C-terminal His-tagged SRC cytoplasmic domain expressed in baculovirus expression system using FAM-Srctide... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |